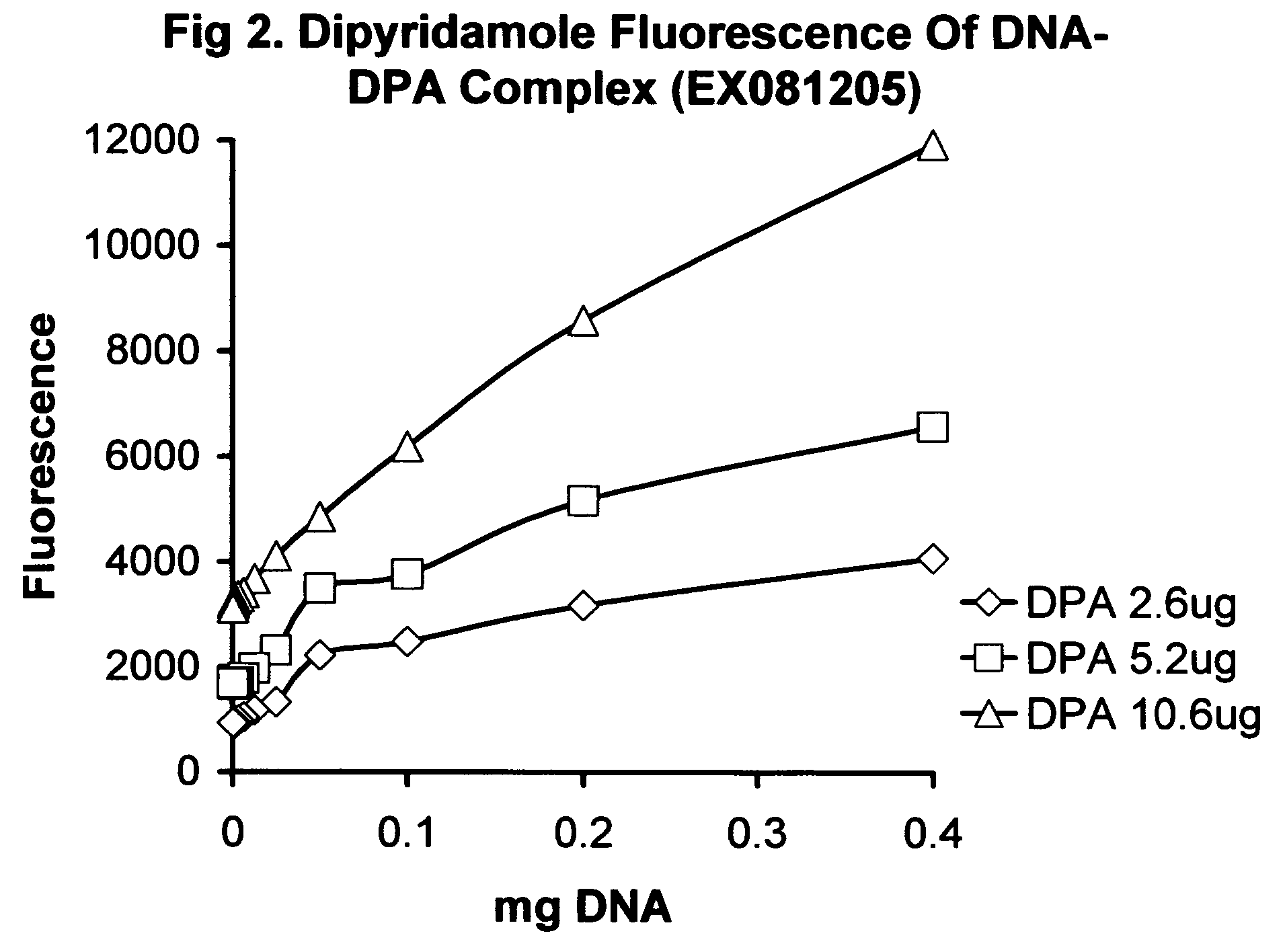
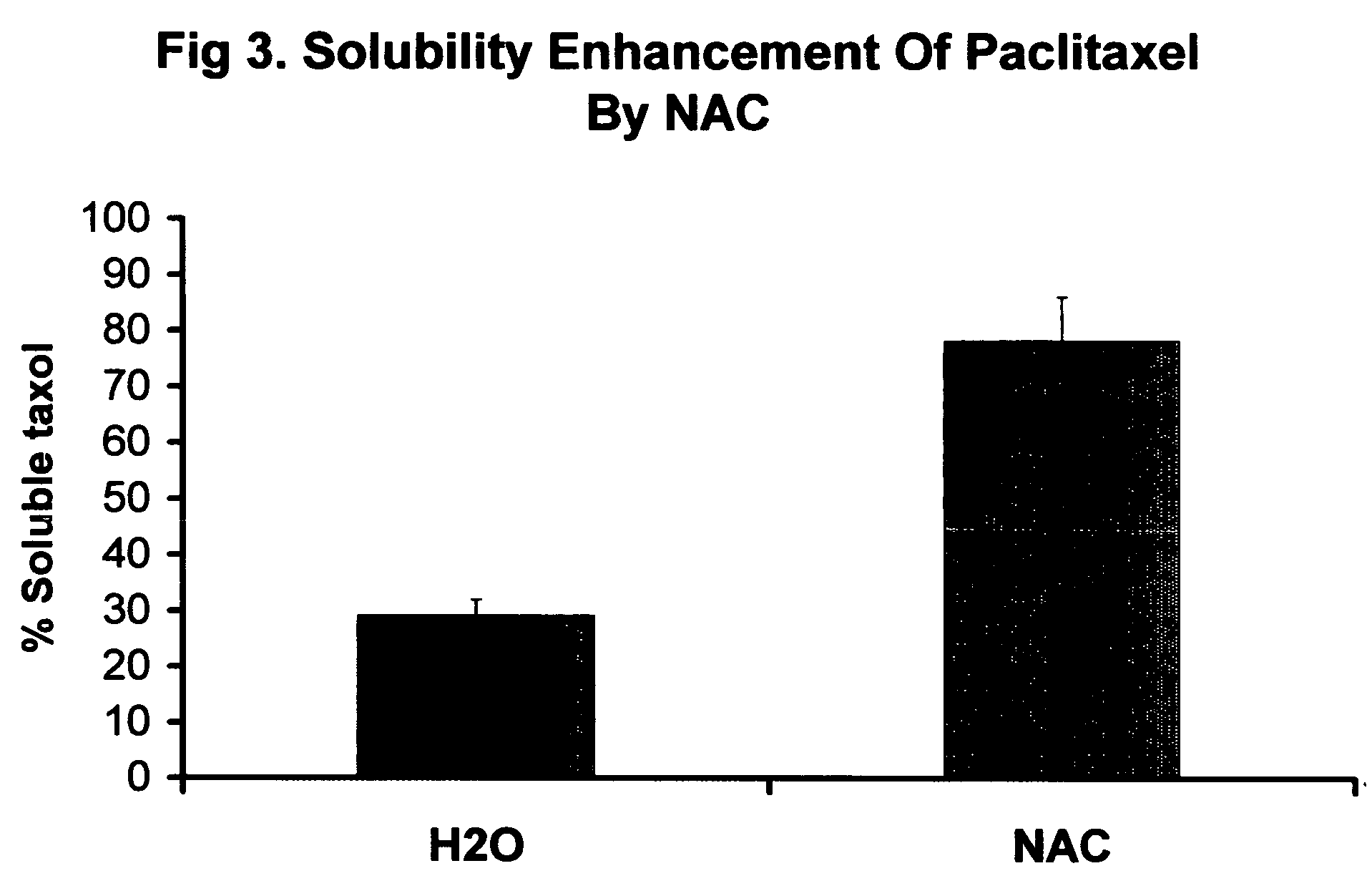
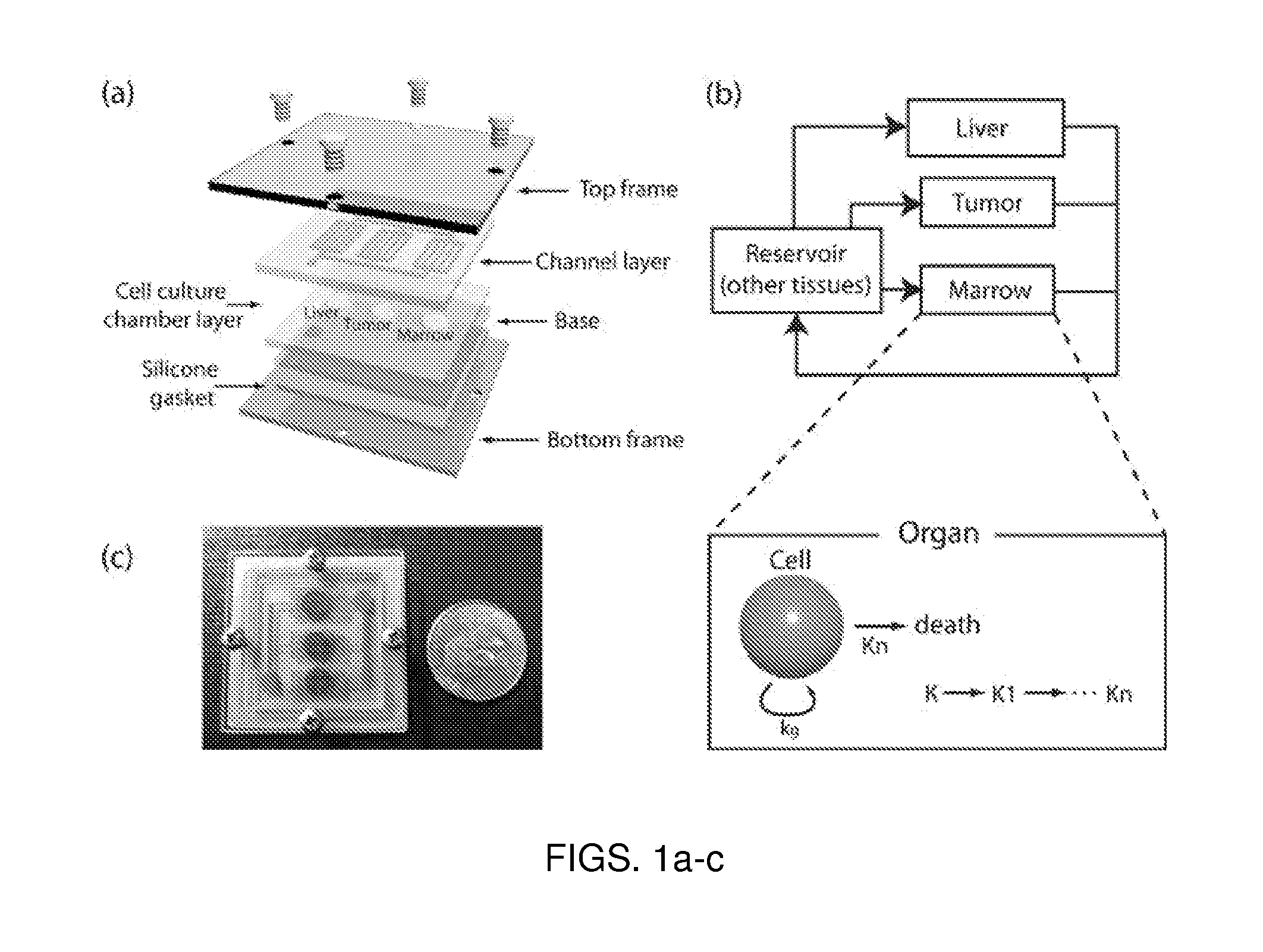
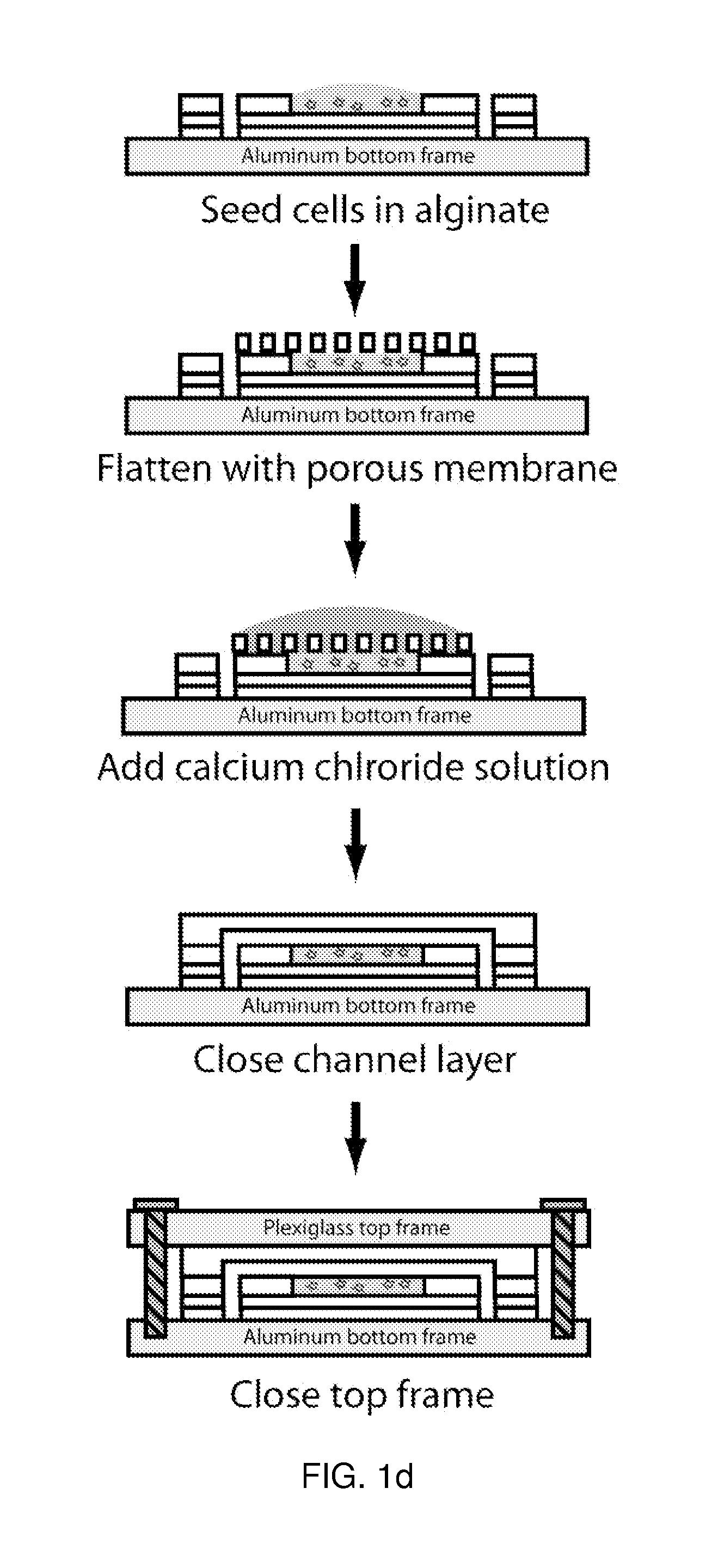
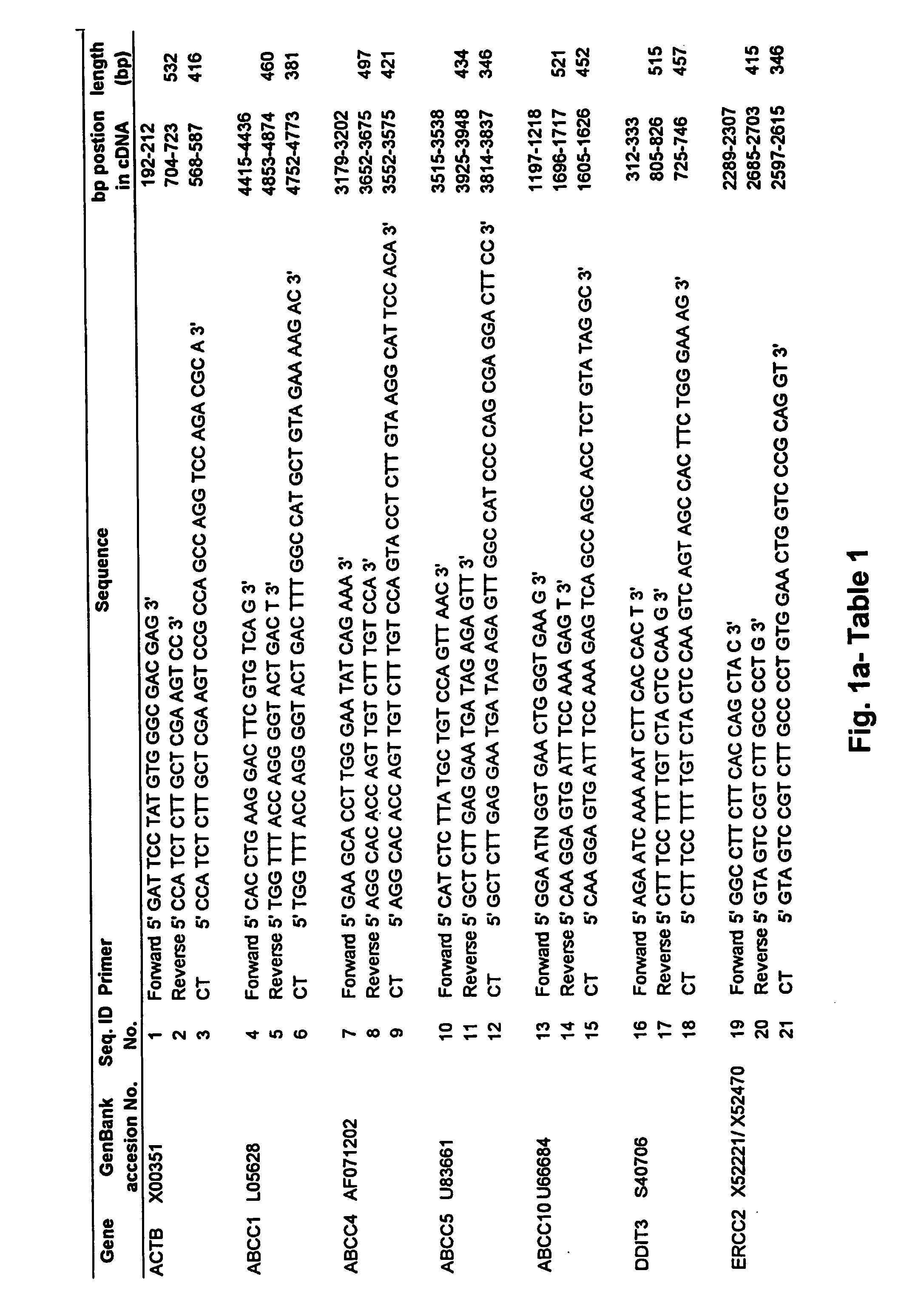
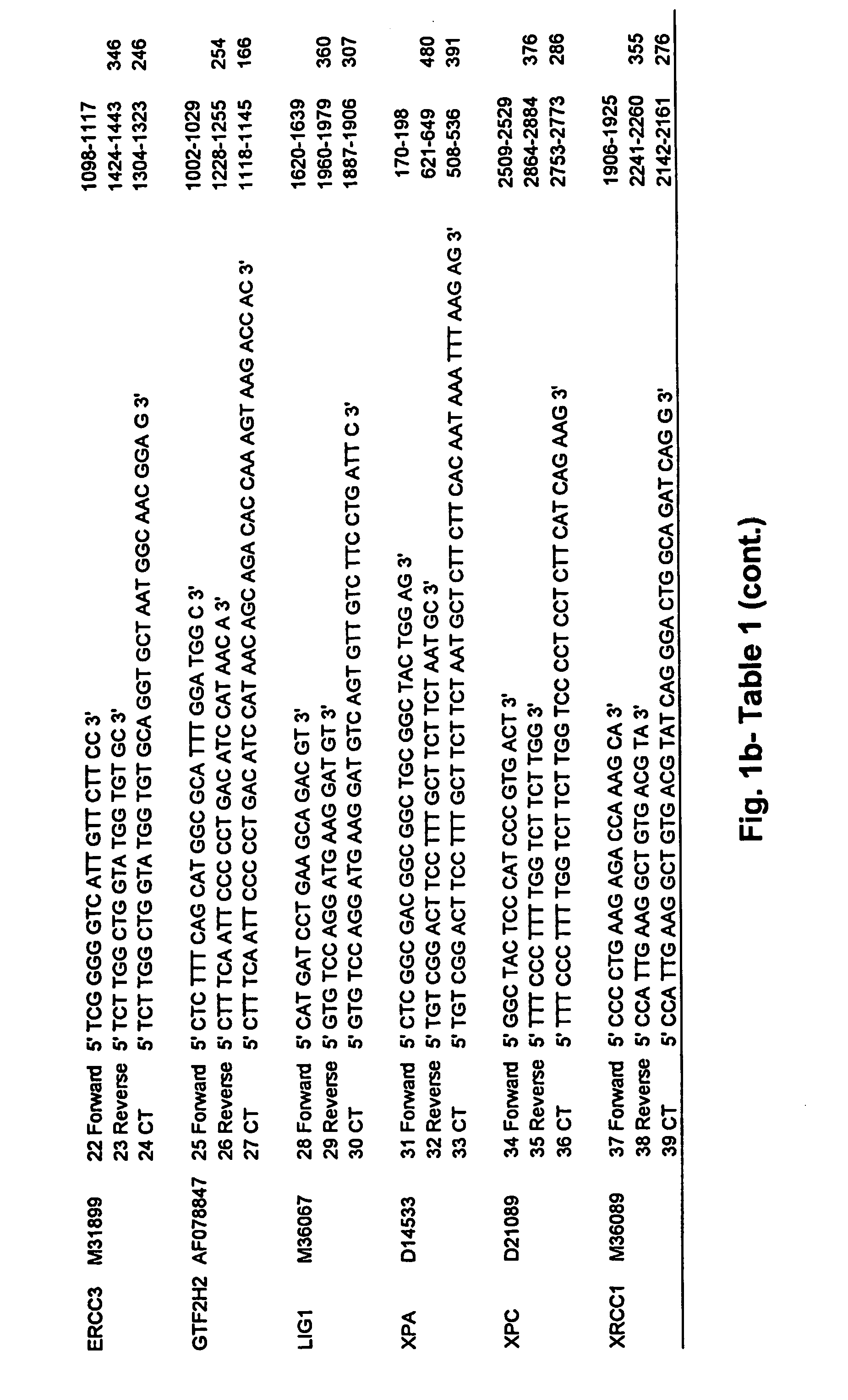
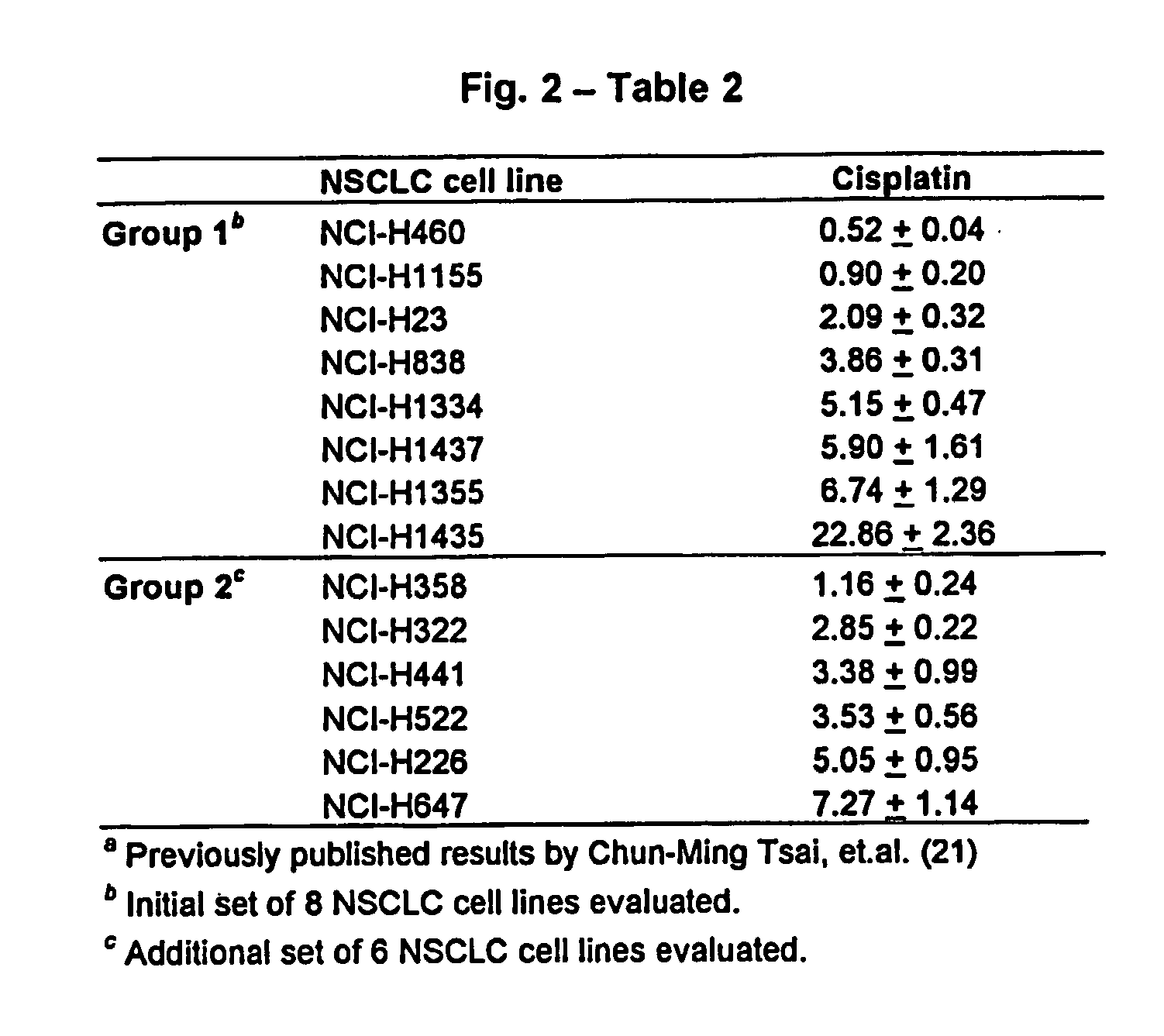
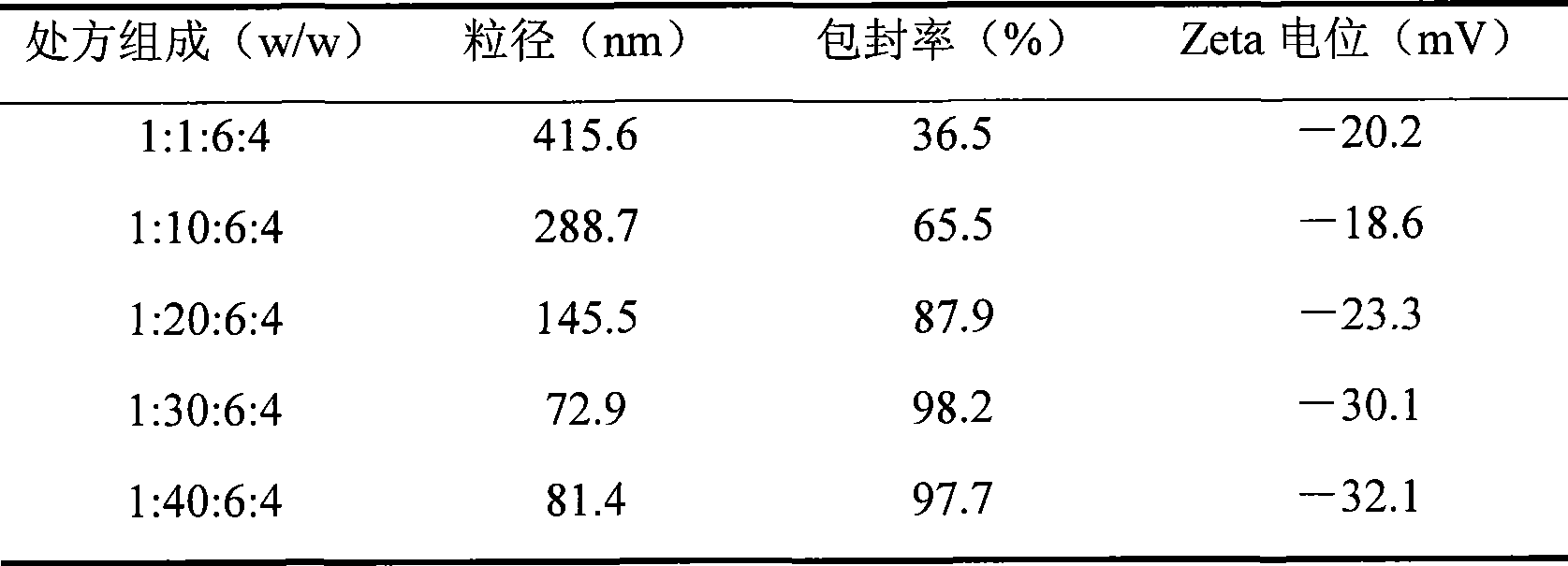
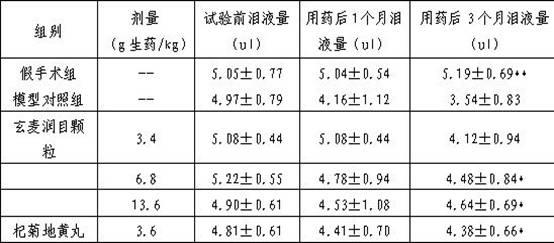
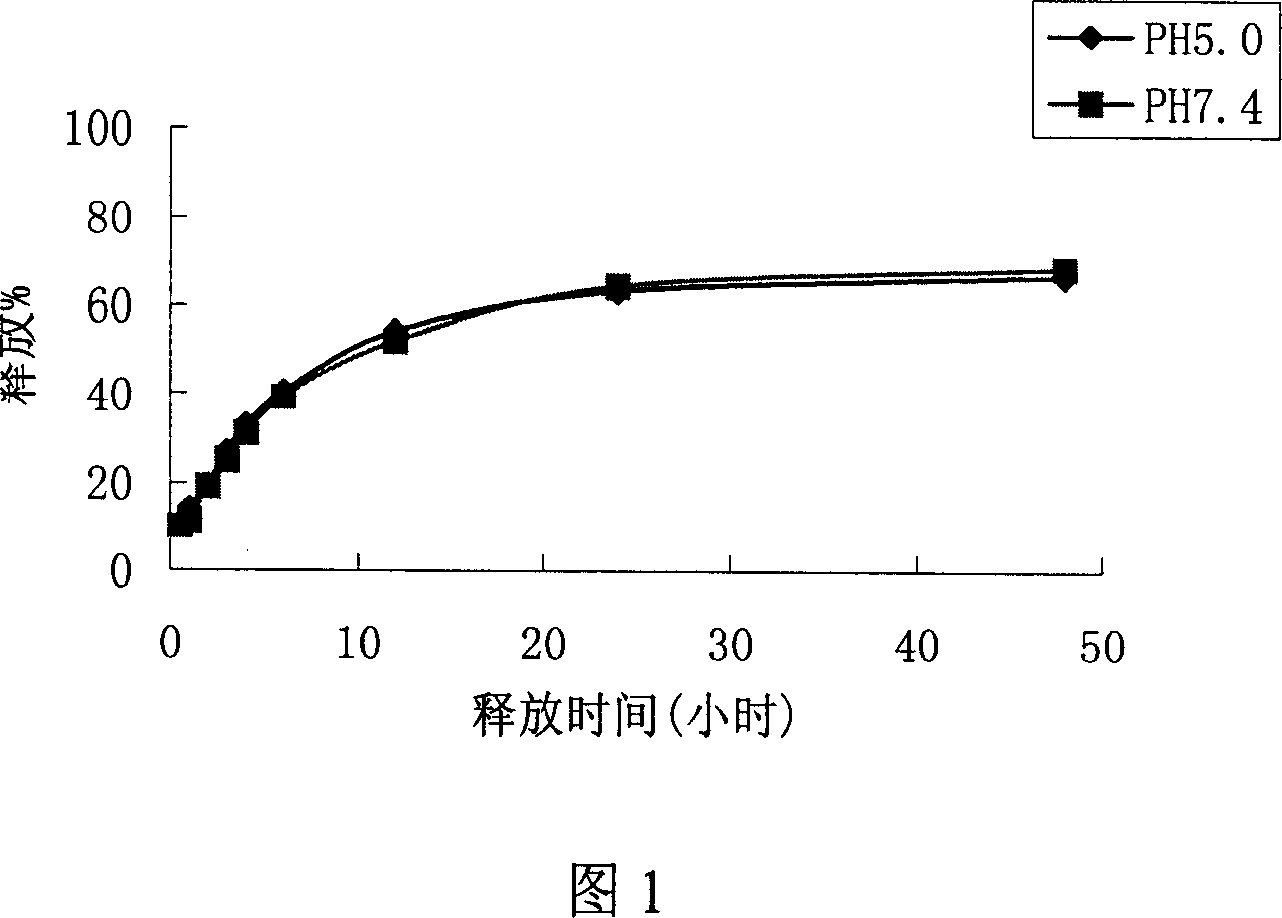
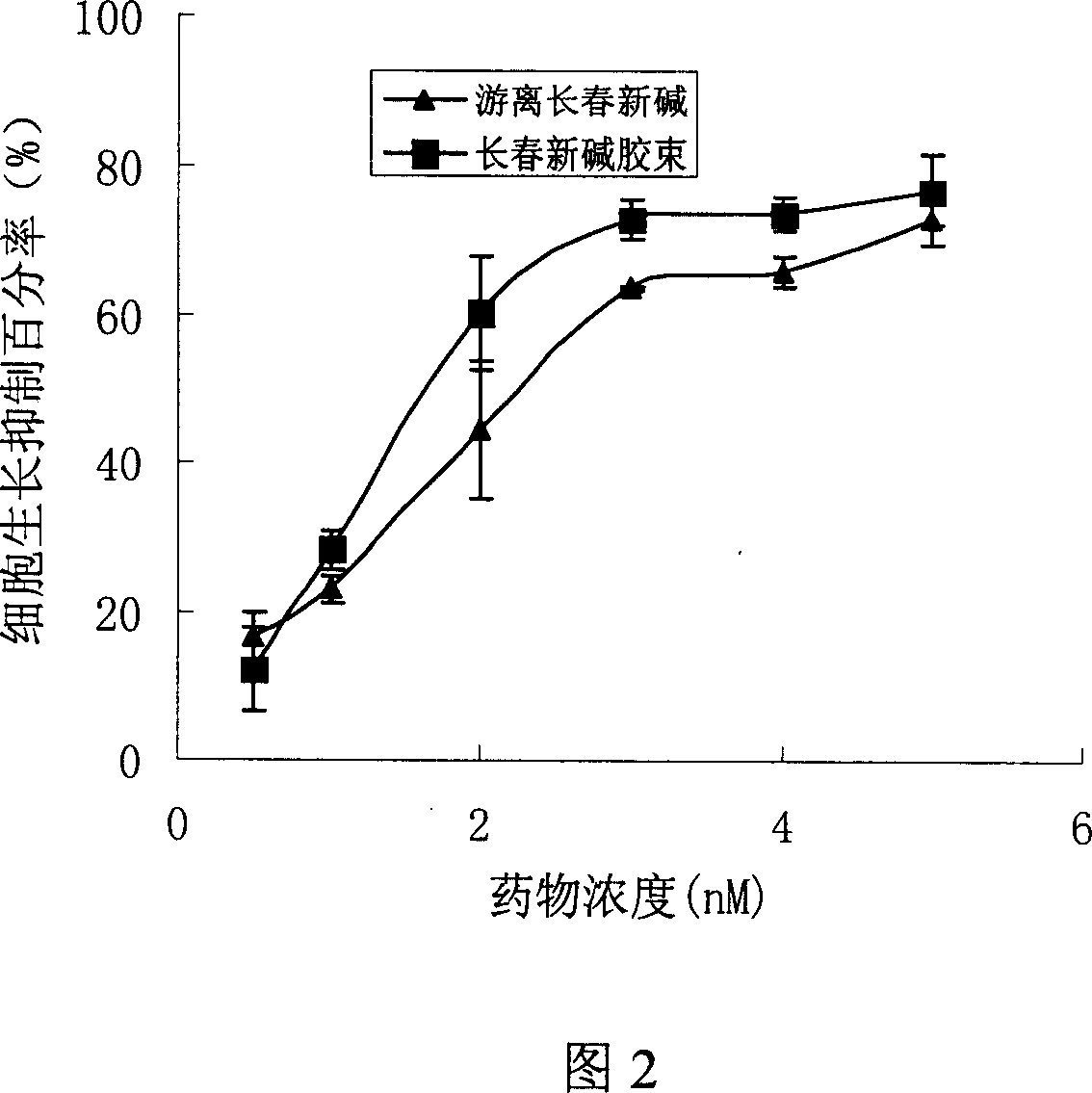
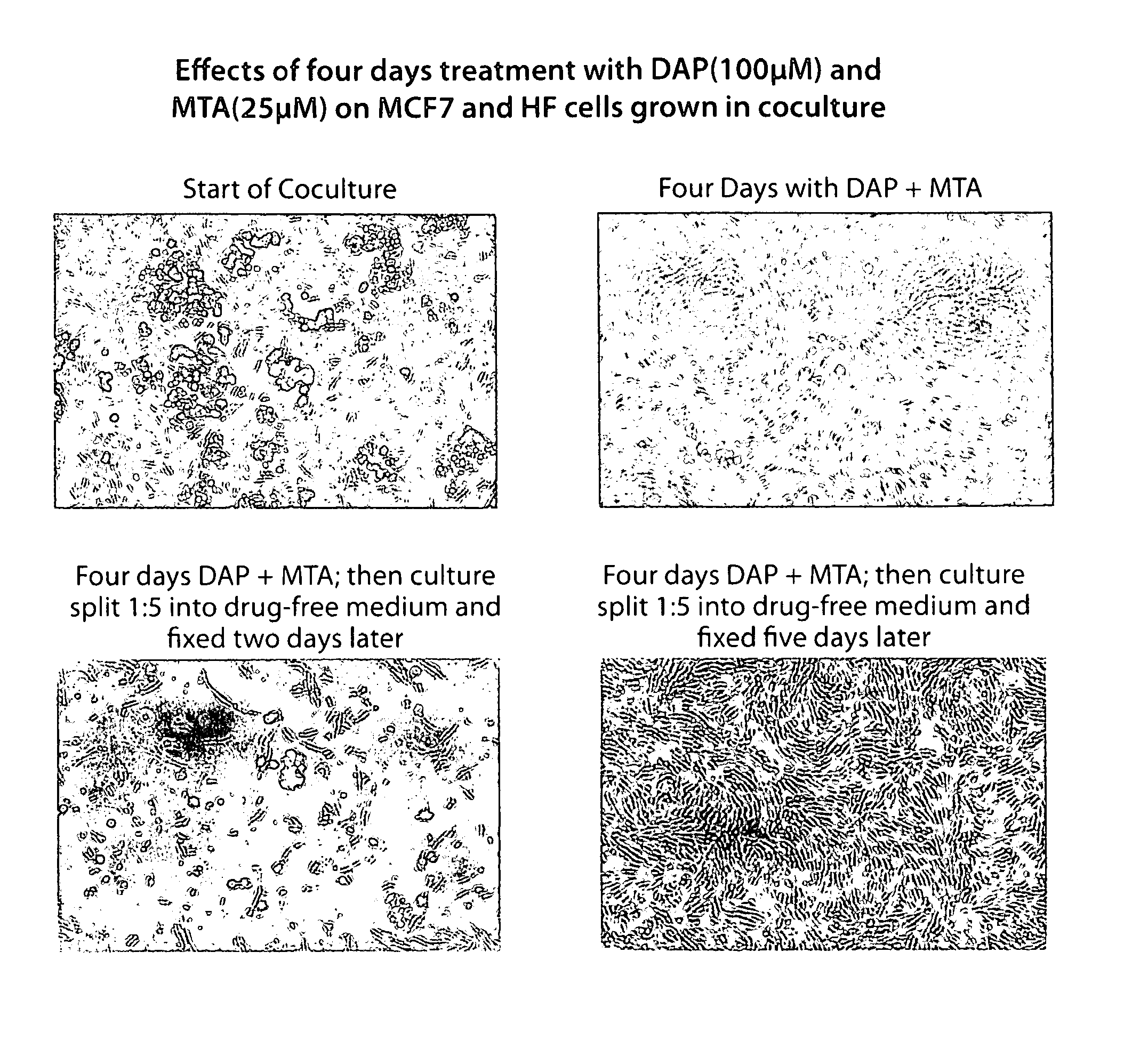
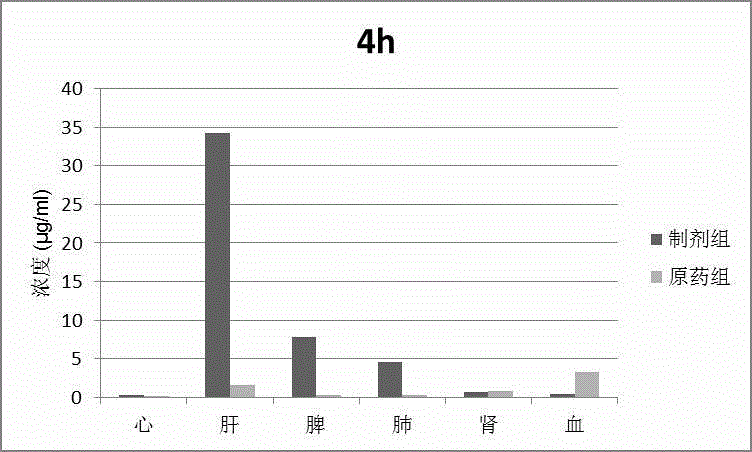
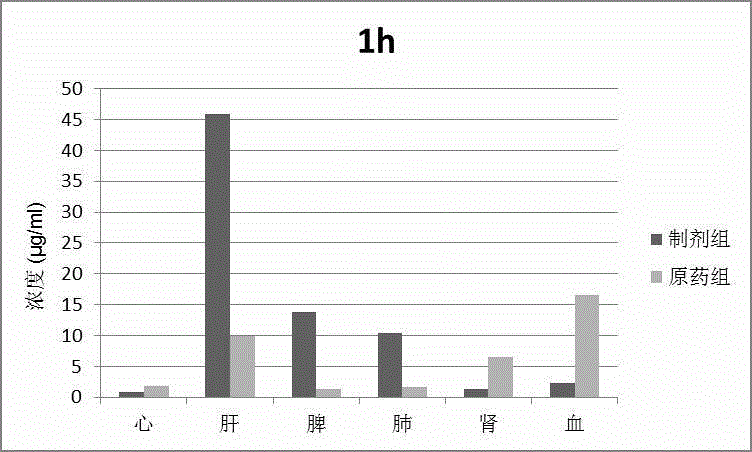
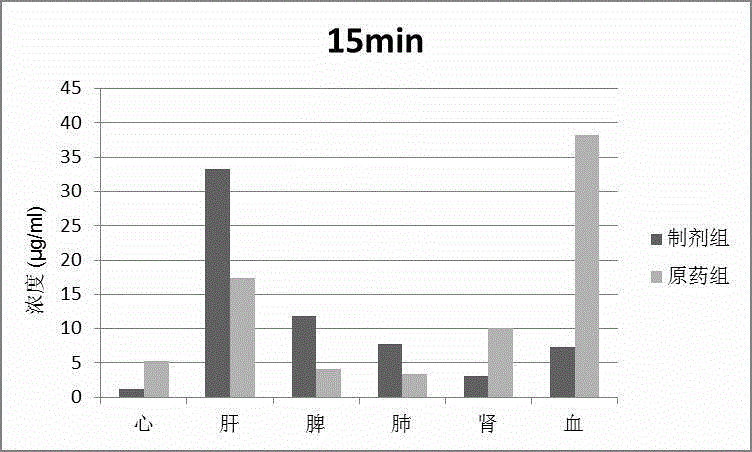
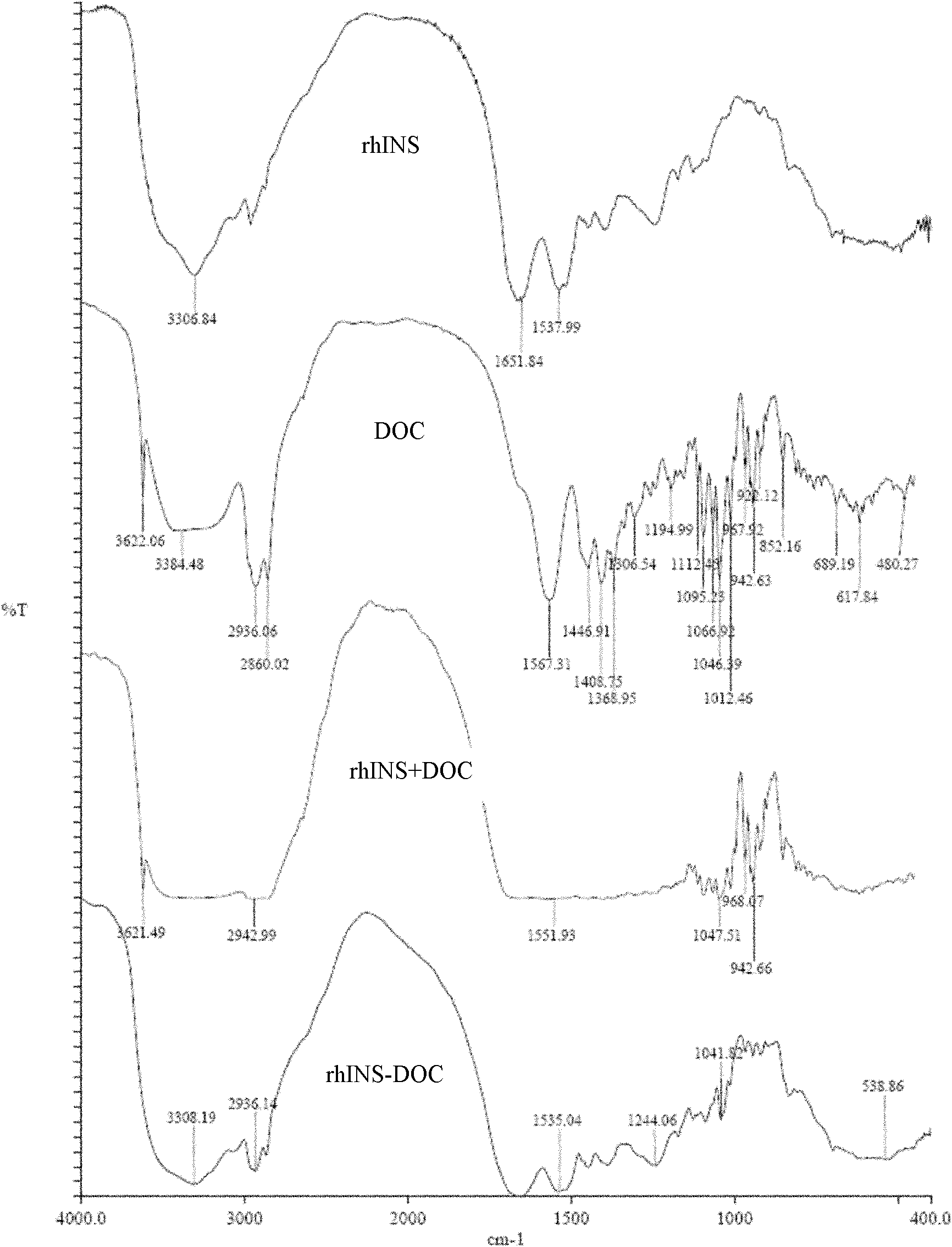
Patents
Literature
350 results about "Drug toxicity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Definition. Drug toxicity refers to the level of damage that a compound can cause to an organism. The toxic effects of a drug are dose-dependent and can affect an entire system as in the CNS or a specific organ such as the liver. Drug toxicity usually occurs at doses that exceed the therapeutic efficacy of a drug; however,...
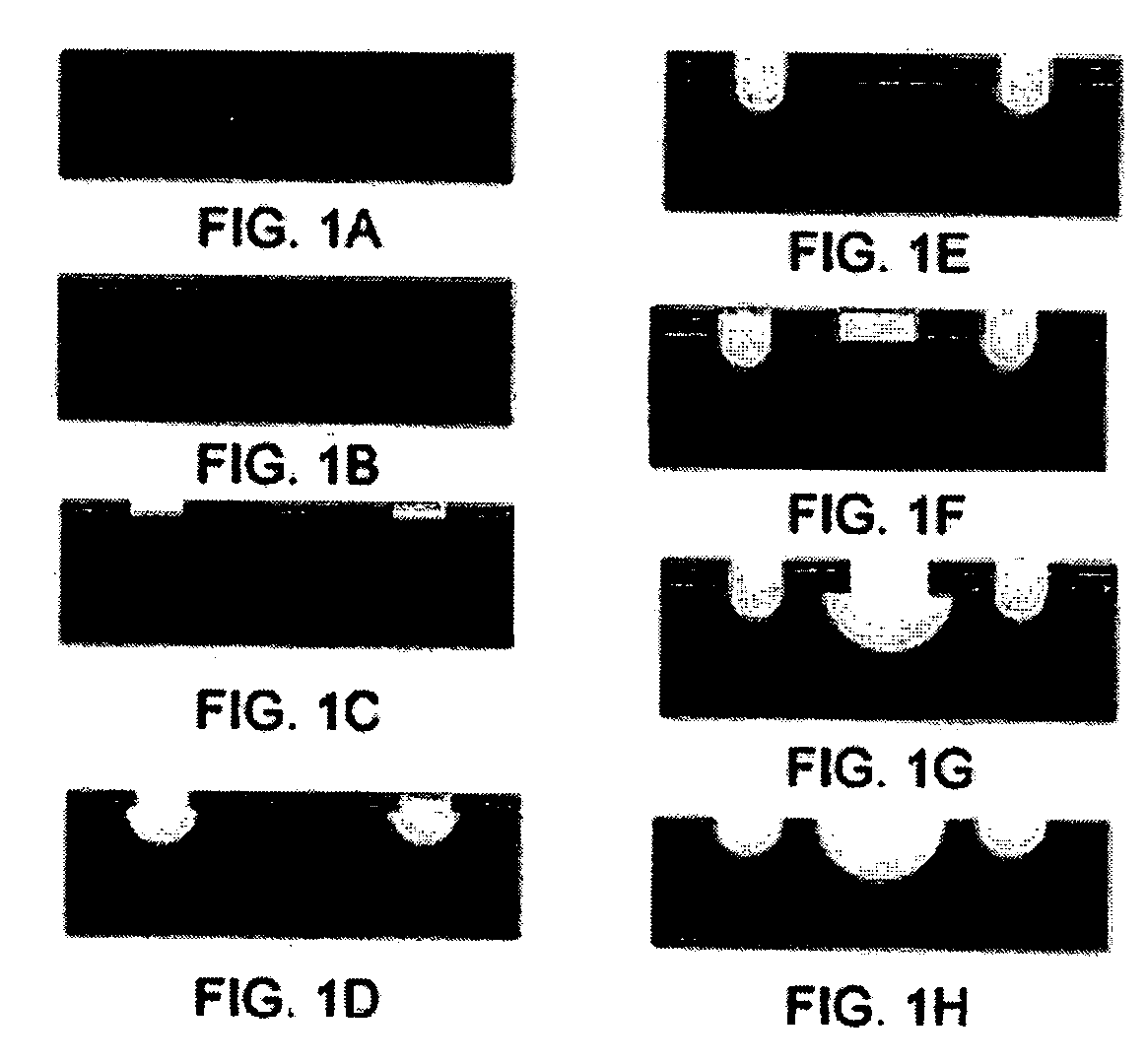
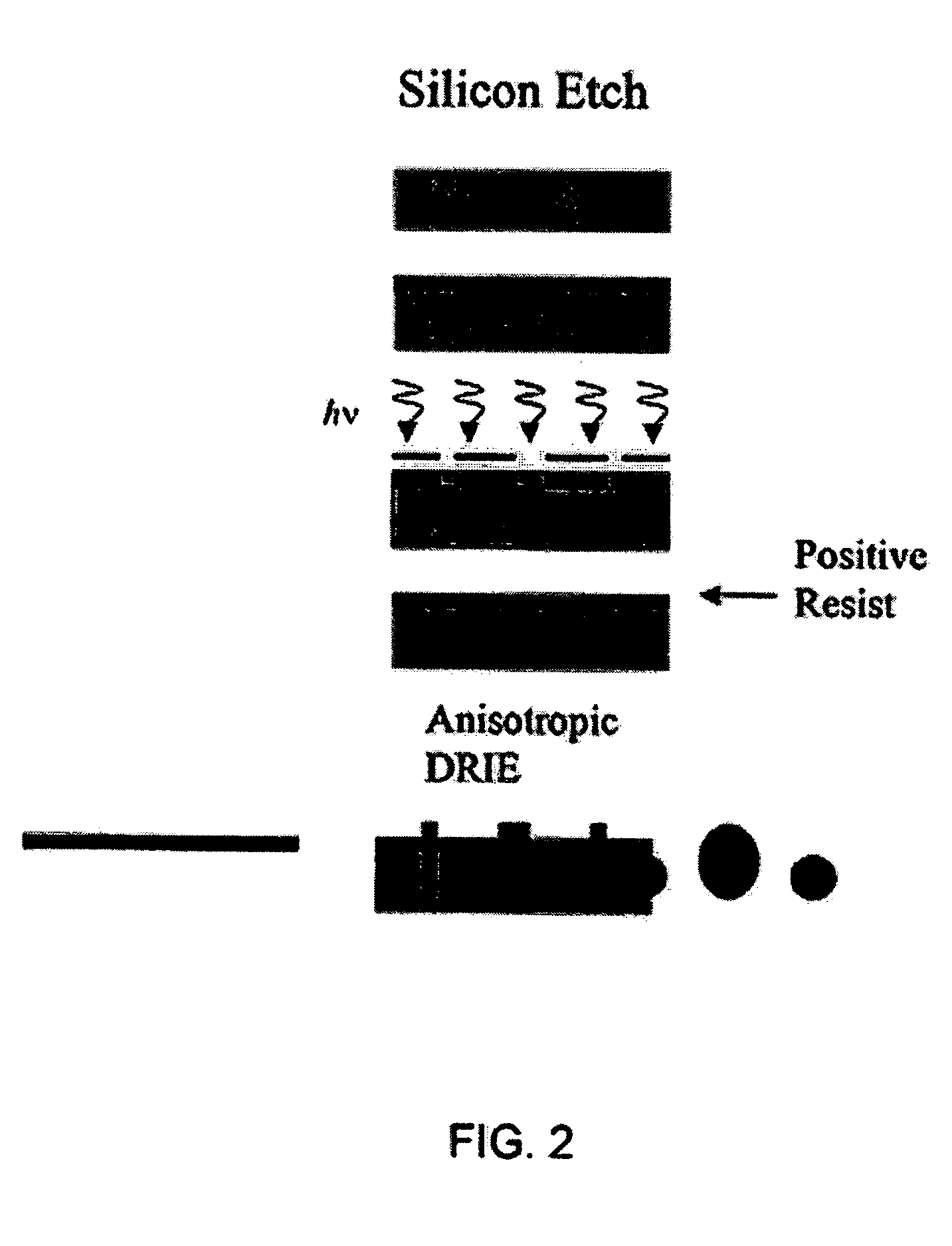
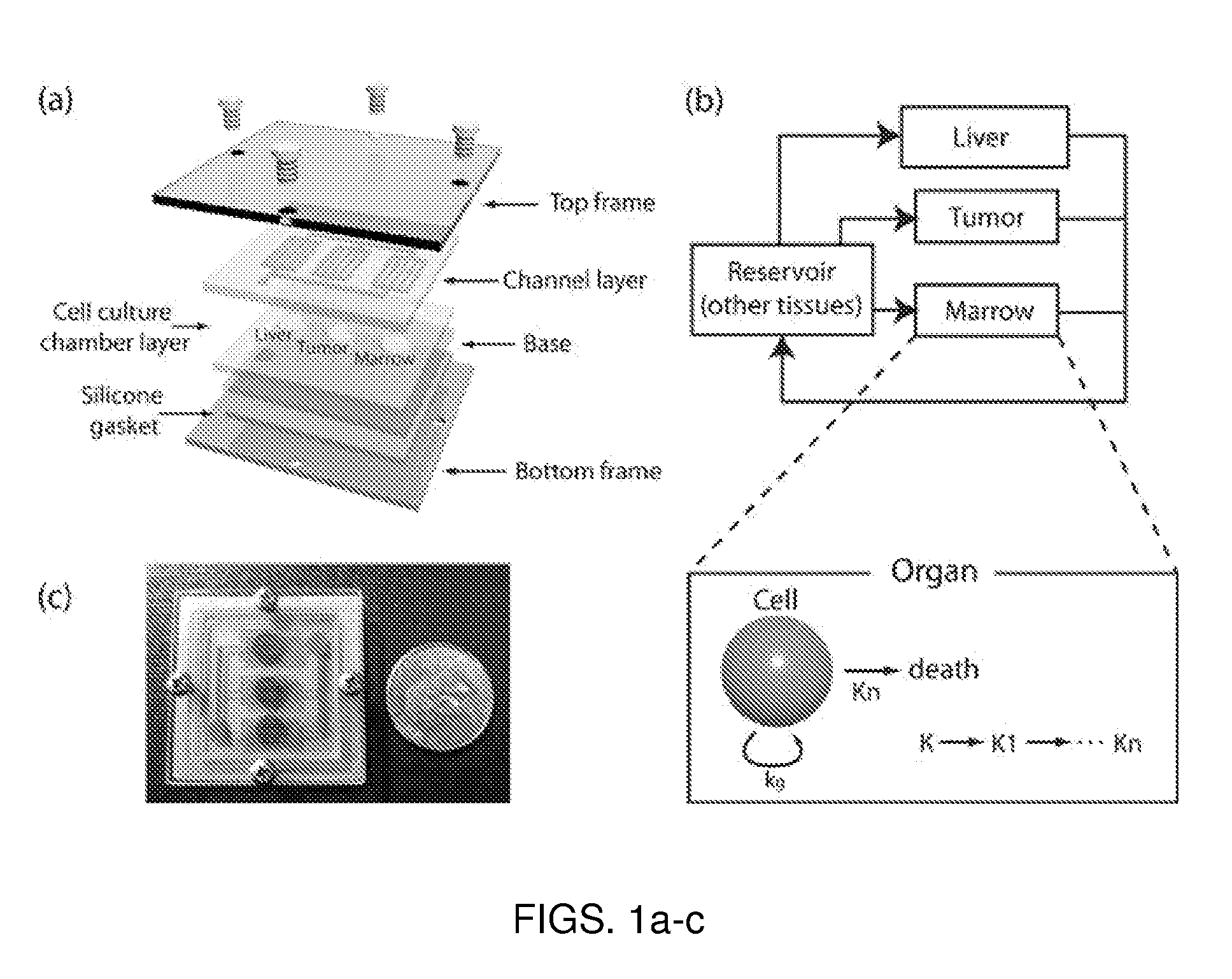
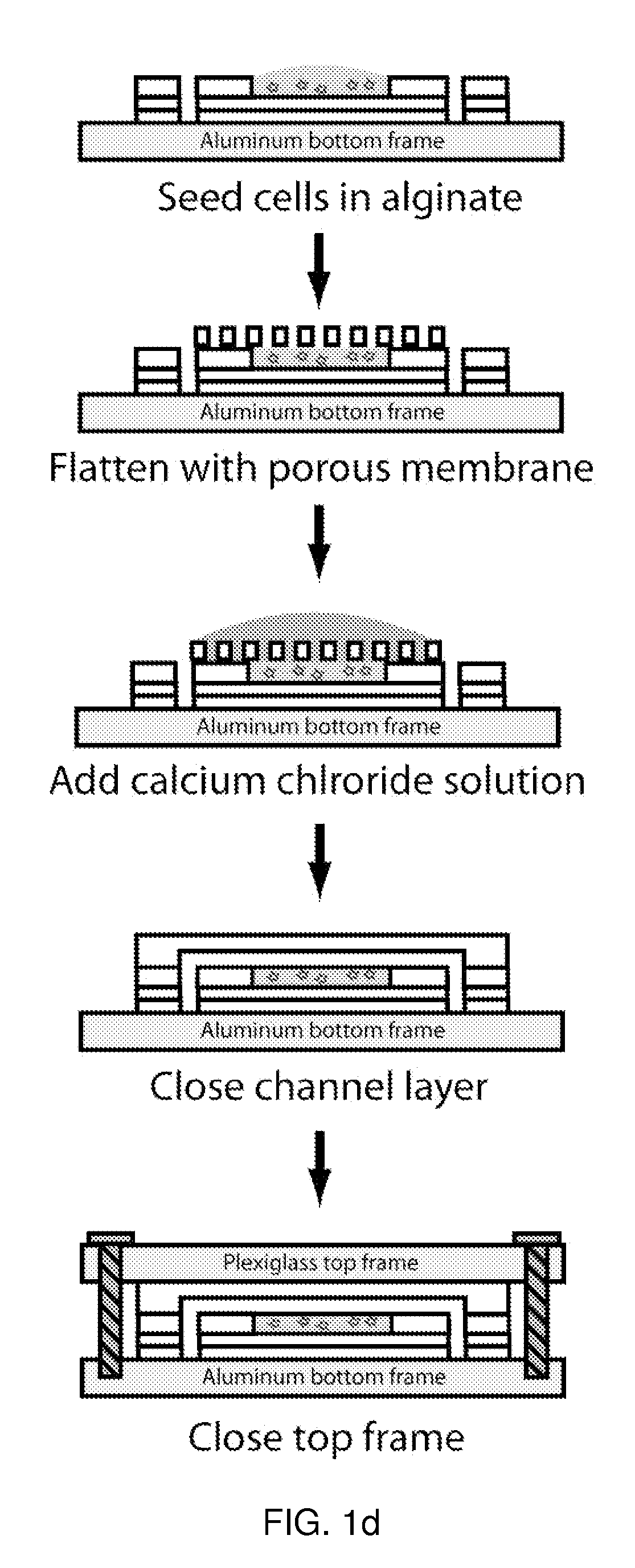
Use of three-dimensional microfabricated tissue engineered systems for pharmacologic applications
ActiveUS20060019326A1Additive manufacturing apparatusMicrobiological testing/measurementExperimental drugSide effect
The present invention generally relates to a combination of the fields of tissue engineering, drug discovery and drug development. It more specifically provides new methods and materials for testing the efficacy and safety of experimental drugs, defining the metabolic pathways of experimental drugs and characterizing the properties (e.g., side effects, new uses) of existing drugs. Preferably, evaluation is carried out in three-dimensional tissue-engineered systems, wherein drug toxicity, metabolism, interaction and / or efficacy can be determined.
Owner:CHARLES STARK DRAPER LABORATORY +1
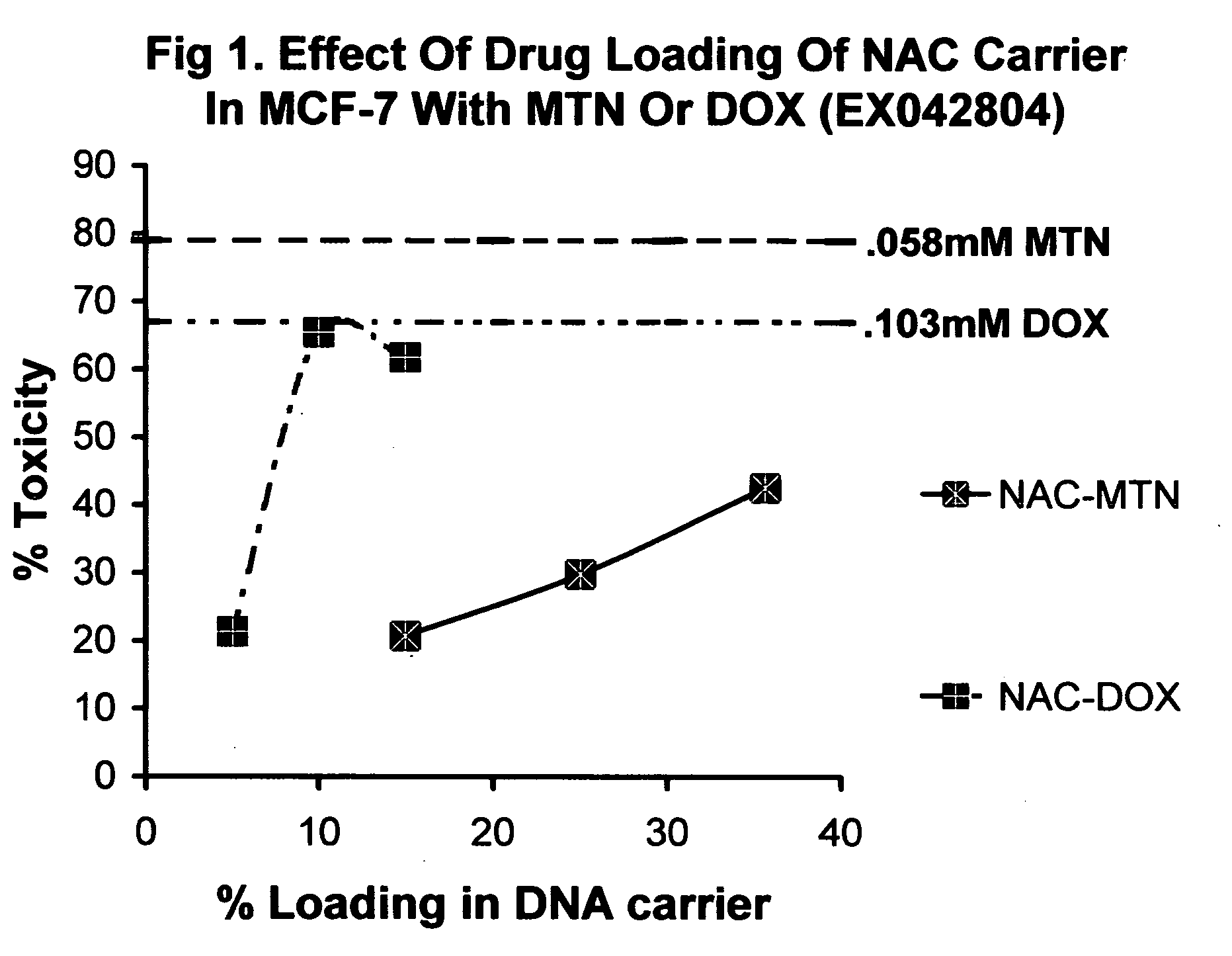
Nucleic acid carriers for delivery of therapeutic agents
InactiveUS20070225213A1Improve solubilityLow biological effectHeavy metal active ingredientsBiocideIn vivoProliferation rate
Nucleic acid drug carriers comprise a nucleic acid carrier complexed with a drug, wherein the nucleic acid carrier and the drug are associated non-covalently, and optionally other agents such as spacer, transfection agents, and targeting agents. The nucleic acid drug complex are discovered to have permissive or refractory uptake depending on many factors including cell type, proliferation rate, among others. The refractive uptake of the nucleic acid drug complex are shown to be useful in the nucleic acid targeting of drugs, both in vitro and in vivo. Novel drug compositions are disclosed that effectively reduce the toxicity of drugs while maintaining drug activity and enhancing a drug's therapeutic index.
Owner:KOSAK MATTHEW K
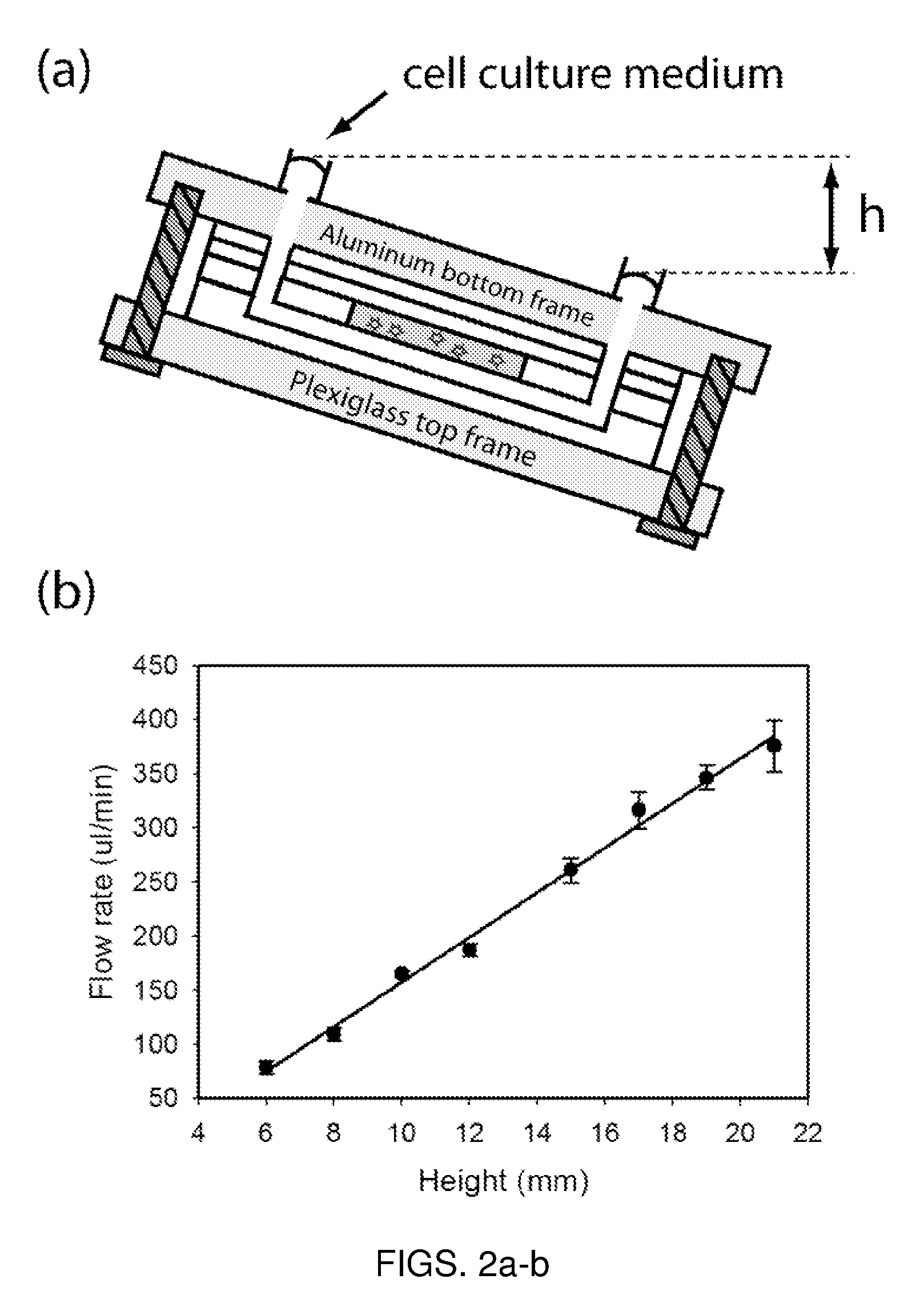
Microfluidic device for pharmacokinetic-pharmacodynamic study of drugs and uses thereof
ActiveUS20120135452A1Bioreactor/fermenter combinationsCompound screeningTissues typesMicrofluidic channel
A microfluidic device for culturing cells, termed a microscale cell culture analog (μCCA), is provided. The microfluidic device allows multiple cell or tissue types to be cultured in a physiologically relevant environment, facilitates high-throughput operation and can be used for drug discovery. The microfluidic device uses gravity-induced fluidic flow, eliminating the need for a pump and preventing formation of air bubbles. Reciprocating motion between a pair of connected reservoirs is used to effect the gravity-induced flow in microfluidic channels. Bacterial contamination is reduced and high throughput enabled by eliminating a pump. The microfluidic device integrates a pharmacokinetic-pharmacodynamic (PK-PD) model to enable PK-PD analyses on-chip. This combined in vitro / in silico system enables prediction of drug toxicity in a more realistic manner than conventional in vitro systems.
Owner:CORNELL UNIVERSITY
Methods and platforms for drug discovery
The present invention involves methods for identifying an agent that corrects a phenotype associated with a health condition or a predisposition for a health condition. The invention also involves methods for identifying a diagnostic cellular phenotype, determining the risk of a health condition in a subject, methods for reducing the risk of drug toxicity in a human subject, and methods for identifying a candidate gene that contributes to a human disease. The invention also discloses human induced pluripotent stem cell lines.
Owner:KYOTO UNIV
Method and compositions for the diagnosis and treatment of non-small cell lung cancer using gene expression profiles
ActiveUS20050260586A1Accurate diagnosisAccurately diagnose lung cancerMicrobiological testing/measurementFermentationDisease progressionBiology
The present invention identifies and quantifies changes in gene expression associated with non-small cell lung cancer NSCLC by examining gene expression in tissue from normal lung and diseased lung. The present invention also identifies and quantifies expression profiles which serve as useful diagnostic markers as well as markers that are useful to monitor disease states, disease progression, drug toxicity, drug efficacy and drug metabolism.
Owner:UNIVERSITY OF TOLEDO
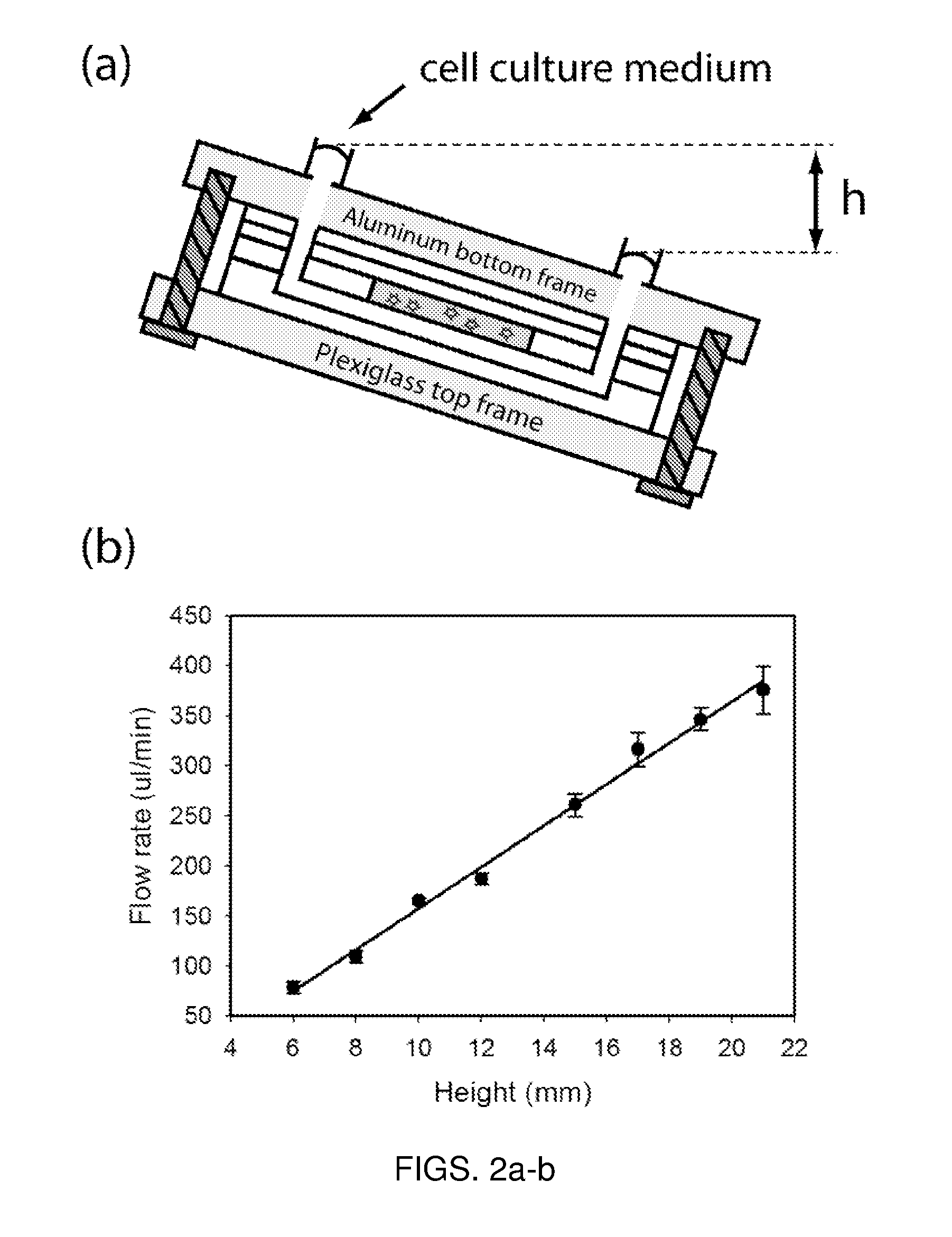
Well-based flow system for cell culture
InactiveUS20110091930A1Improve throughputImproved fluidic routingBioreactor/fermenter combinationsBiological substance pretreatmentsSmooth muscle3D cell culture
A well-based flow system for cell culture is described which provides for flow of culture containing compounds for drug screening to be exposed to cells seeded on a membrane. The flow of medium may be planar or radial and means are provided for the removal of waste media through fluid outlets in fluid communication with the assay well plates through conduits. Methods of using the system for cell culture and drug toxicity screening are also provided including coculturing cells such as hepatocytes, stem cells, fibroblasts and smooth muscle cells and selectively exposing cells to test compounds.
Owner:THE GENERAL HOSPITAL CORP
Biogastrone acid prosome liposome with long circulation function and preparation method thereof
InactiveCN101366698AAchieve long cycleSmall particle sizeOrganic active ingredientsMetabolism disorderFreeze-dryingCholesterol
The invention belongs to the new technical field of a drug preparation and in particular relates to a precursor liposome containing a glycyrrhetinic acid and a method for preparing the same. The precursor liposome containing the glycyrrhetinic acid consists of glycyrrhetinic acid, phospholipids, cholesterol, a surfactant and a water-soluble material; a suspension solution of the glycyrrhetinic acid liposome is prepared by an ethanol injection method or a thin-film dispersion method; and solid liposome powder is prepared by a freeze drying method or a spray drying method. The precursor liposome has simple process and good repeatability, is suitable for industrialized production; through drug administration by intravenous injection, the precursor liposome has long-circulating function, can remarkably reduce the toxicity of drug and achieve the function of prolonging the drug effect; and through oral administration, a solid preparation of the liposome can increase absorption and improve bioavailability by three to five times.
Owner:CHINA PHARM UNIV
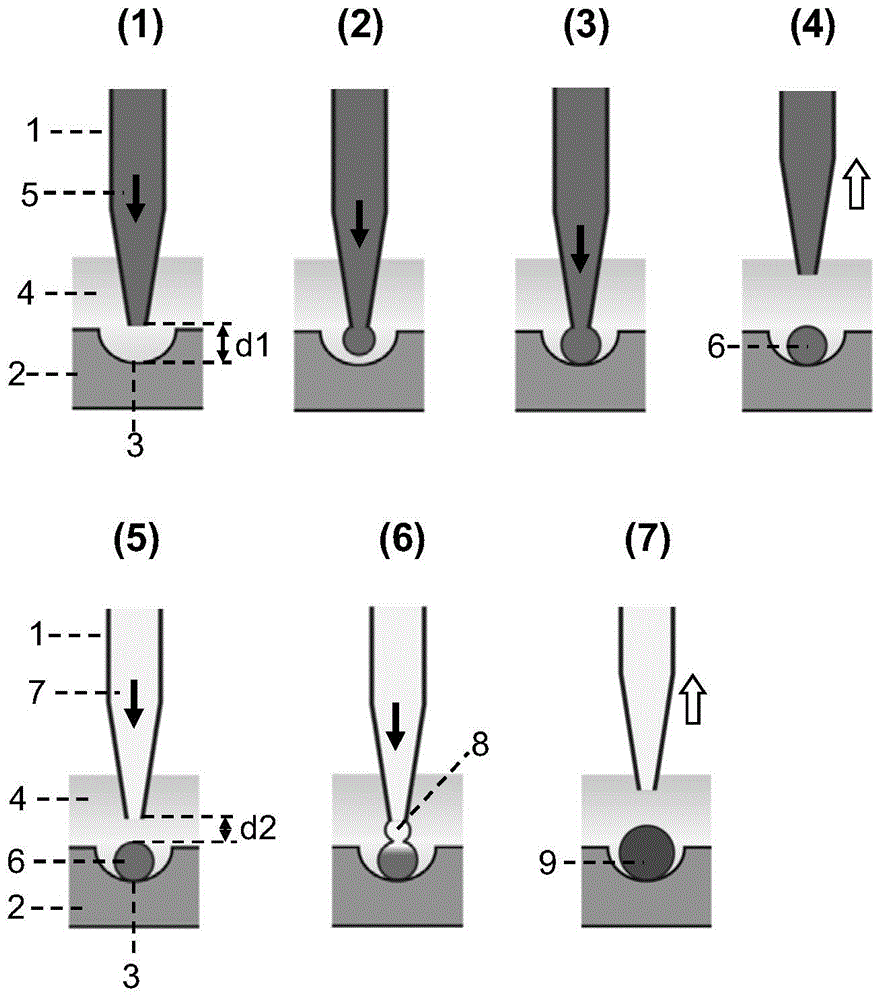
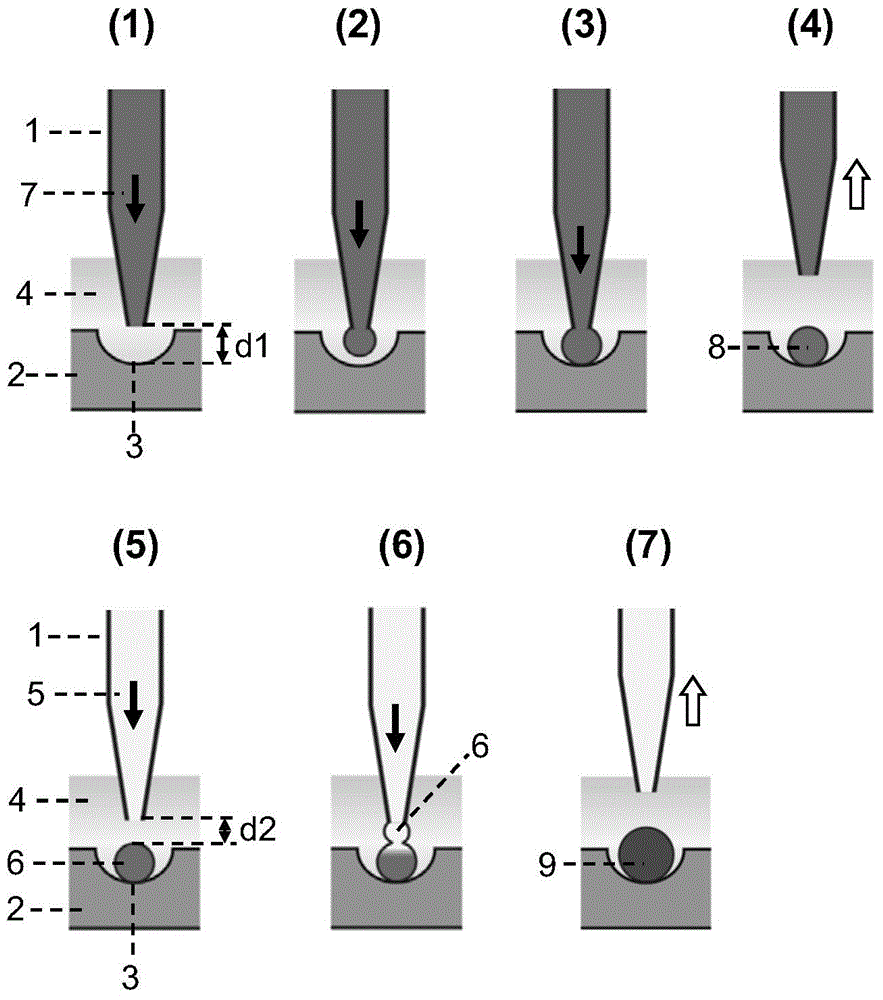
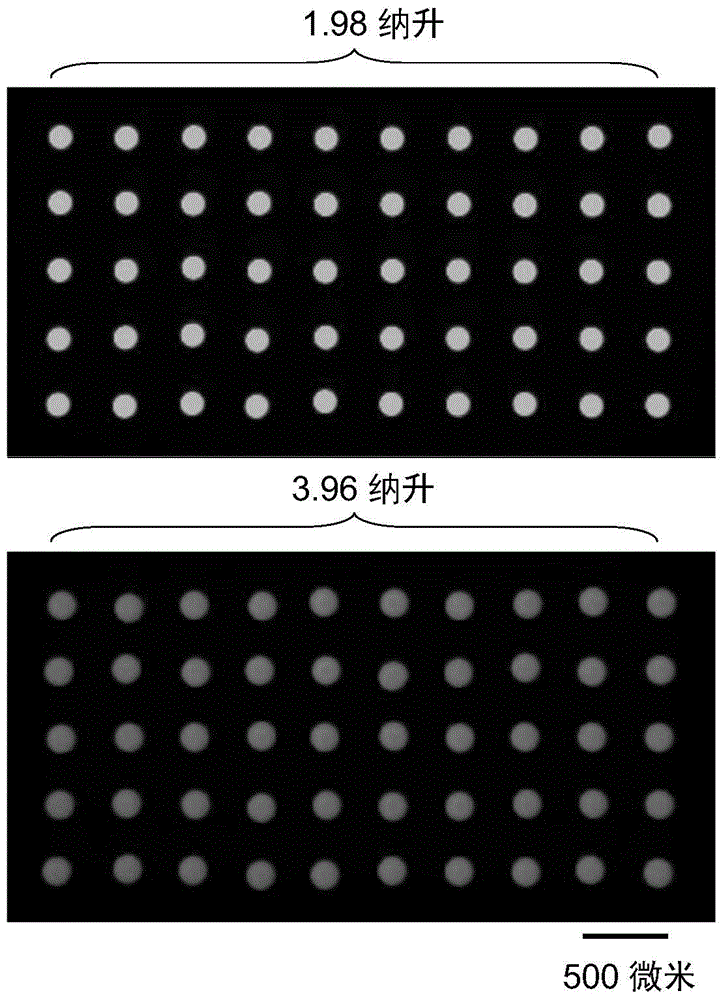
Semi-contact under-oil continuous droplet sample applying and liquid adding method
The invention provides a semi-contact under-oil continuous droplet sample applying and liquid adding method, which is suitable for sequentially operating a droplet array system. According to the method, the distance between the pointed end of a capillary tube sample applying needle and the lower surface of a micro-hole (or a generated droplet) is controlled accurately, and the affinity or interface tension interaction between the droplet and the surface (or the generated droplet) is utilized, so that rapid and reliable continuous droplet sample applying or continuous liquid adding is realized, and the problem of cross infection during sample applying is solved effectively. The method is suitable for biochemical analysis screening researches such as high-flux medicament screening, protein crystallization condition screening, enzyme kinetics research and drug toxicity determination.
Owner:ZHEJIANG UNIV
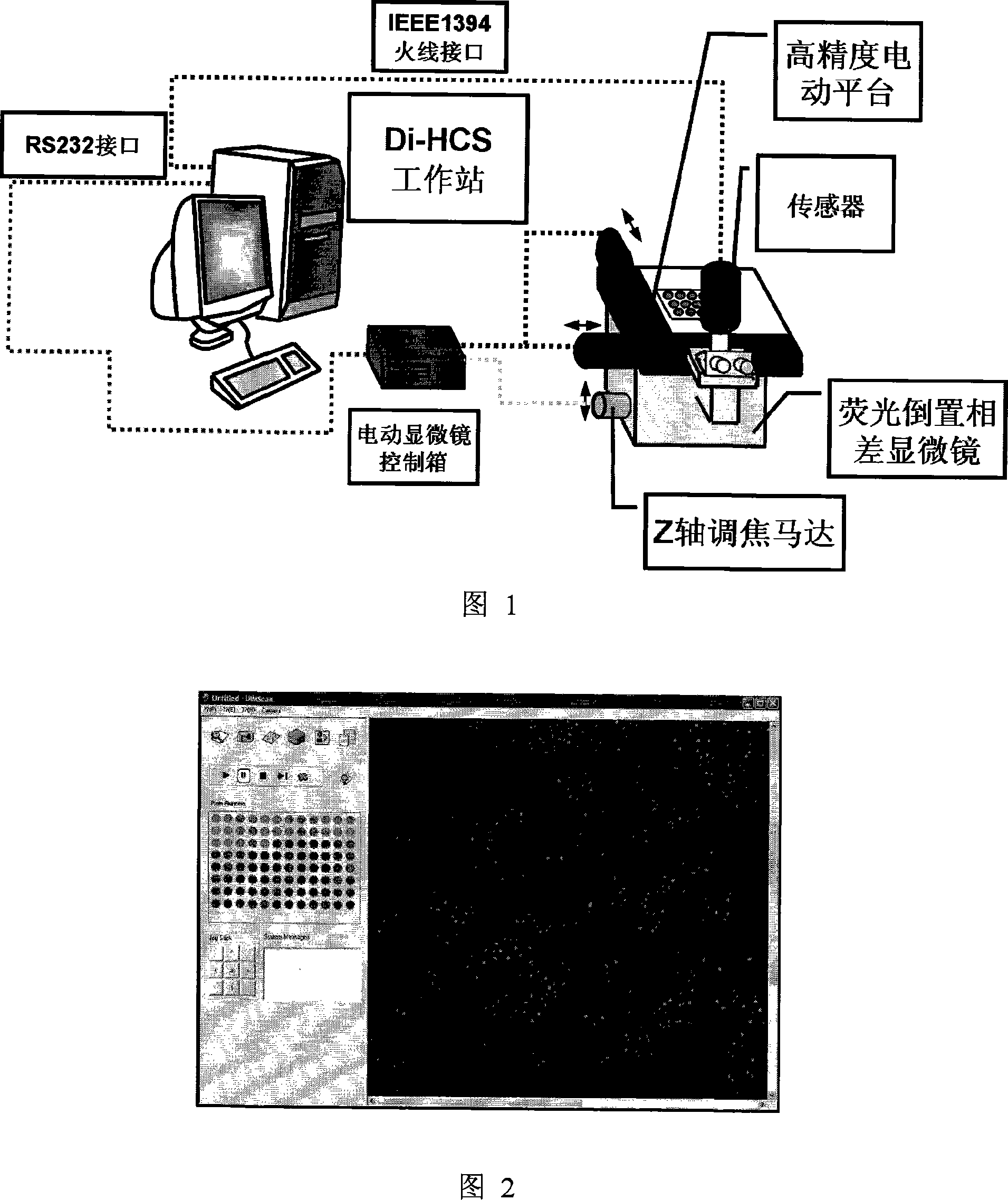
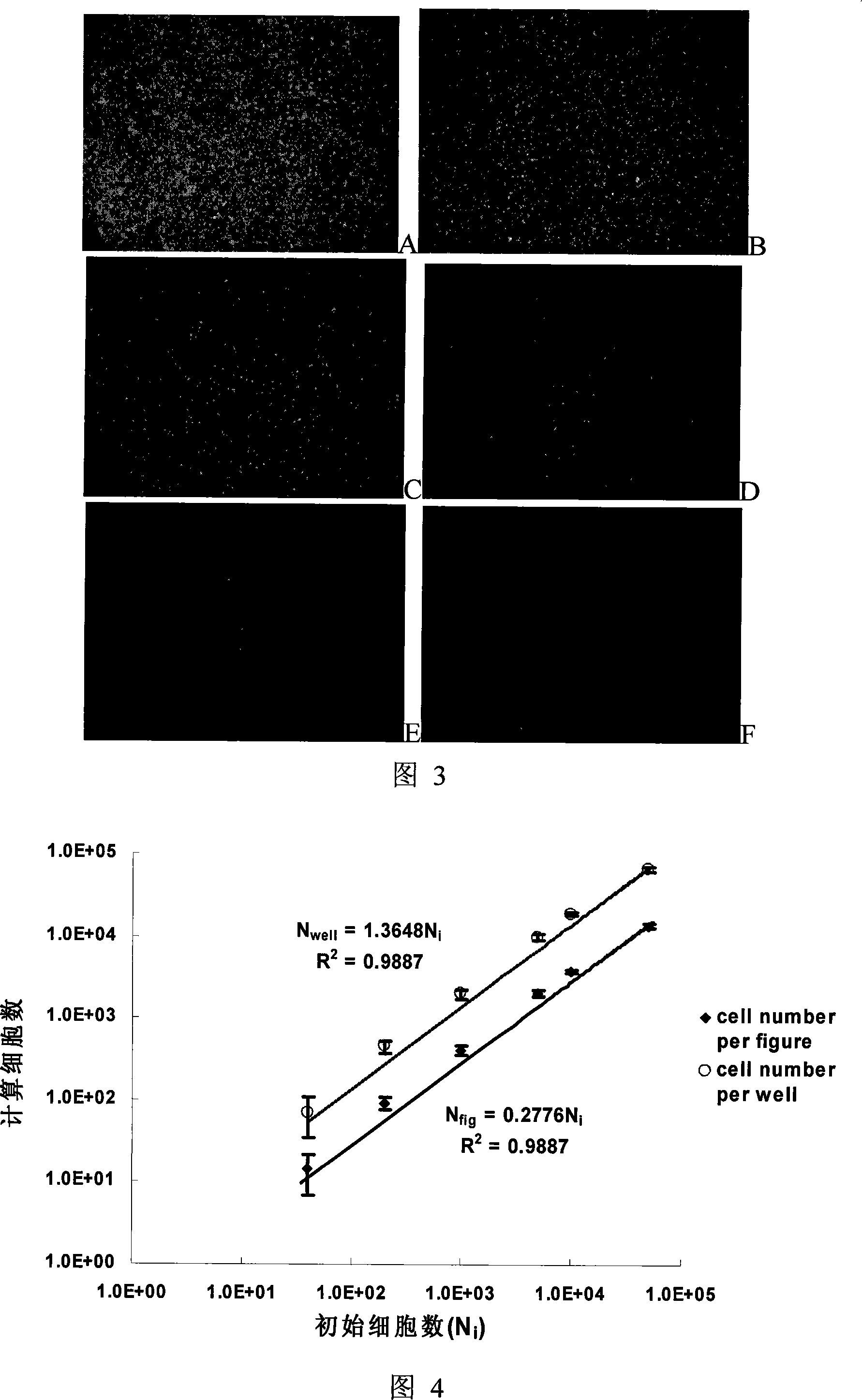
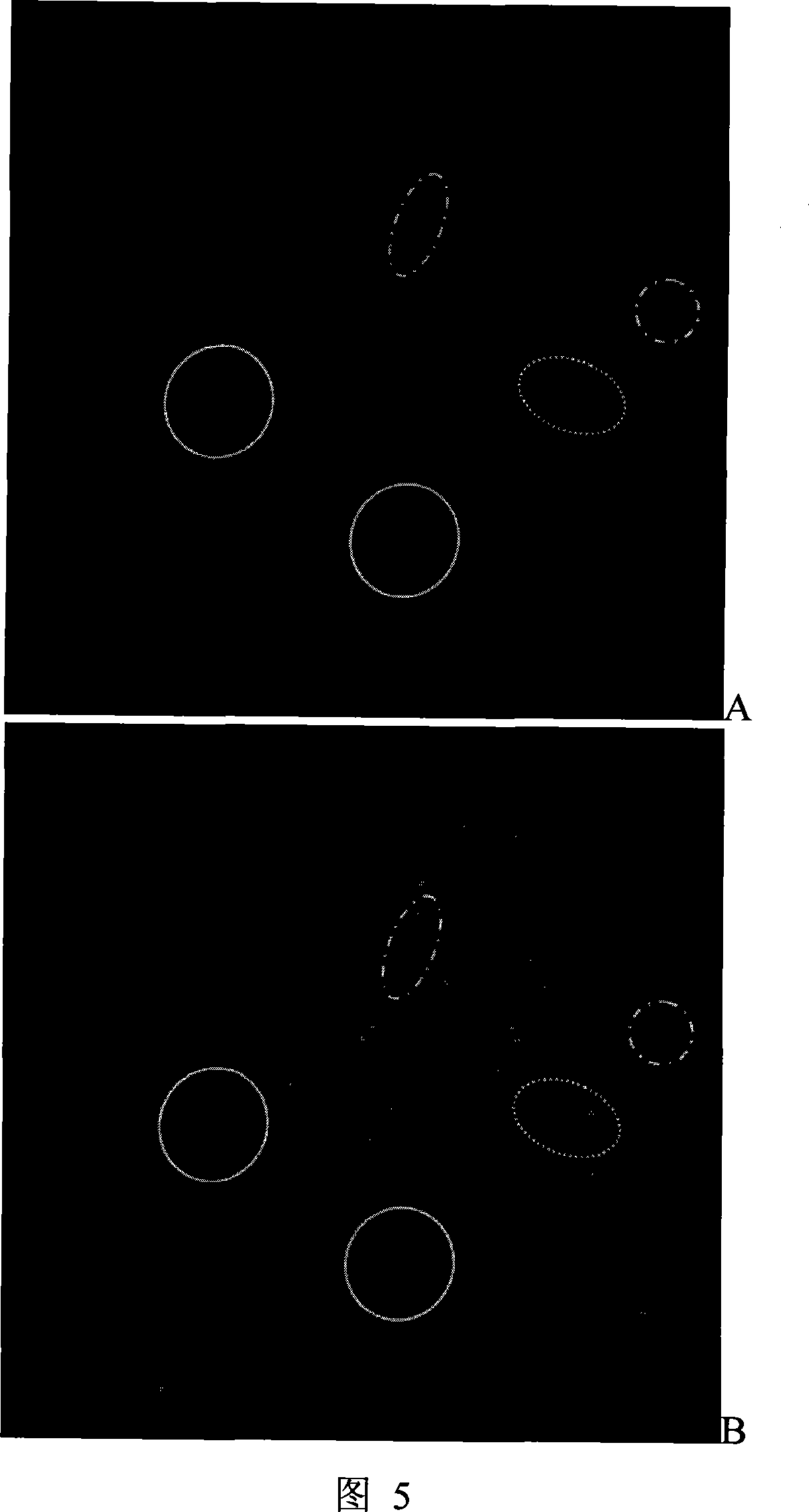
Antineoplastic drug evaluation and screening method based on cell microscopic image information
InactiveCN101149327ARealize program controlRealize control program controlMicrobiological testing/measurementIndividual particle analysisMicroscopic imageFluorescence
The invention provides the appraisal and selective method for the antineoplastic drug based on the cell micrograph information, which uses a sort of selection and appraisal hardware system to appraisal and select the antineoplastic drug by different fluorescence dye marking and measuring the multicellular parameter change in cell. The hardware system is made up of the high precision electric hydrous platform, the fluorescence vision system, the image collecting and processing system and working station. The diacetoxyl fluoresceine dyeing measures the active cell number; the double dyeing method of Hoechst33342 and iodized pyridine appraise the drug inducing the cell die; the three dyeing method of FDA, Hoechst33342 and PI analyzes the die mode induced by drug. The invention can measure at least two kinds of single cell or cell subgroup which expresses different drone cell organ. The method is in reason and can be used in study of drug action mechanism and selecting the high hedonic drug and toxicity analyzing, which can be used in selecting drug and appraising the drug toxicity.
Owner:ZHEJIANG UNIV
Microfluidic device for pharmacokinetic-pharmacodynamic study of drugs and uses thereof
ActiveUS8748180B2Bioreactor/fermenter combinationsCompound screeningTissues typesMicrofluidic channel
A microfluidic device for culturing cells, termed a microscale cell culture analog (μCCA), is provided. The microfluidic device allows multiple cell or tissue types to be cultured in a physiologically relevant environment, facilitates high-throughput operation and can be used for drug discovery. The microfluidic device uses gravity-induced fluidic flow, eliminating the need for a pump and preventing formation of air bubbles. Reciprocating motion between a pair of connected reservoirs is used to effect the gravity-induced flow in microfluidic channels. Bacterial contamination is reduced and high throughput enabled by eliminating a pump. The microfluidic device integrates a pharmacokinetic-pharmacodynamic (PK-PD) model to enable PK-PD analyses on-chip. This combined in vitro / in silico system enables prediction of drug toxicity in a more realistic manner than conventional in vitro systems.
Owner:CORNELL UNIVERSITY
Application of levorotatory oxiracetamto preparation of medicaments for preventing or treating cognitive dysfunction
ActiveCN102204904AReduce dosageGood treatment effectOrganic active ingredientsNervous disorderPharmacyCurative effect
The invention relates to application of levorotatory oxiracetamto to the field of pharmacy, and specifically relates to application of levorotatory oxiracetamto preparation of medicaments for preventing or treating cognitive dysfunction. According to the invention, levorotatory oxiracetamto with a purity higher than 99.3% is used as a material, so that hidden trouble of drug toxicity caused by invalid ingredients in a medicament is eliminated to further ensure medications safety. In addition, levorotatory oxiracetamto is a single active ingredient in the medicament of the invention, so that medicament quality is easier to control, and a curative effect is more obvious.
Owner:CHONGQING RUNZE PHARM CO LTD
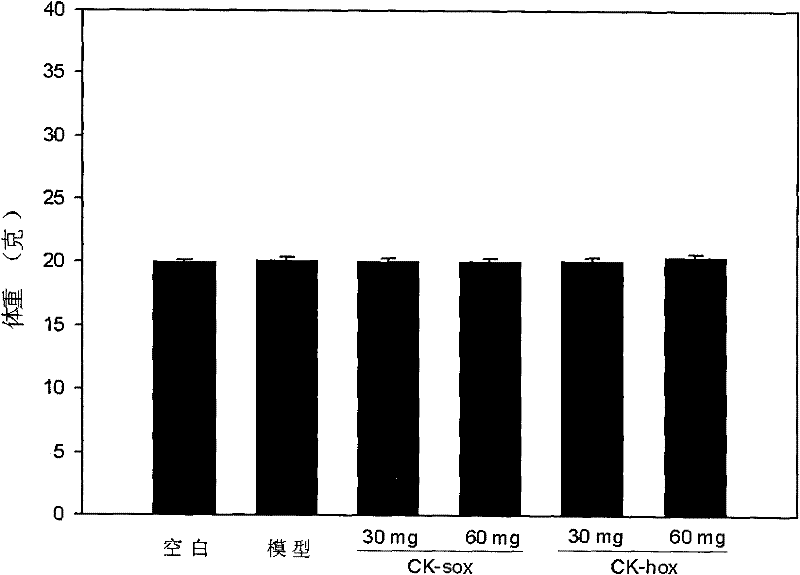
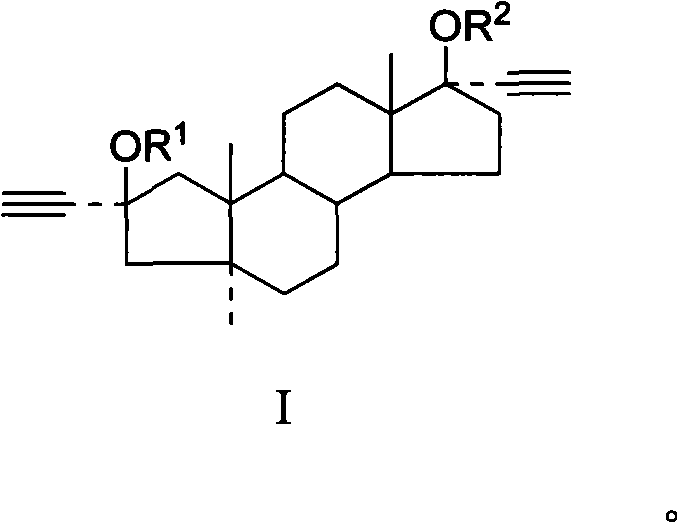
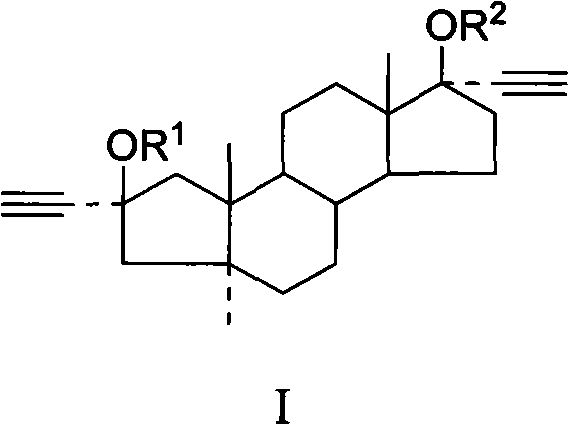
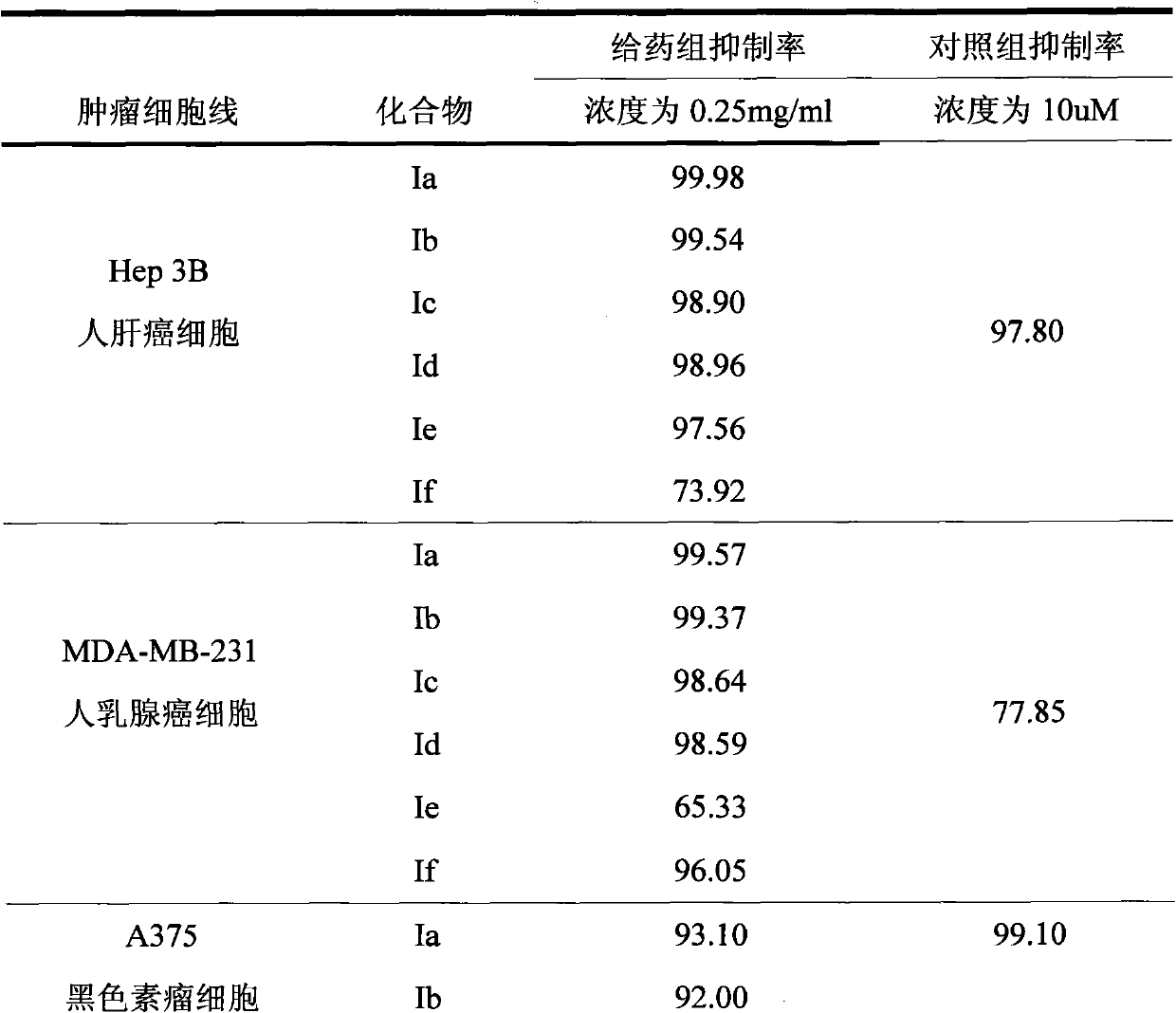
Applicationof A-nor-5 alpha-androstane compounds in preparation of malignant tumor resistant medicaments
ActiveCN102218069APotent tumor suppressor activityBroad-spectrum tumor suppressor activityOrganic active ingredientsSteroidsTreatment effectMda mb 231
The invention discloses application of A-nor-5 alpha-androstane compounds in preparation of malignant tumor resistant medicaments. The compounds have the following general formula I, and comprise Ia, Ib, Ic, Id, Ie and If. The growth inhibition rate of the A-nor-5 alpha-androstane compounds for in-vitro human liver cancer cell Hep 3B, human breast cancer MDA-MB-231, human lung adenocarcinoma A549 and mouse melanoma B16 is higher than 85% on average, and even up to 99.98% to the maximum. The in-vivo test proves that the inhibition rate of the A-nor-5 alpha-androstane compounds for mouse tumors, such as intestinal cancer C26, liver cancer H22, Lewis lung cancer, breast cancer, B16 melanoma and the like, is higher than 50% on average, and even up to 63.19% to the maximum. The result proves that the compounds disclosed by the invention have an obvious malignant tumor resistant action. The A-nor-5 alpha-androstane compounds disclosed by the invention have an obvious and broad-spectrum action on inhibiting growth of malignant tumor cells, and are novel targeted malignant tumor resistant medicaments with low drug toxicity and favorable treatment effect; and the A-nor-5 alpha-androstane compounds just specifically act on tumor cells, but not influence normal cells, thereby having a high clinical application value.
Owner:SHANGHAI AO QI MEDICAL TECH
Anticancer agents
InactiveUS6683083B1Suppressing myocardial hypercontractionSuppressing and preventing myocardial necrosisOrganic active ingredientsOrganic chemistryAnticarcinogenCancer cell
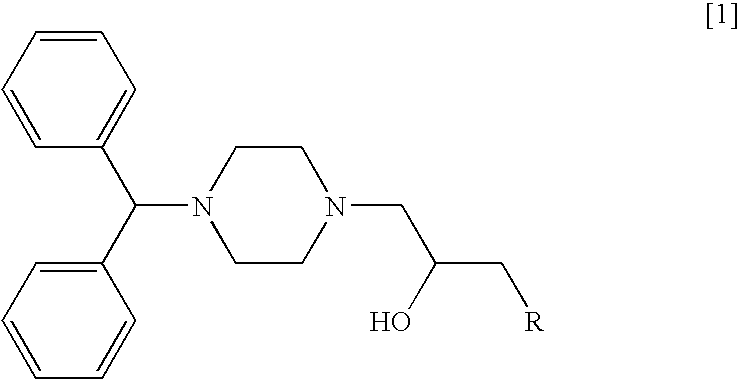
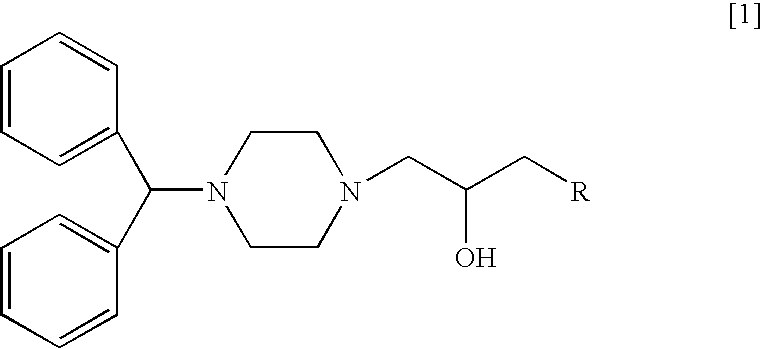
Disclosed herein is a method for suppressing the growth of cancer cells in a mammal in need of such treatment comprising administering to said mammal a cancer cell suppressing amount of a diphenylmethylpiperazine represented by the following general formula [1]:wherein R represents:or a pharmaceutically acceptable salt thereof.Compared with conventional anticancer agents, these agents are less toxic and exert an excellent carcinostatic effect on various solid cancers. Moreover, these anticancer agents inhibit the proliferation of fibroblasts, which makes them efficacious against pulmonary fibrosis and proliferative keloid lesions.
Owner:KANEKO NOBORU
Animal-derived food pathogen identification and drug-resistant and toxic gene detection composite chip
ActiveCN105950732AStrong detection applicabilityHigh selectivityMicrobiological testing/measurementDNA/RNA fragmentationResistant genesSingle strand dna
The invention provides an animal-derived food pathogen identification and drug-resistant and toxic gene detection composite chip. The invention provides a probe for identifying the animal-derived food pathogen, the pathogen drug-resistant gene and / or drug toxicity gene. The probe consists of single-chain DNA (deoxyribonucleic acid) molecules shown by the sequence 1 to sequence 171. Experiment results show that the animal-derived food pathogen identification and drug-resistant and toxic gene detection composite chip provided by the invention can effectively achieve the integral goal of simultaneously identifying the bacteria, the toxicity of the bacteria and the drug-resistant gene.
Owner:CHINA AGRI UNIV
Methods for reducing toxicity of a chemotherapeutic drug
ActiveUS20190099498A1Low toxicityImprove securityPowder deliveryOrganic active ingredientsChemotherapeutic drugsChemotherapy related toxicity
This disclosure relates to methods for improving the therapeutic index of a chemotherapeutic drug in the treatment of patients afflicted with cancer, by reducing chemotherapy-related toxicity to a level that allows the chemotherapeutic drug to be used in humans.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
Pharmaceutical composition for treating xerophthalmia
InactiveCN102145126ASignificant technological progressConvenient sourceSenses disorderPlant ingredientsXerophthalmiaTreatment effect
The invention belongs to a pharmaceutical composition, in particular a pharmaceutical composition for treating xerophthalmia. The pharmaceutical composition provided by the invention is prepared from effective amounts of radix rehmanniae, figwort, ophiopogon root, dendrobium, rhizoma anemarrhenae, radix trichosanthis, chrysanthemum, mulberry leaf, pipewort, radix sileris and other raw medicines. The invention solves the problems of short action time, limited treatment, poor treatment effect and the like in the prior art. The pharmaceutical composition has the advantages of small drug toxicity, obvious treatment effect and the like.
Owner:张铭连
Nano micelle preparation of Catharanthus roseus alkaloids antineoplastic drugs with coating of phospholipid derived from polyethylene glycol
The invention provides intravenous nanomicelle agents of vinca alkaloids antitumor drug, it contains an effective dose for treating of vinca alkaloids antitumor drug (vinblastine and vincristine or vindesine), macrogol derivatization phospholipid, and the pharmaceutical acceptable adjuvants. Its preparation is to pack the drug in the formative nanomicelle agents, prepare and make the intravenous nanomicelle agents of vinca alkaloids antitumor drug. Vinca alkaloids antitumor drug and macrogol derivatization phospholipid form into a very uniform size nanomicelle. In micelles, polyethylene glycol molecule and hydrophobic core for drug packing form a hydrophilicitious inhibitory coating, avoid the drugs contact with the protein such as enzymes in the blood and identified and phagocytized by the endothelial system in vivo, phagocytosis, the cycle time of micellar in vivo is extended. In addition, the micellar drug also increases the storage stability and the effect on the tumor of the drug and reduces drug toxicity.
Owner:BEIJING DEKERUI MEDICAL TECH
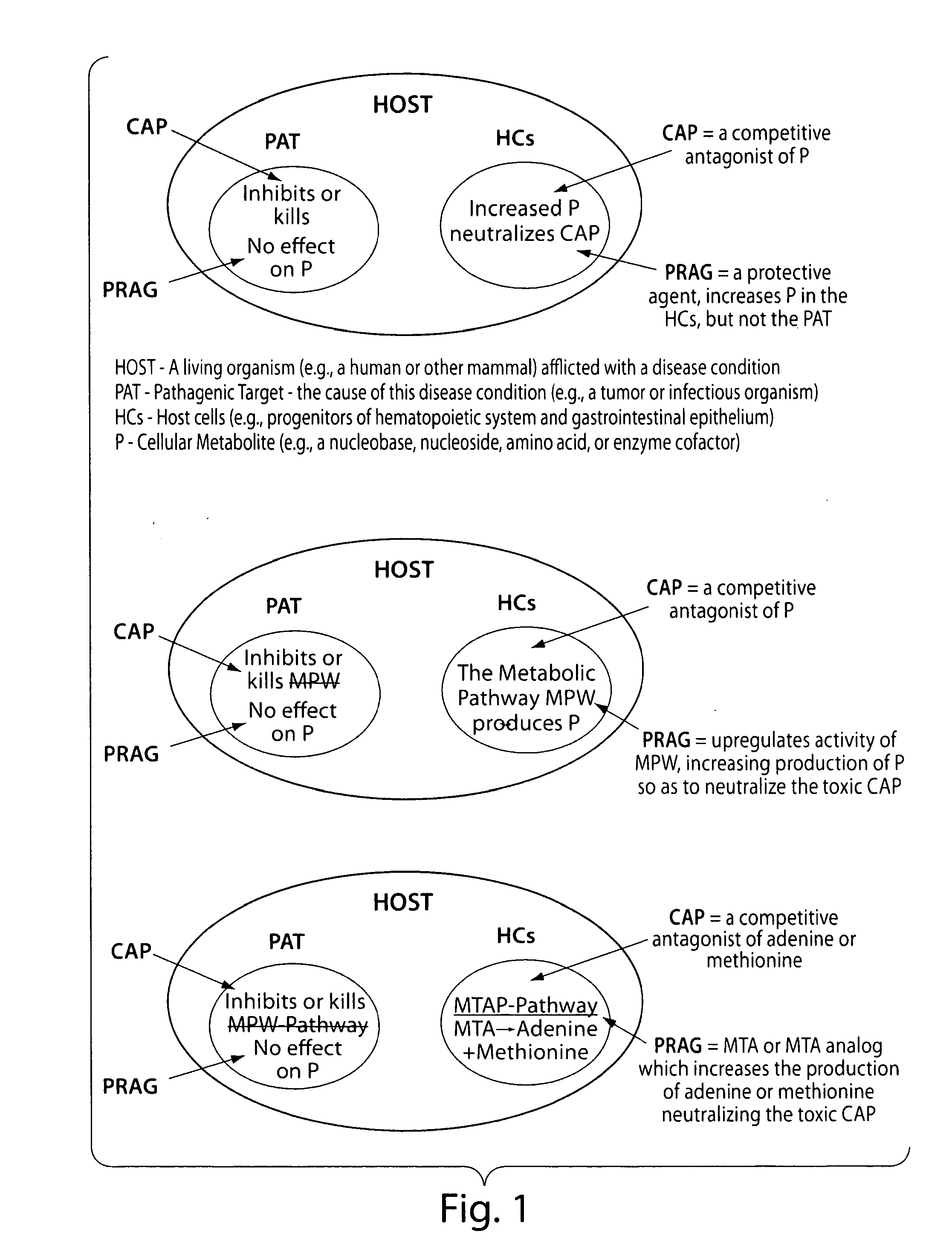
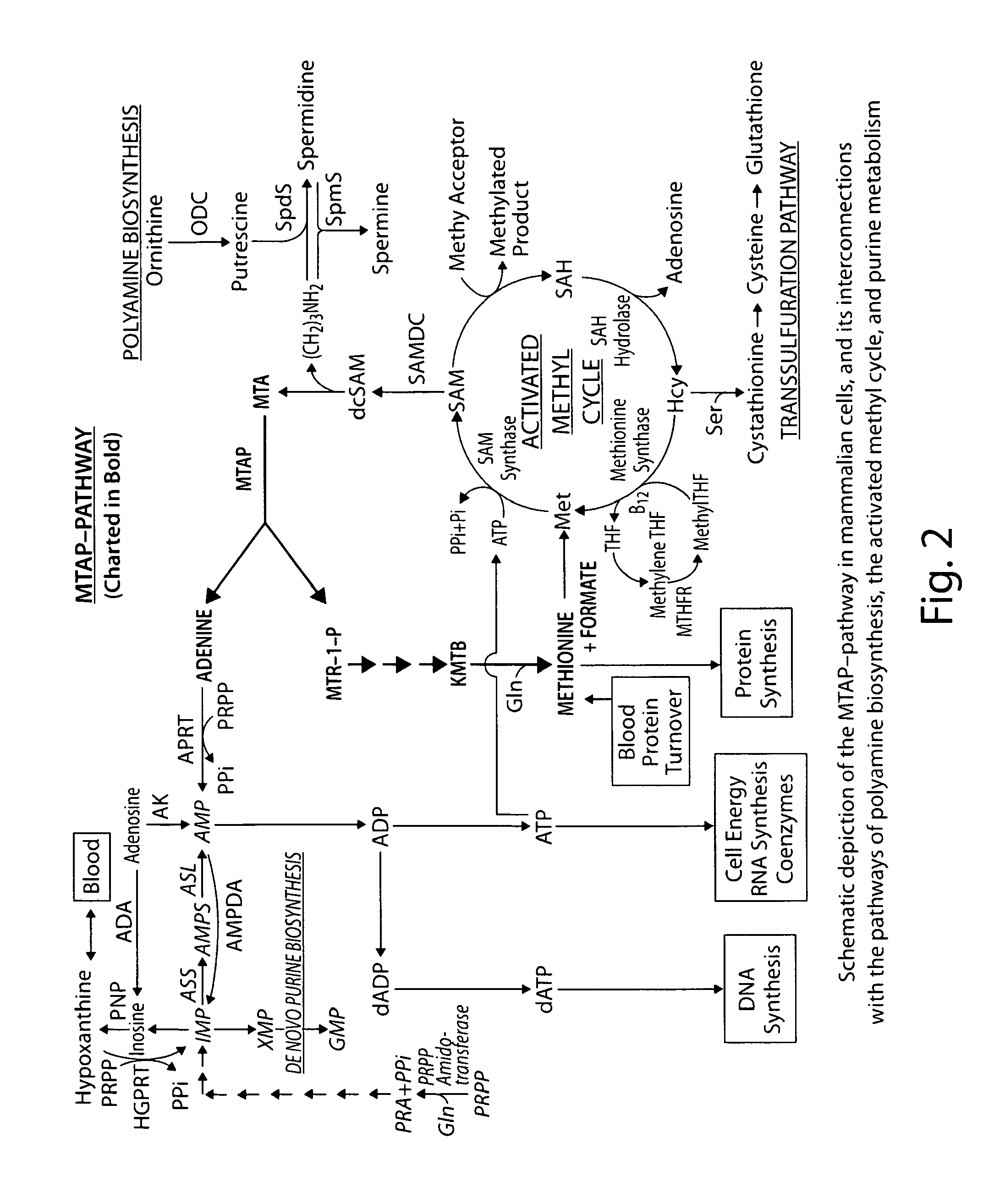
Method for the selective therapy of disease
ActiveUS20120094947A1Low toxicityIncrease level of cellAntibacterial agentsBiocideDiseaseCompetitive antagonist
Methods for treating diseases in humans and vertebrate animals are provided using competitive antagonists of cellular metabolites combined with a protective agent for protecting host cells from toxic effects of the drugs. Also provided are kits comprising competitive antagonists and suitable protective agents. In addition, screening methods for identifying competitive antagonists, protective agents and potentiating agents, for use according to the methods of the invention, are provided.
Owner:LUBIN ADAM +1
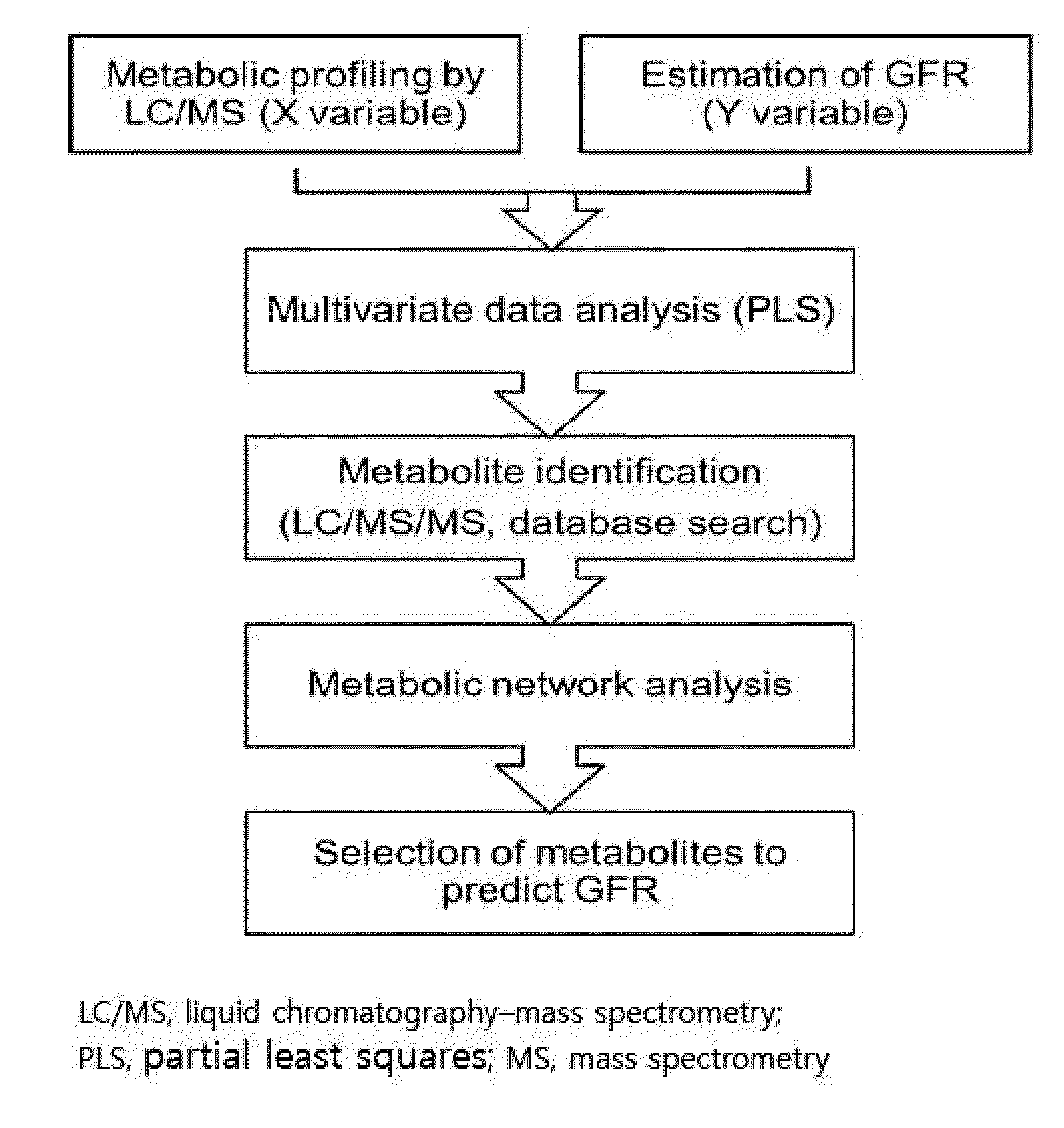
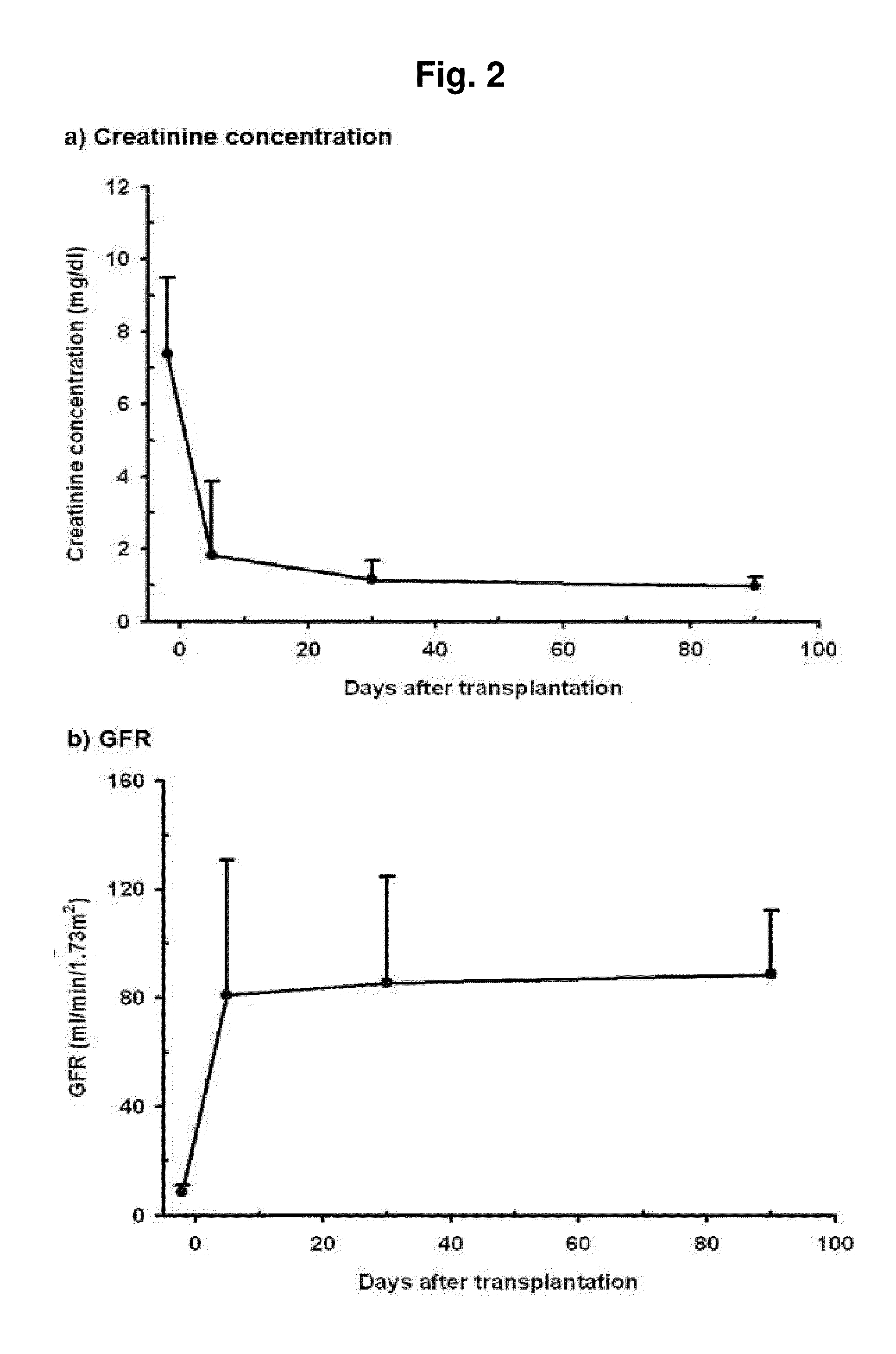
Prediction method of glomerular filtration rate from urine samples after kidney transplantation
Disclosed is a prediction method of glomerular filtration rate (GFR) from urine samples after kidney transplantation to provide an information needed for predict renal function after the transplantation, more particularly to a prediction method of glomerular filtration rate (GFR) from urine samples after kidney transplantation, which comprises detecting metabolic profiles of five biomarkers, 5a-androst-3-en-17-one (AS), glycocholic acid (GC), sphingosine (SG), tryptophan (TR) and histidine (HT), from urine samples of patients. Glomerular filtration rate (GFR) after kidney transplantation can be predicted more rapidly and precisely to provide an information needed for predict renal function after the transplantation by using five metabolites as biomarkers. The method provides more specific, sensitive, and reliable biomarkers that monitor clinical outcomes and adverse renal events after kidney transplantation, such as rejection, drug toxicity, delayed graft function, and infection.
Owner:KYUNGPOOK NAT UNIV IND ACADEMIC COOP FOUND
Application of cepharanthine in preparing medicine for resisting SARS virus
InactiveCN1452967AHigh inhibition rateRelieve symptomsOrganic active ingredientsAntiviralsCepharanthineVirology
Cepharanthine is used as effective component in preparing medicine for resisting SARS virus. Within the safe dosage range, the present invention has high inhibition rate to SARS virus and powerful effect of preventing SARS virus infection. The medicine can alleviate the syndromes of SARS patient and has fast after cure, less toxicity, high stability and other advantages.
Owner:GUANGZHOU JINAN BIOMEDICINE RES & DEV CENT
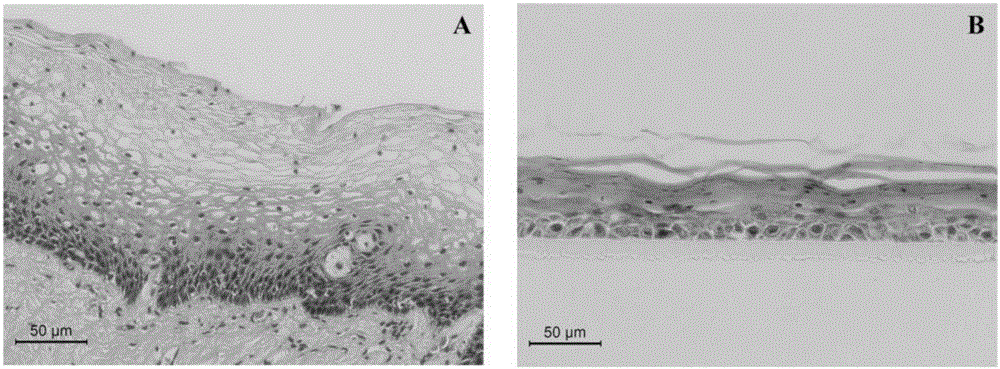
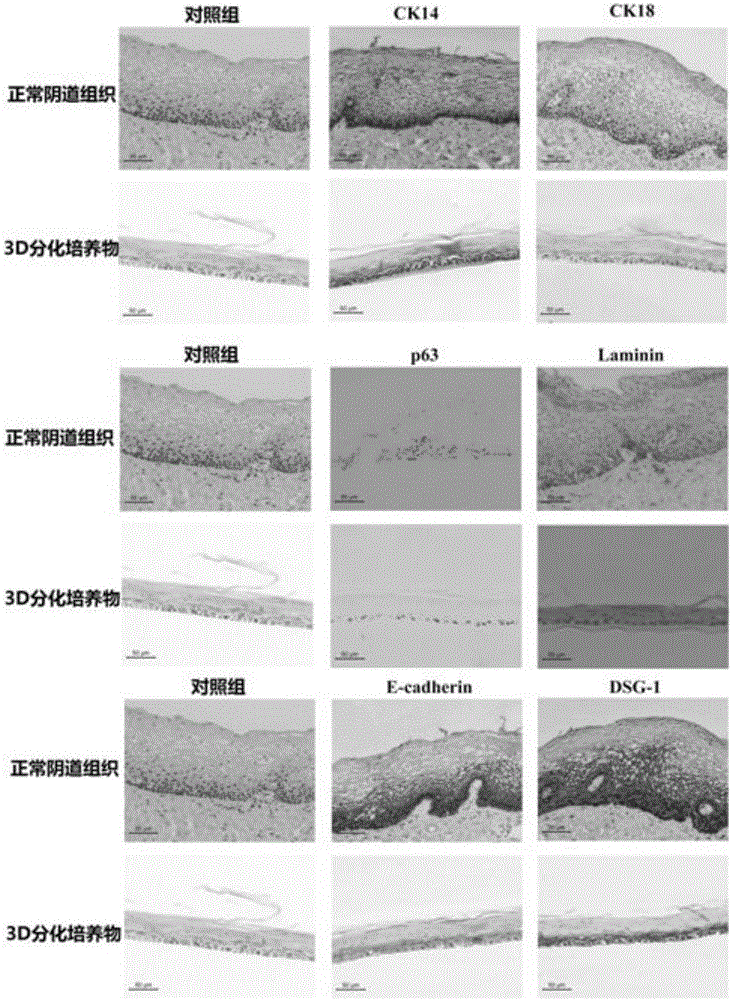
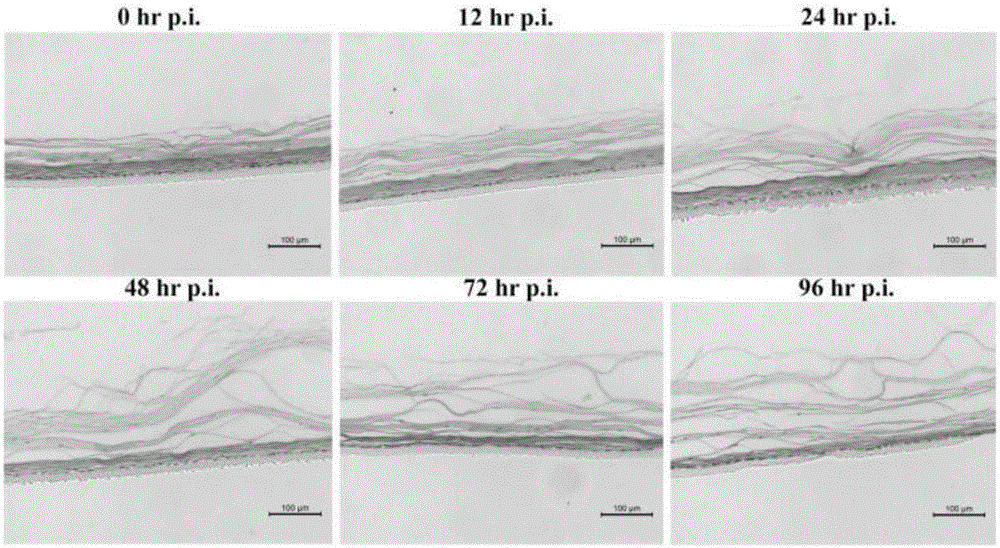
Construction method and application of human normal vaginal epithelium 3D (Three Dimensional) differentiation culture model
The invention belongs to the field of biomedicines and discloses a construction method and application of a human normal vaginal epithelium 3D (Three Dimensional) differentiation culture model. A 2D (Two Dimensional) growth culture medium is provided and normal vaginal epithelial cells separated and cultured from normal tissues beside female vaginal cancer are obtained; any exogenous gene is not introduced and the normal vaginal epithelial cells have a physiological function of normal differentiation. The method for constructing the human normal differentiation vaginal epithelium 3D model comprises the following steps: re-suspending single cells by the 2D culture medium and inoculating the single cells into a gas-liquid culture device; replacing the growth culture medium with a differentiation culture medium and culturing for 14 to 21 days; after completely differentiating 3D gas-liquid culture of the human vaginal epithelial cells, inoculating HSV-2 (Herpes Simplex Virus-2) virus liquid to obtain an HSV-2 virus infection 3D model. The two types of 3D models can be used for physiological studies and drug toxicity safety evaluation of human normal reproductive tract epithelium, researches of pathogenic mechanisms of HSV-2 virus infected diseases and sexual transmission pathogen infected diseases and researches and development of anti-viruses medicines.
Owner:深圳涌泰生物科技有限公司
Materials and methods for treating coagulation disorders
InactiveUS6864279B2Treatment safetyImprove the quality of lifeBiocideOrganic chemistryWarfarinCoagulation Disorder
This invention is drawn to compounds which are more easily metabolized by the metabolic drug detoxification systems. Particularly, warfarin analogs which have been designed to include esters within the structure of the compounds are taught. The invention teaches methods of reducing the toxicity of drugs comprising the introduction of ester groups into drugs during the synthesis of the drug. This invention is also drawn to methods of treating coagulation disorders comprising the administration of compounds which have been designed to be metabolized by serum or intracellular hydrolases and esterases. Pharmaceutical compositions of the ester containing warfarin, analogs are also taught.
Owner:ARMETHEON INC
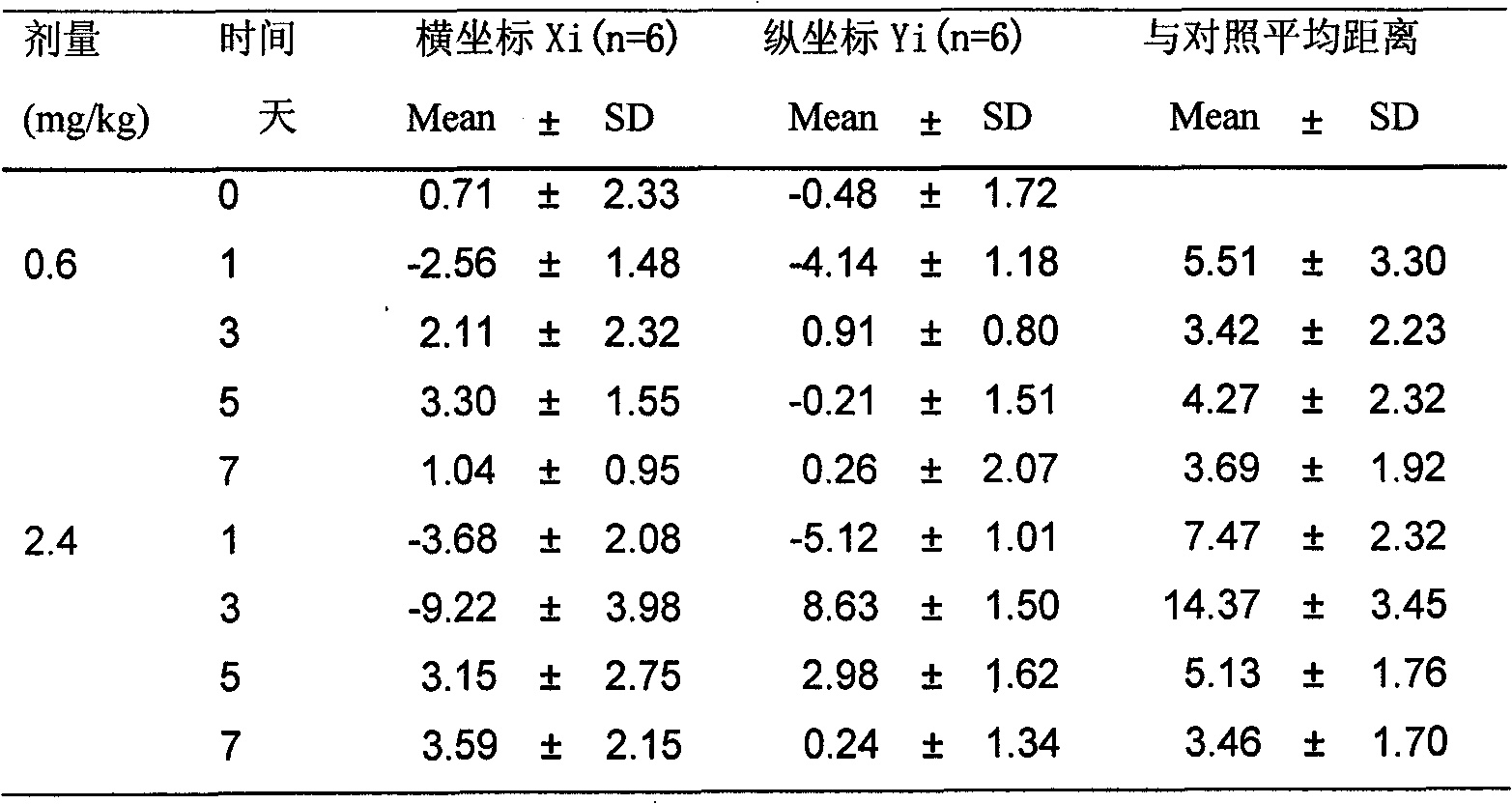
Method for quantitatively evaluating medicament toxicity by using metabonomic technology
InactiveCN101813680AReduce deviationImprove objectivityComponent separationNMR - Nuclear magnetic resonanceMathematical model
The invention discloses a method for quantitatively evaluating medicament toxicity by using metabonomic technology, which is characterized by comprising the following steps: comprehensively and quantitatively measuring small molecule compounds in a biological sample by using the measuring technology based on mass spectrum, nuclear magnetic resonance and the like, then establishing a multi-dimensional spatial mathematical model by adopting a multi-variable data processing method, calculating a relative distance between an administration group and a control group and taking the relative distance as a quantitative index for evaluating the medicament toxicity so as to solve the difficult problem that the medicament toxicity evaluation lacks the quantitative evaluating index. Compared with the conventional toxicity evaluating method, the method has the advantages of wide application, sensitivity, simple and convenient sampling, no harm to the body, and capability of reflecting the toxicity function more comprehensively, and providing a comprehensive and reliable quantitative evaluating method for toxicity evaluation in new medicament research and development and pharmacologic research.
Owner:CHINA PHARM UNIV
Tibetan medicinal composition for expectorant, antitussive, antiasthmatic and its preparation method
This invention provides a Tibetan medicinal composition for expectorant, antitussive, antiasthmatic and its preparation method. It is prepared by using the following medical ingredients in accordance to the amount by weight: gentian flower 90-135, savoury rhododendron leaf 445-665, licorice root 45-65, Japanese bulbocapnine 90-135, trendrilleaf fritillary bulb 55-75, larberry root 450-660, plumieride,45-65, crab shell 45-65, racemose inula root, 70-110, horse ladder, 2.0-2.0. It is proved by tests that the composition of this invention can be used for treating cough, sound breathe, yellow sputum due to accumulation of phlegm and heat in the lung, or running nose, sourer throat, thirst, reddish urine, dry stool due to heat, and acute tracheitis, acute attack of bronchitis and those of the above symptom groupings. The medicine is of short toxin, safe for oral taking. It can be provided to the clinical use, it has high biological use with excellent curative effect.
Owner:西藏藏药集团股份有限公司
Method for evaluating and predicating toxicity and efficacy of medicament by using metabonomic technology
InactiveCN102339356AAccurate evaluationAccurate predictionComponent separationMaterial analysis by electric/magnetic meansEndogenous metabolismSample Measure
The invention relates to a method for evaluating and predicating the toxicity and the efficacy of a medicament based on an endogenous metabolism signal which is acquired by eliminating an exogenous metabolism signal in a biological sample, in particular to the method for evaluating and predicating the specific toxicity and the non-specific toxicity of the medicament by using a metabonomic technology. In the method, the biological sample depended by a metabolism group is obtained according to a blank control group, a model toxic substance group and a toxicity test group which are set in the metabolism group; and a sample measuring signal matrix is detected and acquired for evaluating and predicating the toxicity of the medicament. The invention simultaneously discloses a method for evaluating and predicating the efficacy by using the metabonomic technology. In the method, a biological sample depended by the met toxic substance group, a treatment group and a positive medicament control group which are set in the metabolism group; and a sample measuring signal matrix is detected and acquired for evaluating and predicating the efficacy of the medicament. According to the methods disclosed by the invention, the toxicity and the efficacy of the medicament can be evaluated more comprehensively and accurately.
Owner:SICHUAN YUANDASHUYANG PHARM CO LTD
Targeted administration preparation of epipodophyllotoxins medicine
ActiveCN104523597ALow toxicityReduce inhibitionOrganic active ingredientsPowder deliveryNanoparticlePhospholipid formation
The invention discloses a phospholipid complex albumin nanoparticle containing epipodophyllotoxins medicine. The nanoparticle contains a complex formed by epipodophyllotoxins medicine and phospholipid and albumin. The nanoparticle comprises the following raw materials in parts by weight: 1 part of epipodophyllotoxins medicine, 1-50 parts of phospholipid and 2-100 parts of albumin. The phospholipid complex albumin nanoparticle can be used for remarkably reducing the toxicity of the epipodophyllotoxins medicine, especially bone marrow suppression; meanwhile, the phospholipid complex albumin nanoparticle has liver-lung targeting property.
Owner:SICHUAN UNIV
Insulin nanoparticle and preparation method thereof
ActiveCN102614498AHigh encapsulation efficiencyHigh drug loadingPowder deliveryPeptide/protein ingredientsCell membraneEntrapment
The invention relates to the technical field of drugs, and especially relates to an insulin nanoparticle and a preparation method thereof. The preparation method comprises the following steps: preparation of an insulin ion pair compound and preparation of the insulin nanoparticle. According to the insulin nanoparticle of the invention, the entrapment rate and the drug loading are high, the particle size distribution is uniform, and a certain slow releasing effect is possessed, so drugs can be effectively prevented from being degraded by proteases, the stability of the drugs can be increased, and a case that the drugs permeate a biomembrane is promoted, thereby curative effects of the drugs are improved, the toxicity of the drugs is reduced, the biological utilization degree of the drugs is increased, and the insulin nanoparticle is suitable for various administration approaches of the drugs; preparation conditions are mild, no violent conditions of high temperature, and high speed shearing force or the like are needed, so the biological activities of the drugs can be guaranteed; and a surfactant Cremophor EL used in the preparation process can inhibit P-glycoprotein, so the permeability of the drugs going through cell membranes is improved, and the absorption of the drugs going through intestinal tract walls to enter blood is increased.
Owner:SICHUAN KELUN PHARMA RES INST CO LTD
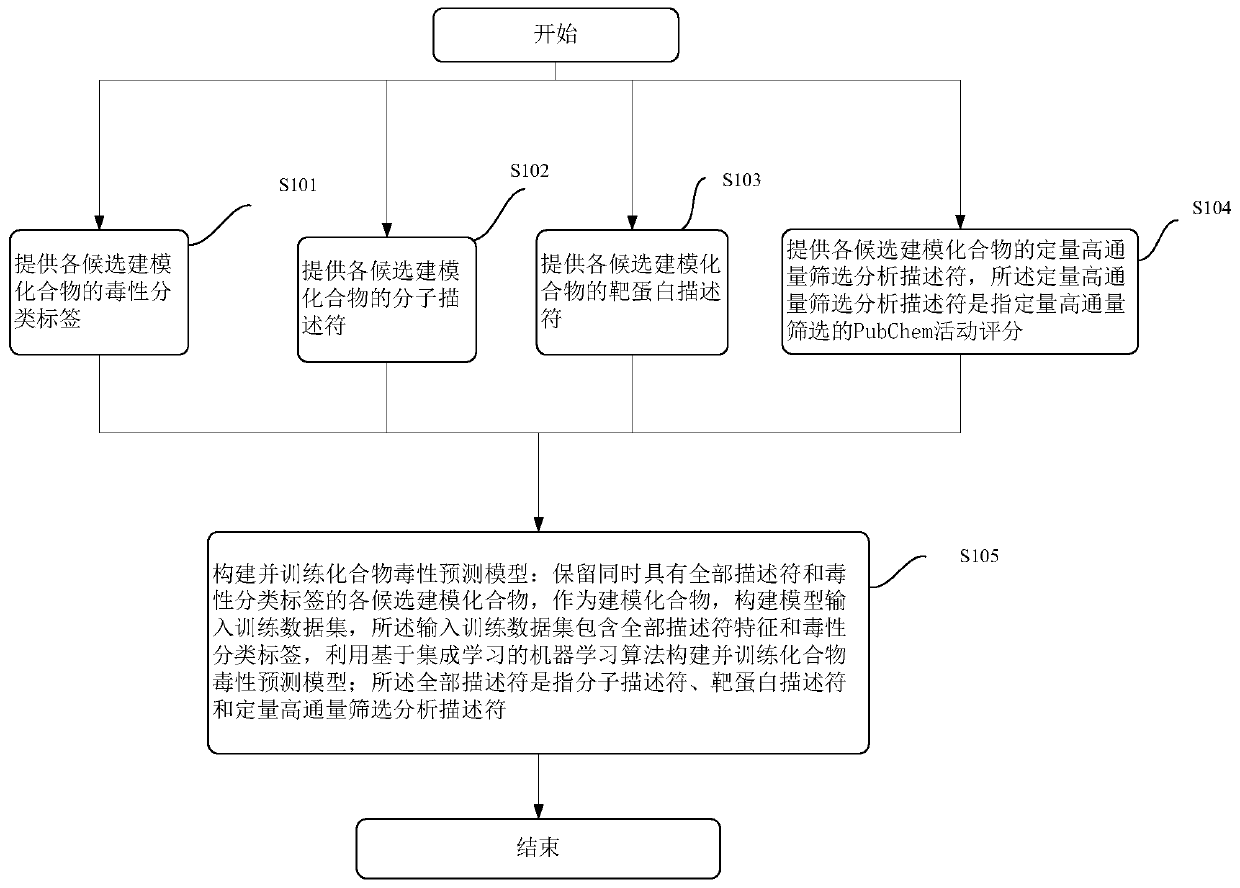
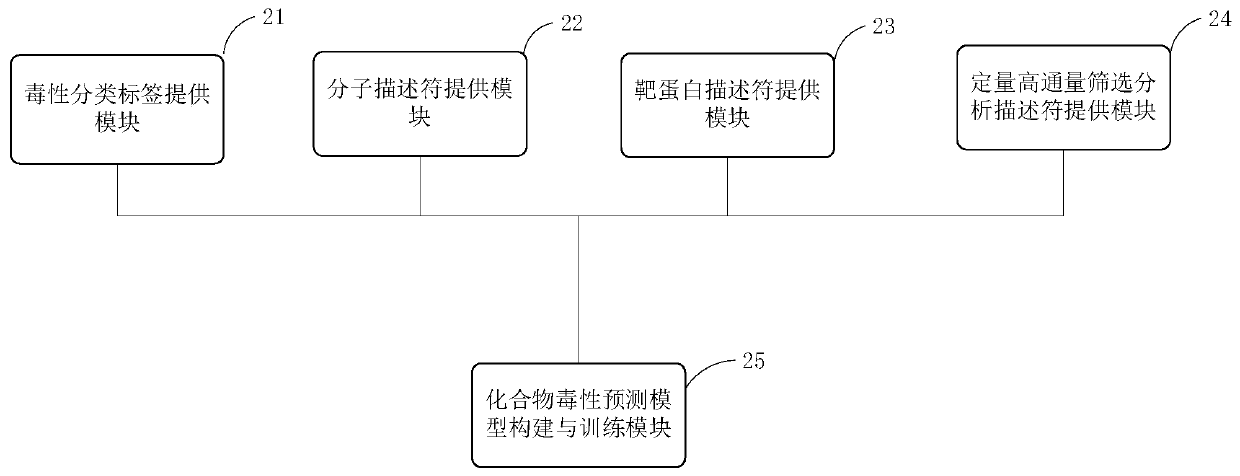
Modeling method and device of compound toxicity prediction model and application of compound toxicity prediction model
InactiveCN110890137AReliable Toxicity Prediction ResultsReduce false positive rateMolecular designSystems biologyProtein targetDrug biological activity
The invention provides a modeling method of a compound toxicity prediction model. The modeling method at least comprises the following steps of: S101, providing toxicity classification labels of candidate modeling compounds; S102, providing a molecular descriptor of each candidate modeling compound; S103, providing a target protein descriptor of each candidate modeling compound; S104, providing aquantitative high-throughput screening analysis descriptor of each candidate modeling compound, wherein the quantitative high-throughput screening analysis descriptor is a PubChem activity score of aspecified amount of high-throughput screening; and S105, constructing and training a compound toxicity prediction model. According to the method, physicochemical properties, biological activity and target protein action properties of drug candidate compounds can be fully utilized, and a drug toxicity prediction system is constructed by utilizing statistical modeling advantages of a machine learning algorithm based on ensemble learning, so that the model has interpretability and prediction performance, and has the better physicochemical and biological significance and research value.
Owner:上海尔云信息科技有限公司
Injection carbazochrome sodium sulfonate suspension and preparation method thereof
ActiveCN102600074AImprove stabilityImprove solubilityOrganic active ingredientsSolution deliveryPharmaceutical medicineImmunogenicity
The invention discloses an injection carbazochrome sodium sulfonate suspension and a preparation method thereof, relating to the technical field of medicines. The injection carbazochrome sodium sulfonate suspension is a powder injection and comprises the following components in parts by weight: 1 part of carbazochrome sodium sulfonate, 1.5-8.5 parts of pharmaceutically acceptable biological carrier, 0.1-1.8 parts of stabilizing agent and 2-5 parts of freezing protective agent. According to the invention, the stability and the dissolvability of the carbazochrome sodium sulfonate are improved, the injection carbazochrome sodium sulfonate suspension has no remarkable change of various indexes through detection after being placed for a long time, the quality of the injection carbazochrome sodium sulfonate suspension within a valid period is ensured to be qualified; and the injection carbazochrome sodium sulfonate suspension can be slowly administrated for a long time, thus bioavailability is greatly improved. The pharmaceutically acceptable biological carrier, i.e. protein, is degraded in vivo without toxicity and immunogenicity; and meanwhile, medicine therapeutic indexes can be effectively increased, and medicine toxicity is lowered and medicine side effects are reduced.
Owner:SHANGHAI JINCHENG PHARMACEUTICAL CO LTD
Externally used medicine for expelling wind and clearing away cold, activating meridians to stop pain and preparation method thereof
InactiveCN101559128ANo allergiesAvoid stimulationAnthropod material medical ingredientsAntipyreticDiseaseIrritation
The invention provides an externally used medicine for expelling wind and clearing away cold, activating meridians to stop pain and a preparation method thereof. The medicine is prepared by 25 types of medicinal materials such as radix aconiti preparata, wild aconite root, nux vomica (processed), epimedium, achyranthes root, notopterygium root, cyrtomium fortunei, phellodendron, zaocys dhumnade, hairy antler, dipsacus root, dark plum, asarum, Chinese ephedra, cassia twig, safflower, acanthopanax, honeysuckle, earth worm, loranthus, licorice, drynaria (scald), anisetree bark, myrrh gum (processed) and red ginseng. The medicine links closely with pathogen and pathogenesis of a disease and a plurality of medicines are compatible reasonably, thus expelling wind and clearing away cold as well as activating meridians to stop pain. As an externally used cataplasm, the medicine is taken through skin, thus avoiding the irritation of an oral preparation to gastrointestinal tract; in addition, relatively slow percutaneous absorption process inevitably greatly mitigates drug toxicity to the whole body. Neither skin sensibility nor skin irritation occurs. Therefore, the transdermal drug delivery of new preparation improves the safety of medicine taking to a certain extent and provides new choices for safe clinical medicine use.
Owner:潘首德
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com