Application of levorotatory oxiracetamto preparation of medicaments for preventing or treating cognitive dysfunction
A technology for cognitive dysfunction and drugs, applied in drug combinations, pharmaceutical formulations, medical preparations containing active ingredients, etc., can solve problems such as toxic side effects and poor efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
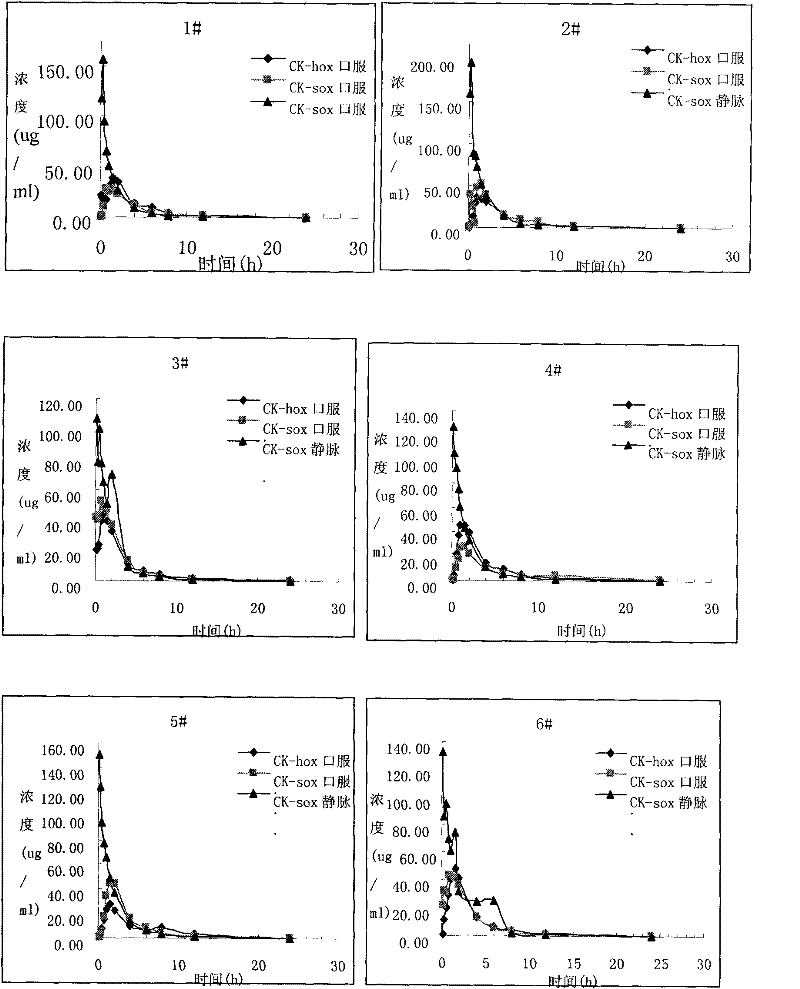
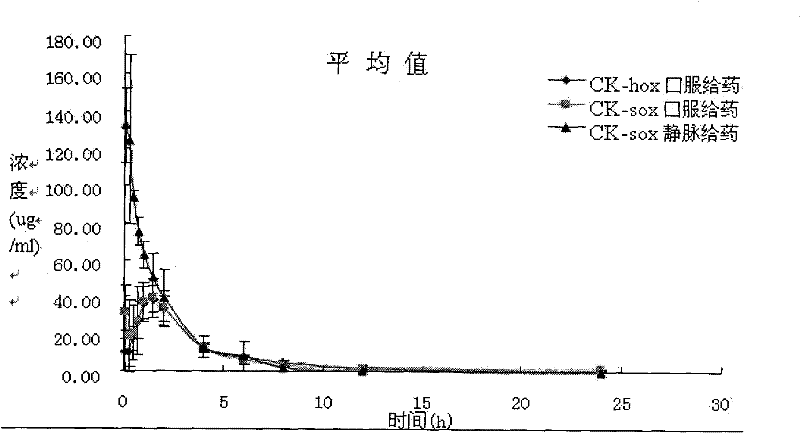
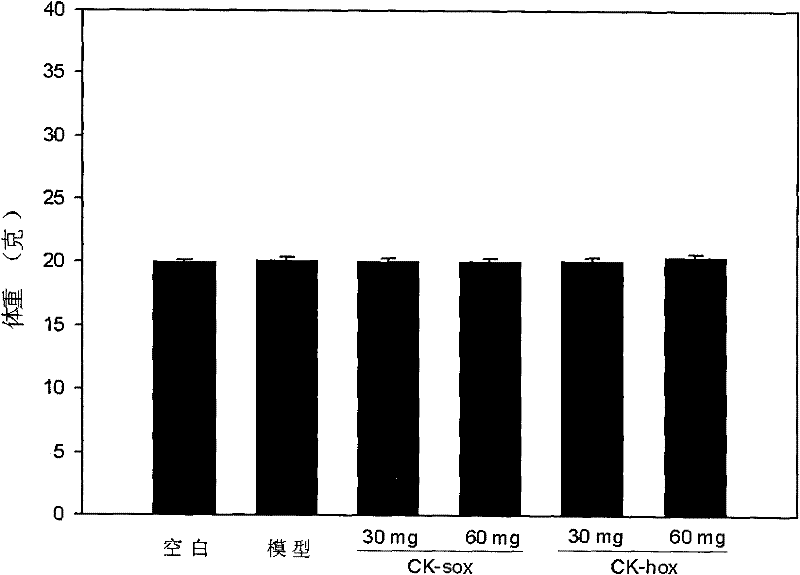
Image
Examples
Embodiment 1
[0092] The prescription consists of:
[0093] (a) Levoxiracetam (99.5% pure) 200mg / capsule
[0094] (b) Lactose 80mg / capsule
[0095] (c) Microcrystalline cellulose 70mg / capsule
[0096] Taking the preparation of 1000 levoxiracetam capsules as an example, the specific preparation method is: first pass the raw and auxiliary materials through an 80-mesh sieve, weigh the prescribed amount of levoxiracetam, lactose, and microcrystalline cellulose and mix evenly, directly Fill the capsule.
Embodiment 2
[0098] The prescription consists of:
[0099] (a) Levoxiracetam (99.6% pure) 200mg / tablet
[0100] (b) Starch 34mg / tablet
[0101] (c) Microcrystalline cellulose 60mg / tablet
[0102] (d) Talc powder 6mg / tablet
[0103] (e) 2% hydroxypropyl methylcellulose (K4M type) appropriate amount
[0104] Taking the production of 1000 levoxiracetam tablets as an example, the specific preparation method is: first pass the raw and auxiliary materials through an 80-mesh sieve, weigh the prescribed amount of levoxiracetam, starch, and microcrystalline cellulose and mix evenly, add 2% HPMC aqueous solution is used to prepare soft materials, granulate, dry, and granulate, add talcum powder in the prescribed amount to the granules, mix evenly, and compress into tablets.
Embodiment 3
[0106] The prescription consists of:
[0107] (a) Levoxiracetam (99.3% pure) 200mg / capsule
[0108] (b) Lactose 80.8mg / capsule
[0109] (c) Sodium Carboxymethyl Starch 72mg / capsule
[0110] (d) Talc powder 7.2mg / grain
[0111] (e) 10% polyvinylpyrrolidone appropriate amount
[0112] Taking the preparation of 1000 levoxiracetam capsules as an example, the specific preparation method is as follows: first the raw and auxiliary materials are passed through an 80 mesh sieve, and the levoxiracetam, lactose, and carboxymethyl starch sodium are mixed evenly by weighing the prescribed amount, Add 10% PVP alcohol solution to make soft material, granulate, dry, granulate, add prescription amount of talcum powder to the granules, mix evenly, and fill the capsules.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| optical rotation | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com