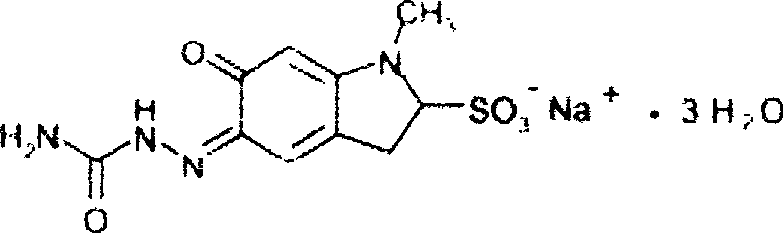
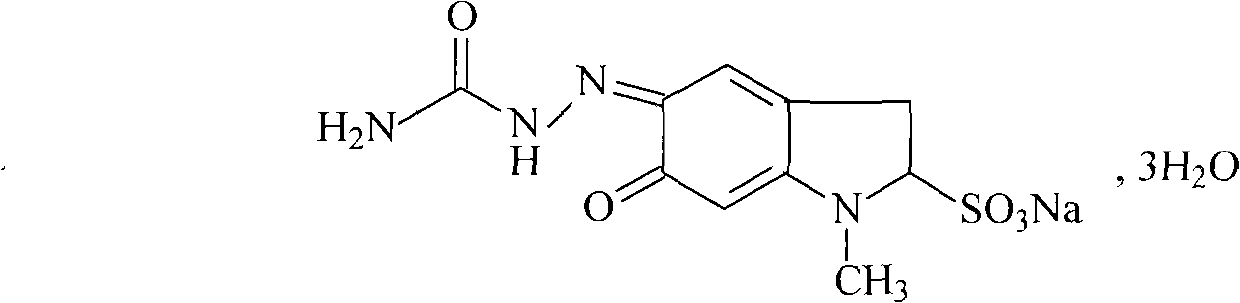
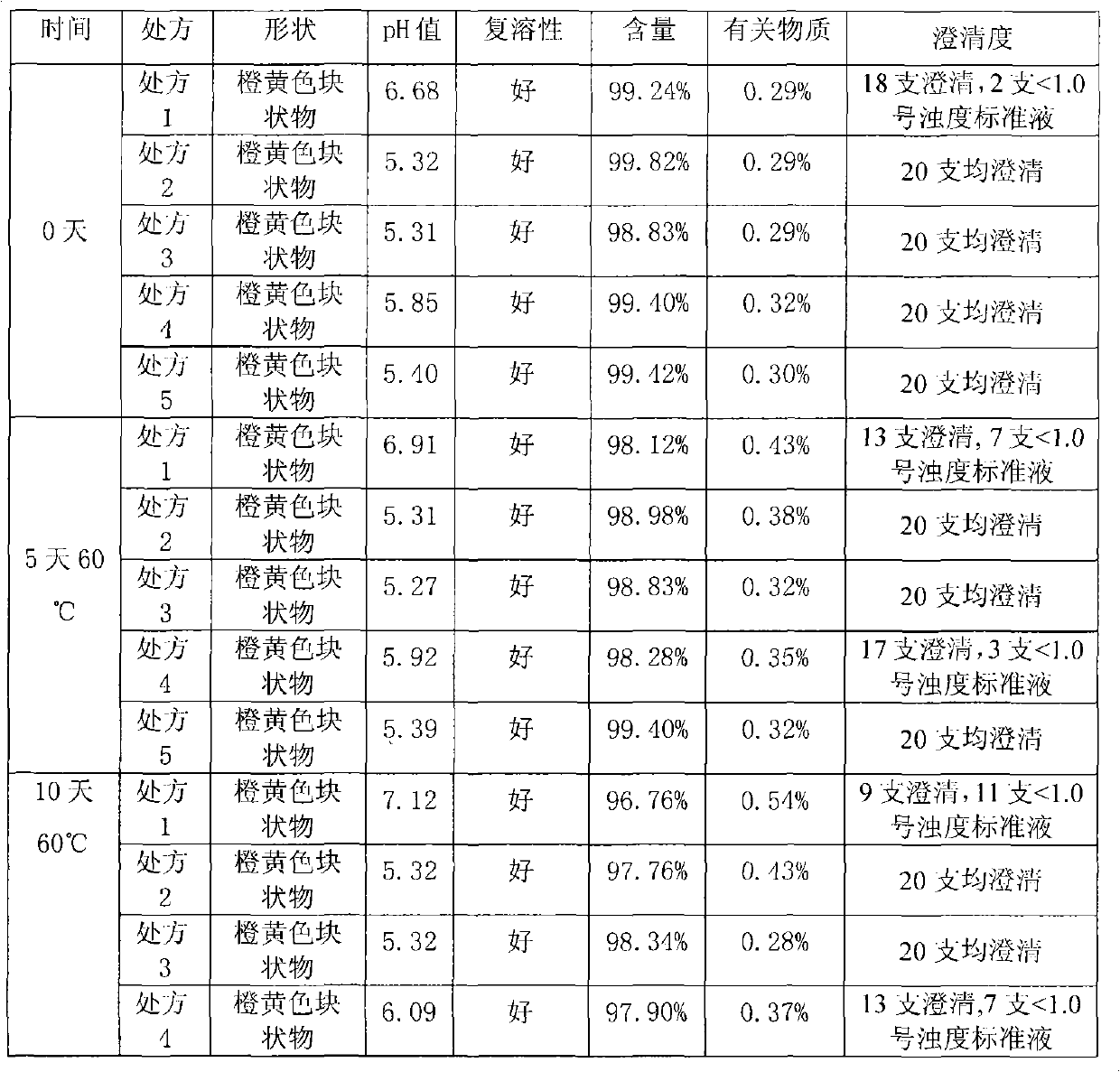
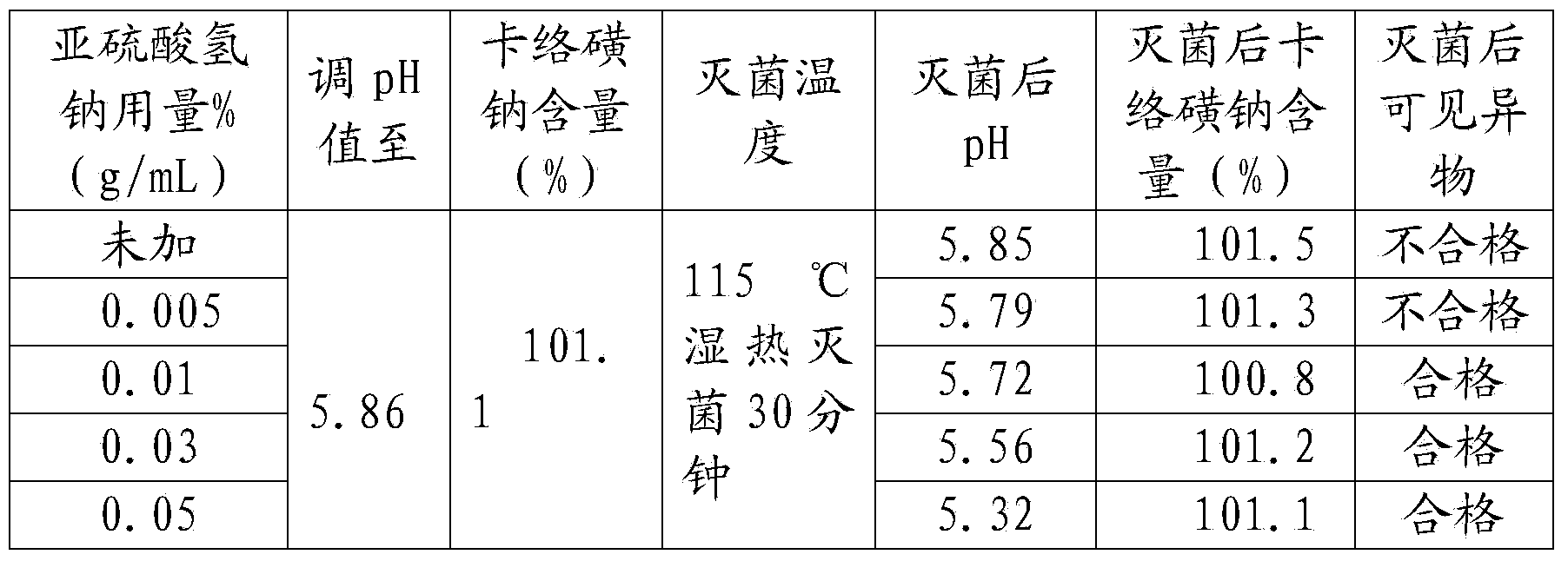
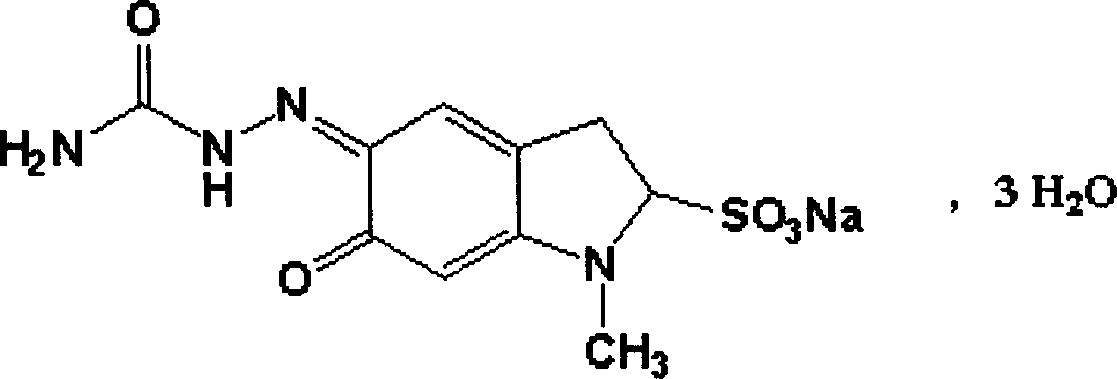
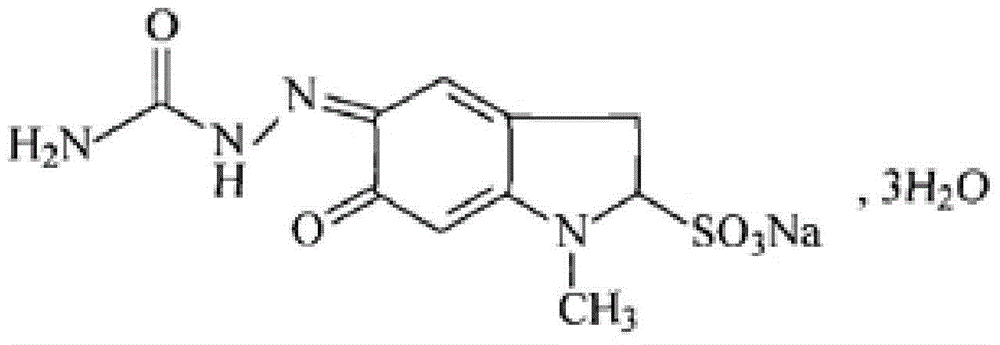
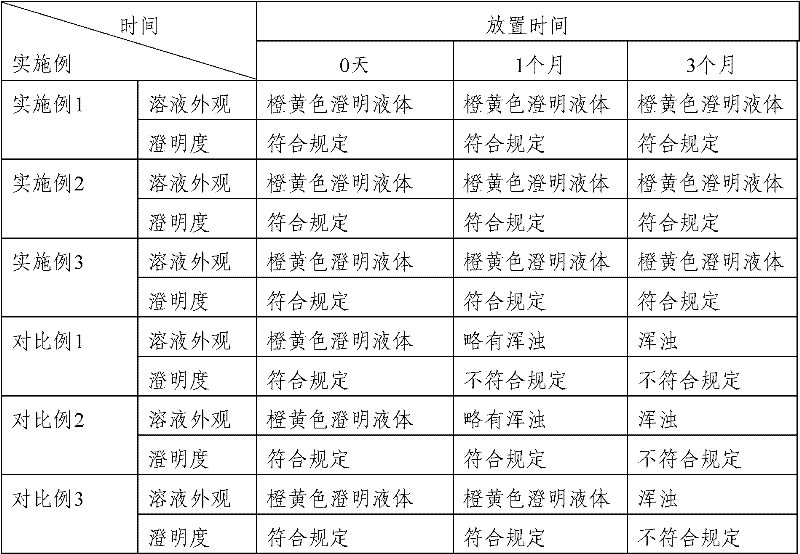
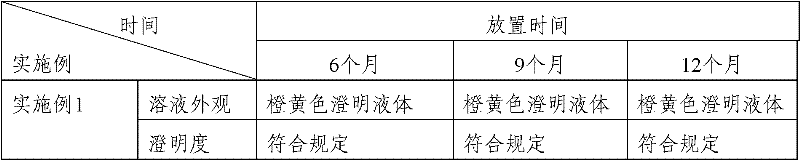
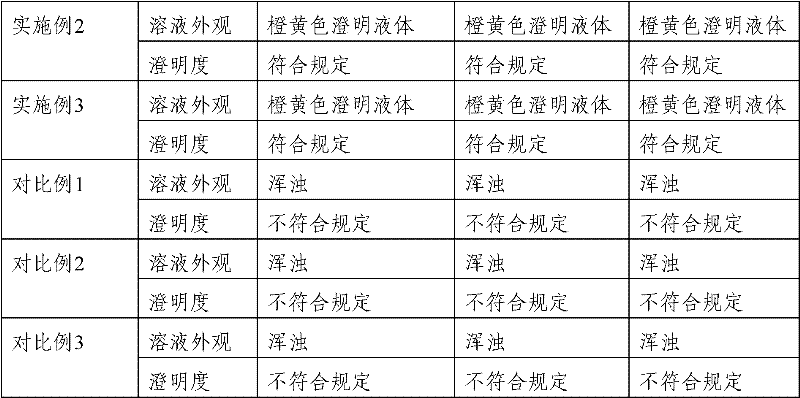
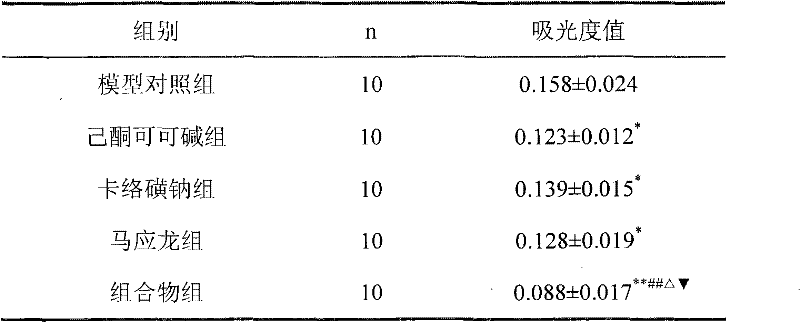
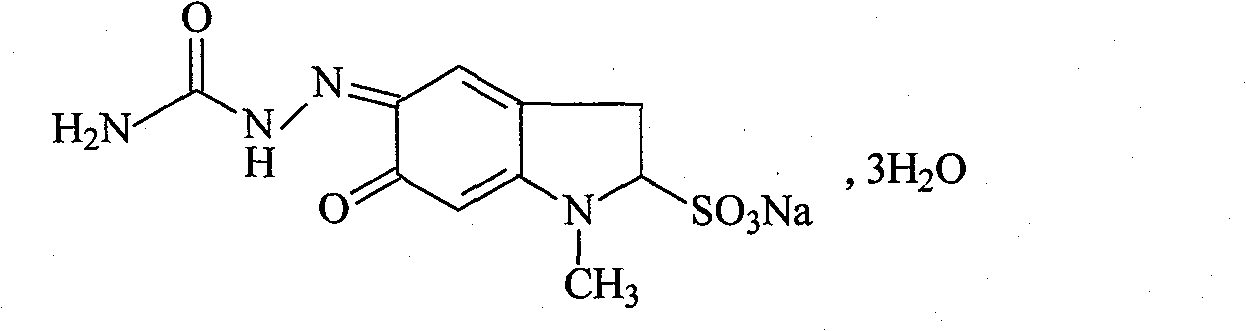
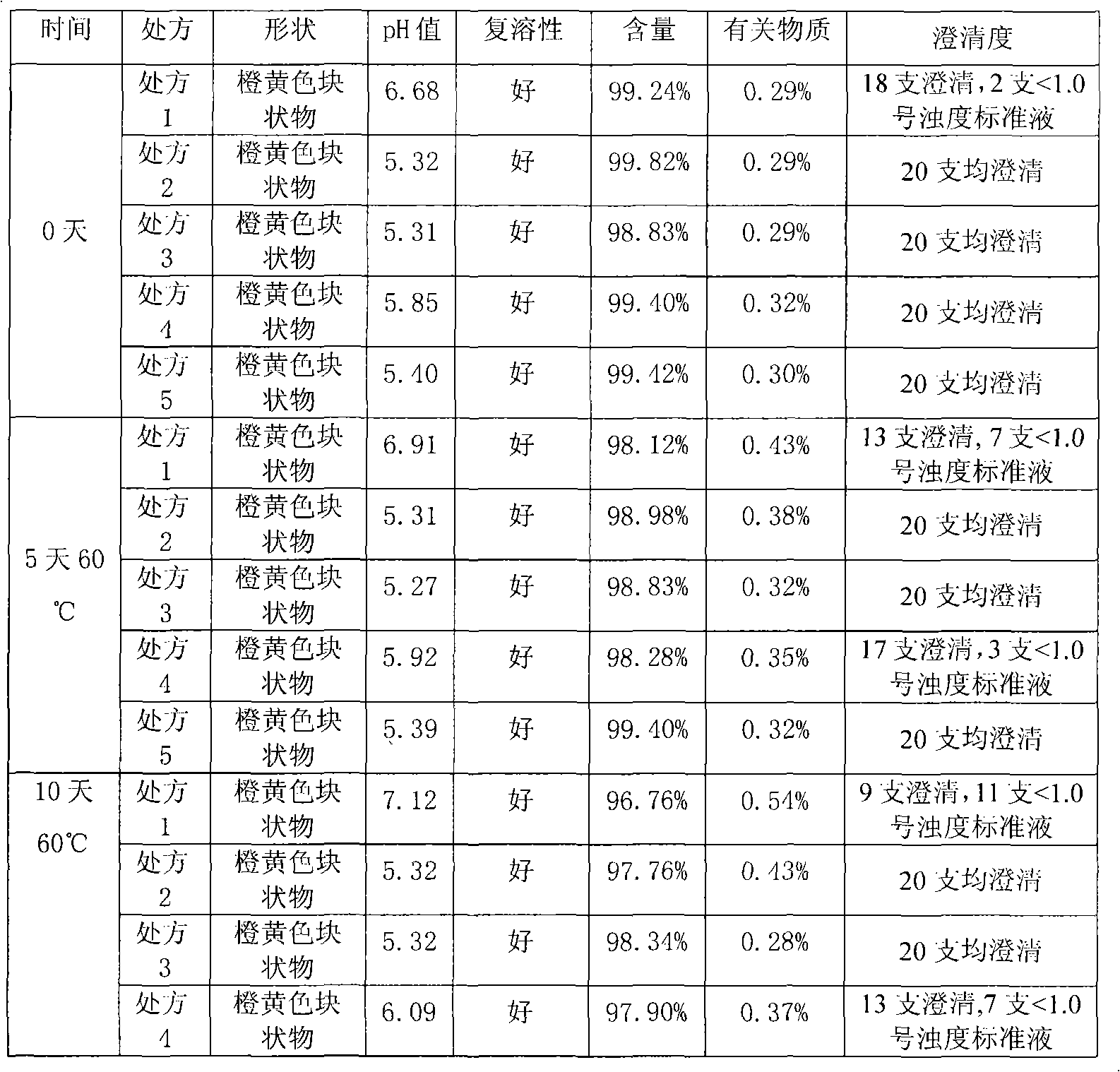
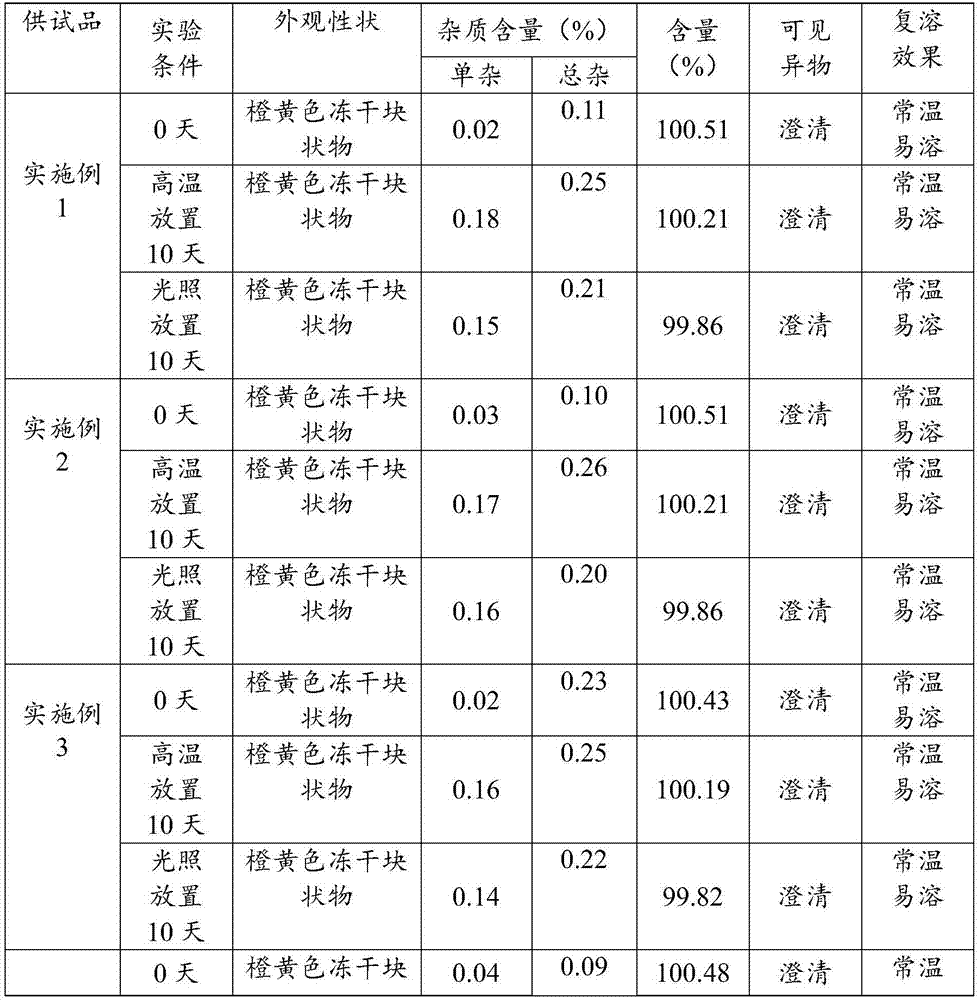
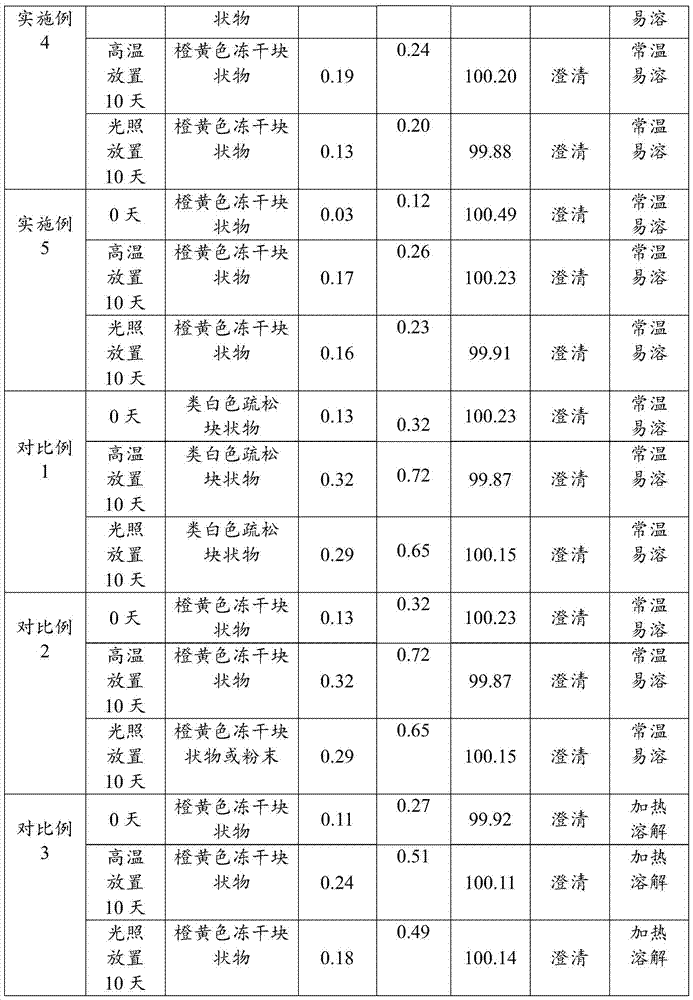
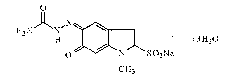
Patents
Literature
55 results about "Carbazochrome Sodium Sulfonate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
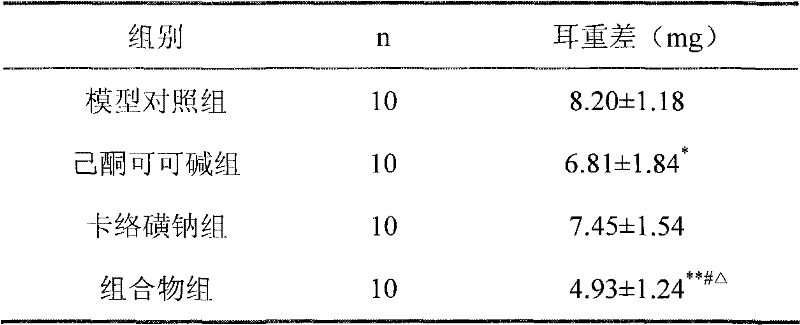
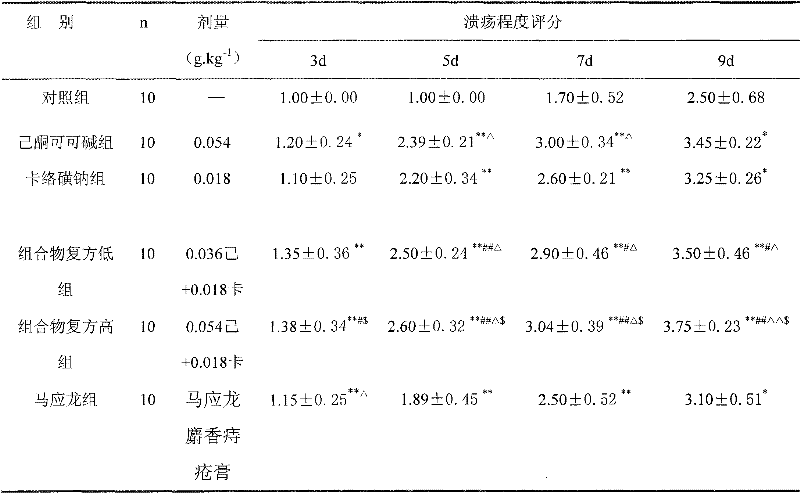
Carbazochrome is an antihemorrhagic, or hemostatic, agent that will cease blood flow by causing the aggregation and adhesion of platelets in the blood to form a platelet plug, ceasing blood flow from an open wound.
Carbazochrome Sodium Sulfonate infusion and its preparation method
InactiveCN1557302AGood effectEasy to operateOrganic active ingredientsPharmaceutical delivery mechanismDiseaseUpper gastrointestinal
The present invention belongs to the field of medicine technology. The Carbazochrome liquid contains Carbazochrome 0.002-0.5 wt%, antioxidant 0-10 wt%, osmotic pressure regulator 0.8-75 wt%, and injection water 20-99.1 wt%. The preparation process of the Carbazochrome liquid includes dissolving osmotic pressure regulator with partial injection water in a compounding tank; adding injection level active carbon through heating and stirring for adsorption for 30 min; filtering and decarbonizing with titanium rod and adding rest injection water to obtain the solution I; dissolving Carbazochrome and antioxidant in solution I, adding injection level active carbon for adsorption for 30 min, filtering and decarbonizing with titanium rod, regulating pH value, fine filtering, detecting, packing in bottle, disinfecting and other steps. The medicine is used in intravenous transfusion for treating various hemorrhage diseases.
Owner:肖广常 +1
Hemostatic injection of carbazochrome sodium sulfonate and method for preparing the same
ActiveCN1827101AOrganic active ingredientsPharmaceutical delivery mechanismCarbazochrome Sodium SulfonateActivated carbon
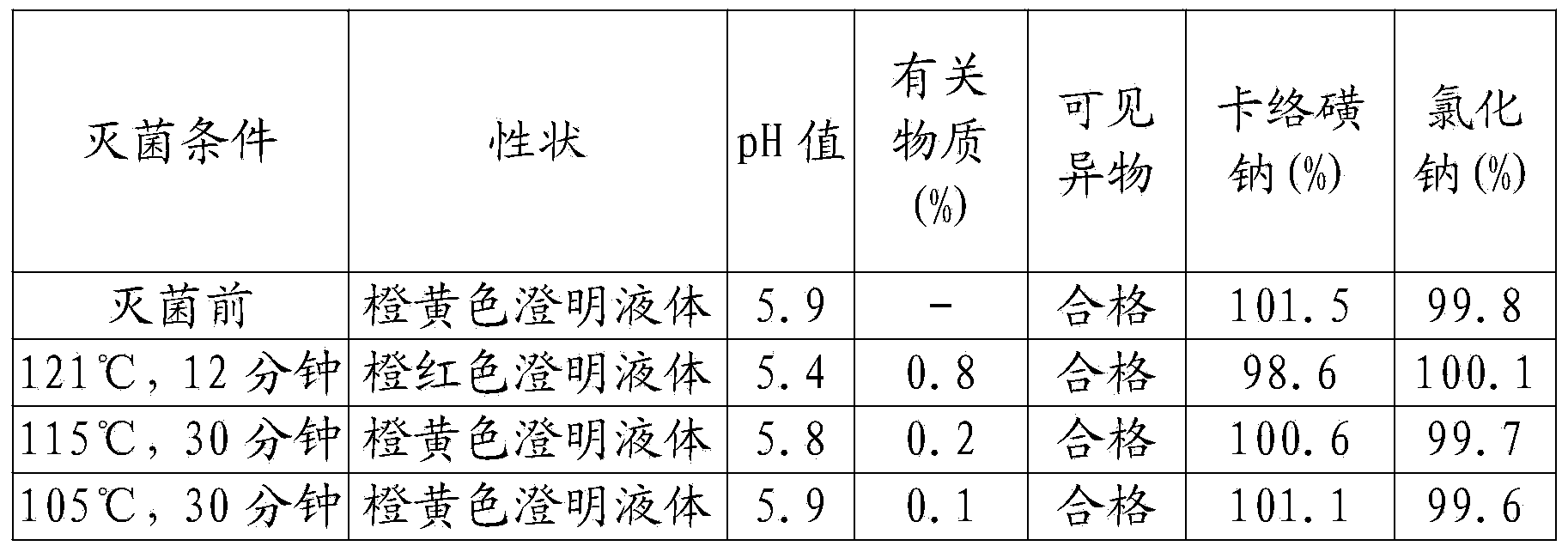
Disclosed is a hemostatic injection of carbazochrome sodium sulfonate and method for preparation, wherein the injection comprises carbazochrome sodium sulfonate 0.005-0.5 wt%, the optimum content being 0.008-0.2%, auxiliary solvent 0.001-0.5 wt%, the optimum content being 0.002-0.1%, osmoregulation agent 0.8-15 wt%, and water for injection 84.0-99.1%. The preparing process consists of boiling, filtering, removing charcoal, adjusting pH to 4.5-6.5, passing soup through a charcoal layer, loading into transfusion bottles, venting nitrogen, rolling the caps, and sterilizing.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD +1
Injection carbazochrome sodium sulfonate suspension and preparation method thereof
ActiveCN102600074AImprove stabilityImprove solubilityOrganic active ingredientsSolution deliveryPharmaceutical medicineImmunogenicity
The invention discloses an injection carbazochrome sodium sulfonate suspension and a preparation method thereof, relating to the technical field of medicines. The injection carbazochrome sodium sulfonate suspension is a powder injection and comprises the following components in parts by weight: 1 part of carbazochrome sodium sulfonate, 1.5-8.5 parts of pharmaceutically acceptable biological carrier, 0.1-1.8 parts of stabilizing agent and 2-5 parts of freezing protective agent. According to the invention, the stability and the dissolvability of the carbazochrome sodium sulfonate are improved, the injection carbazochrome sodium sulfonate suspension has no remarkable change of various indexes through detection after being placed for a long time, the quality of the injection carbazochrome sodium sulfonate suspension within a valid period is ensured to be qualified; and the injection carbazochrome sodium sulfonate suspension can be slowly administrated for a long time, thus bioavailability is greatly improved. The pharmaceutically acceptable biological carrier, i.e. protein, is degraded in vivo without toxicity and immunogenicity; and meanwhile, medicine therapeutic indexes can be effectively increased, and medicine toxicity is lowered and medicine side effects are reduced.
Owner:SHANGHAI JINCHENG PHARMACEUTICAL CO LTD
Carbazochrome sodium sulfonate freeze-dried powder injection and preparation method thereof
The invention discloses a carbazochrome sodium sulfonate freeze-dried powder injection and preparation method thereof, and the carbazochrome sodium sulfonate freeze-dried powder injection is composed of carbazochrome sodium sulfonate, mannitol, and buffer salts, wherein the solvent for preparation is injection water. Through a great number of tests, the problems of unstable pH value during the preparation of the carbazochrome sodium sulfonate freeze-dried powder injection and unqualified clarity during storage are solved only by adding buffer salts. The process of the prescription of the present invention overcomes the disadvantages of the prior arts, and provides a carbazochrome sodium sulfonate freeze-dried powder injection with the advantages of simple prescription, guaranteed quality and good stability; and is suitable for clinical applications. The present invention also achieves the advantages that: (1) the prescription is simple, wherein in addition to using carbazochrome sodium sulfonate as the main drug, the excipients in the prescription are only mannitol and buffer salts; (2) raw materials and excipients used in the prescription are all medicinal injection grade prescribed by the State Food and Drug Administration so as to eliminate the risk of unpredictable adverse reactions caused by non-pharmaceutical excipients; (3) the stability is good and the prescription is convenient for transportation and storage; (4) no hemolysis, agglutination, irritation, or allergic reactions are observed throughout special safety tests such as allergenicity, hemolytic activity andvascular irritation tests.
Owner:HAINAN LEVTEC PHARMA
Carbazochrome sodium sulfonate sodium chloride injection and preparation method thereof
ActiveCN103961310ASolve the sterilization that cannot tolerate F0≥8Solve productivityOrganic active ingredientsPharmaceutical delivery mechanismCarbazochrome Sodium SulfonateSodium Chloride Injection
The invention discloses a carbazochrome sodium sulfonate sodium chloride injection and a preparation method thereof. The product quality and stability are significantly improved by adding a stabilizer A and a stabilizer B, and controlling the pH value and the sterilization condition in the production process. The carbazochrome sodium sulfonate sodium chloride injection is prepared by adopting the formula, and the problems that the carbazochrome sodium sulfonate sodium chloride injection in the prior art cannot tolerate sterilization of which F0 is greater than or equal to 8, and the product is poor in stability in the production and storage processes are solved.
Owner:HUAREN PHARMA (RIZHAO) CO LTD
Freeze-dried injection containing carbazochrome sodium sulfonate and method of preparing the same
InactiveCN101254174AImprove solubilityHigh clarityOrganic active ingredientsPowder deliveryCarbazochrome Sodium SulfonateSolubility
The invention relates to the pharmaceutical preparation field, particularly to carbazochrome sodium sulfonate lyophilized powder for injection, which is characterized in that the stability of carbazochrome sodium sulfonate is improved by adding thiourea and / or sodium formaldehyde sulfoxylate, the solubility of the lyophilized powder for injection is improved by adding mannitol, and the obtained carbazochrome sodium sulfonate lyophilized powder for injection has stable quality.
Owner:SUZHOU SIXTH PHARMA PLANT OF JIANGSU WUZHONG PHARMA GROUP
Carbazochrome sodium sulfonate pharmaceutical composition with good stability and high safety as well as preparation method and application thereof
ActiveCN108938626AImprove stabilityStructure ConfirmationOrganic active ingredientsOrganic chemistryCarbazochrome Sodium SulfonateOrganic chemistry
The invention provides a carbazochrome sodium sulfonate pharmaceutical composition with good stability and highsafety as well as a preparation method and application thereof. The pharmaceutical composition contains carbazochrome sodium sulfonate and a compound represented by a formula III as shown in the specification, wherein the content of the compound is less than 0.5%.
Owner:CHENGDU TIANXIN PHARMA HEALTH CARE CO LTD
Carbazochrome sodium sulfonate slow-released tablet and its preparing method
InactiveCN1985808AImprove complianceGood hemostatic effectOrganic active ingredientsPharmaceutical delivery mechanismCarbazochrome Sodium SulfonateProlonged-release tablet
The present invention is slow released carbazochrome sodium sulfonate matrix tablet and its preparation process. The recipe of the slow released carbazochrome sodium sulfonate matrix tablet includes carbazochrome sodium sulfonate 5-85 wt%, matrix material 10-90 wt%, diluent 0-80 wt%, and proper amount of adhesive and lubricant. It is prepared through mixing material powder and direct tabletting; or pelletizing the mixture of materials except lubricant, mixing with lubricant and tabletting; or preparing fast release part, preparing slow releasing part and double-layered tabletting. The pelletizing may be wet pelletizing, dry pelletizing, smelting palletizing, etc.
Owner:SHANDONG INST OF PHARMA IND
Carbazochrome sodium sulfonate powder injection for injection and preparation method thereof
The invention relates to a carbazochrome sodium sulfonate powder injection for injection and a preparation method thereof. A freeze drying powder injection of carbazochrome sodium sulfonate is basically free of freeze-drying excipients, wherein the content of carbazochrome sodium sulfonate is above 90%. When injection water for the freeze-dried powder injection is dissolved in a carbazochrome sodium sulfonate 10 mg / ml concentration solution, the pH value of the solution ranges from 5.0 to 6.0. The carbazochrome sodium sulfonate powder injection for injection can be applied to bleeding of the urinary system, the upper gastrointestinal tract, the respiratory tract and obstetrical and gynecological diseases, has a remarkable curative effect on bleeding of the urinary system, can be applied to traumatic bleeding and operation bleeding, and has an excellent physicochemical property.
Owner:CHENGDU TIANTAISHAN PHARMA
Special ultrafine carbazochrome sodium sulfonate powder freeze-dried preparation and preparation method thereof
InactiveCN104127388AImprove stabilityHigh purityPowder deliveryOrganic active ingredientsCarbazochrome Sodium SulfonateFreeze-drying
The invention discloses a special ultrafine carbazochrome sodium sulfonate powder freeze-dried preparation and a preparation method thereof. The method comprises the following steps: step 1, dissolving carbazochrome sodium sulfonate in water, adding sodium bisulfite and carrying out a reaction so as to prepare a mixed liquor; step 2, adding a decolorant into the mixed liquor for decoloring and carrying out filtering so as to obtain a filtrate; step 3, adjusting the pH value of the filtrate by using sodium hydroxide, carrying out cooling, allowing a crystal to be precipitated and then successively carrying out filtering, washing and vacuum drying so as to obtain carbazochrome sodium sulfonate; and step 4, carrying out air jet pulverization on dried carbazochrome sodium sulfonate, dissolving special ultrafine powder of carbazochrome sodium sulfonate in injection water, carrying out low-temperature pre-freezing, then carrying out low-temperature pressure-reduced vacuum drying and finally, carrying out high temperature drying so as to prepare the special ultrafine carbazochrome sodium sulfonate powder freeze-dried preparation. The special ultrafine carbazochrome sodium sulfonate powder freeze-dried preparation prepared in the invention has the advantages of high stability, high purity, a few impurities, a small size, a great specific surface area, good dissolvability, small toxic and side effects, uneasy incurrence of irritability, etc.
Owner:杭州长典老一元健康管理有限公司
Preparation method of carbazochrome sodium sulfonate freeze-dried powder injection
ActiveCN105343019AControl contentShort reconstitution timePowder deliveryOrganic active ingredientsCarbazochrome Sodium SulfonateFreeze-drying
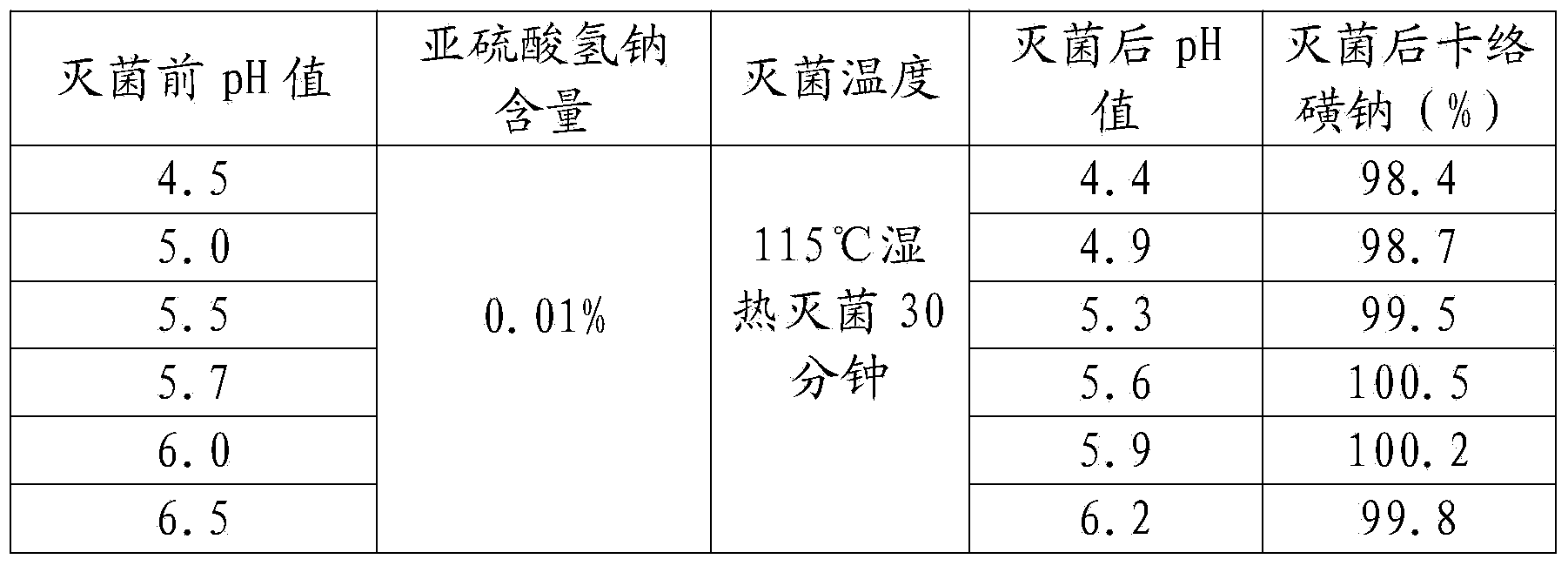
The invention provides a preparation method of a carbazochrome sodium sulfonate freeze-dried powder injection. The method comprises the following steps: (a) mixing carbazochrome sodium sulfonate with first injection water, and sequentially carrying out pH value adjustment and adsorption treatment to obtain a mixed solution A; and (b) mixing the mixed solution A with second injection water, and sequentially freezing and drying to obtain the carbazochrome sodium sulfonate freeze-dried powder injection, wherein the volume ratio of the first injection water to the second injection water is 6 to (3-5). Compared with the prior art, according to the preparation method provided by the invention, the content of impurities in the carbazochrome sodium sulfonate freeze-dried powder injection can be effectively controlled, and the re-dissolving time of a product is shortened. An experiment result shows that the content of the impurities in the carbazochrome sodium sulfonate freeze-dried powder injection prepared by the preparation method provided by the invention is lower than 0.5%, and the re-dissolving time is within 8 seconds.
Owner:HUNAN KELUN PHARMA
Pesticide for preventing and treating ichthyophthiriasis of pet fish
InactiveCN102670649APromote healthy developmentEffective controlInorganic active ingredientsAntiparasitic agentsDiseaseSodium bicarbonate
The invention relates to the technical field of pesticides for preventing and treating diseases of aquatic animals, which particularly discloses a pesticide for preventing and treating ichthyophthiriasis of pet fish. The pesticide consists of sodium chloride, sodium bicarbonate, magnesium chloride, formaldehyde, polyiodide, potassium permanganate, carbazochrome sodium sulfonate, copper sulfate, vitamin K1, ferrous sulfate and water in balance, wherein the concentration of sodium chloride is 15-25g / L, the concentration of sodium bicarbonate is 4.6-5.4g / L, the concentration of magnesium chloride is 0.12-0.25g / L, the concentration of formaldehyde is 20-40mg / L, the concentration of polyiodide is 6-15mg / L, the concentration of potassium permanganate is 1.6-3.0mg / L, the concentration of carbazochrome sodium sulfonate is 1.2-2.0mg / L, the concentration of copper sulfate is 0.05-0.15mg / L, the concentration of vitamin K1 is 0.6-1.0mg / L, and the concentration of ferrous sulfate is 0.02-0.07mg / L.The pesticide provided by the invention can efficiently prevent and treat ichthyophthiriasis of pet fish, guarantee healthy development of pet fish cultivation, and has extensive market prospect and economic benefit.
Owner:HENAN UNIV OF SCI & TECH
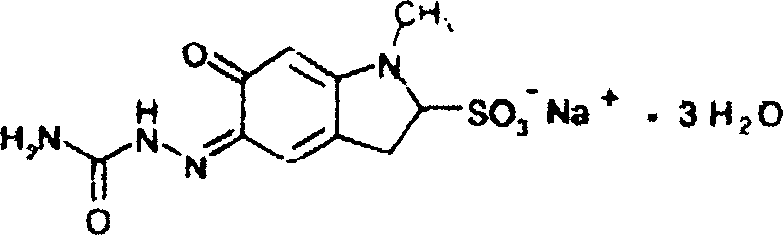
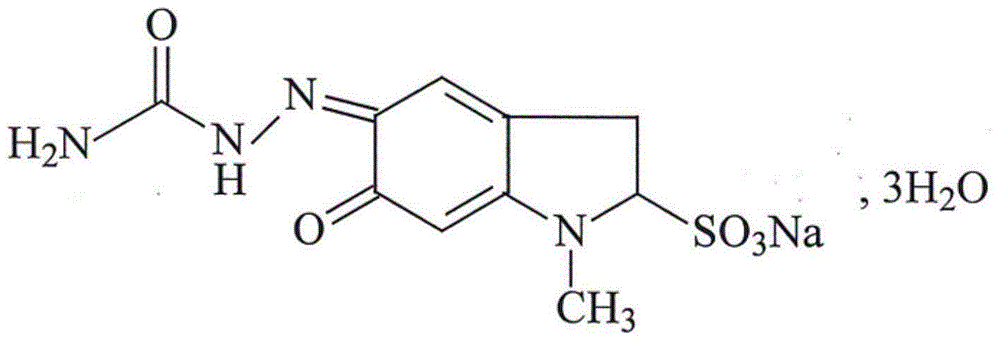
Carbazochrome sodium sulfonate compound and composition thereof
ActiveCN102718693ASimple prescriptionReduced stabilityOrganic active ingredientsOrganic chemistryCarbazochrome Sodium SulfonateX-ray
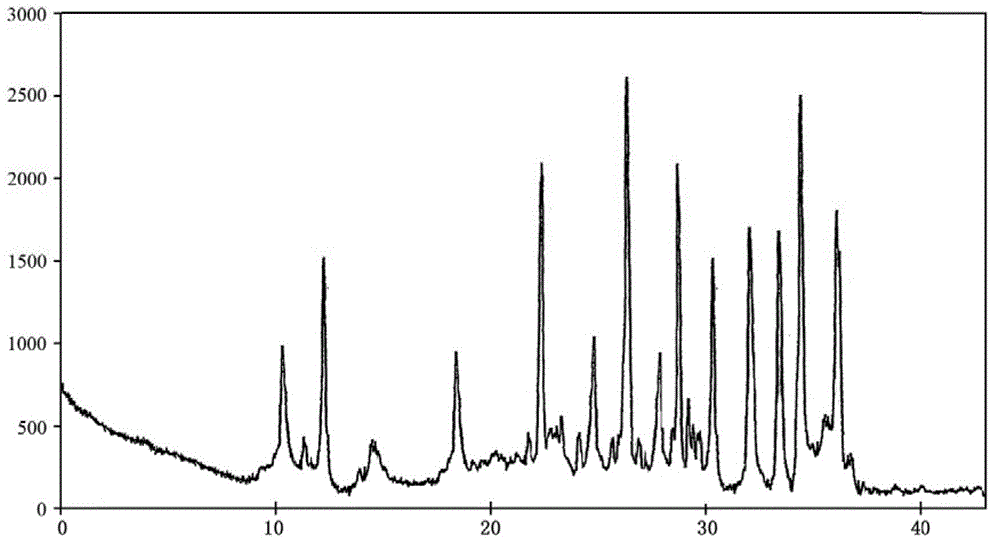
The invention relates to a carbazochrome sodium sulfonate compound and a composition thereof. The carbazochrome sodium sulfonate compound is measured by a powder X-ray diffraction measurement method; and the diffraction angle of 2 theta + / - 0.2 degrees can be used for showing that an X-ray powder diffraction pattern has characteristic diffraction peaks at the parts including 10.23 degrees, 12.12 degrees, 18.39 degrees, 22.32 degrees, 24.79 degrees, 26.23 degrees, 28.60 degrees, 30.25 degrees, 31.98 degrees, 33.32 degrees, 34.32 degrees and 36.02 degrees. The carbazochrome sodium sulfonate compound provided by the invention has the remarkably improved stability and does not change after being placed for a long time, so that the drug safety of patients can be greatly improved.
Owner:江西璟瑞药业有限公司
Carbazochrome sodium sulfonate compound and medical composition thereof
ActiveCN103145603AThe prescription process is simpleImprove stabilityOrganic active ingredientsOrganic chemistryCarbazochrome Sodium SulfonateChemical compound
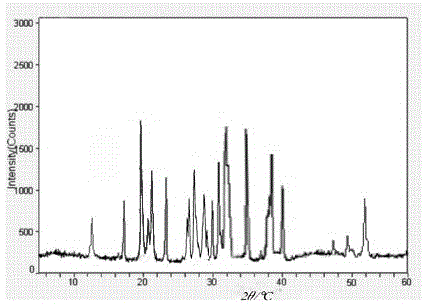
The invention relates to a carbazochrome sodium sulfonate compound which is a crystal. The characteristic peak in a map through X-ray powder diffraction determination is 12.5 degrees, 17.4 degrees, 19.8 degrees, 21.0 degrees, 21.5 degrees, 23.4 degrees, 26.2 degrees, 26.8 degrees, 27.7 degrees, 29.0 degrees, 3.01 degrees, 31.6 degrees, 32.1 degrees, 35.0 degrees, 38.5 degrees, 40.3 degrees and 51.9 degrees at 2theta+ / -0.2 degrees. The invention further provides a medical composition preparation containing the carbazochrome sodium sulfonate compound. The medical composition preparation is a freeze-dried powder injection, a water injection and a troche. The compound provided by the invention can be prepared in various medical forms and is extremely high in stability. The carbazochrome sodium sulfonate freeze-dried powder, water injection and troche preparations provided by the invention are simple in formulation and technology, the stability is remarkably improved, and the pharmaceutical safety and effectiveness are improved.
Owner:HUNAN WUZHOUTONG PHARMA
Carbazochrome sodium sulfoate dripping pill and its preparing method
InactiveCN1562001ADisintegration and dissolution fastHigh dissolution rateOrganic active ingredientsPill deliveryCarbazochrome Sodium SulfonateBiomedical engineering
A dripping pill of carbazochrome sodium sulfonate is prepared through superfine pulverizing and conventional steps for preparing dripping pills.
Owner:HONGYI SCI & TECH CO LTD NANCHANG
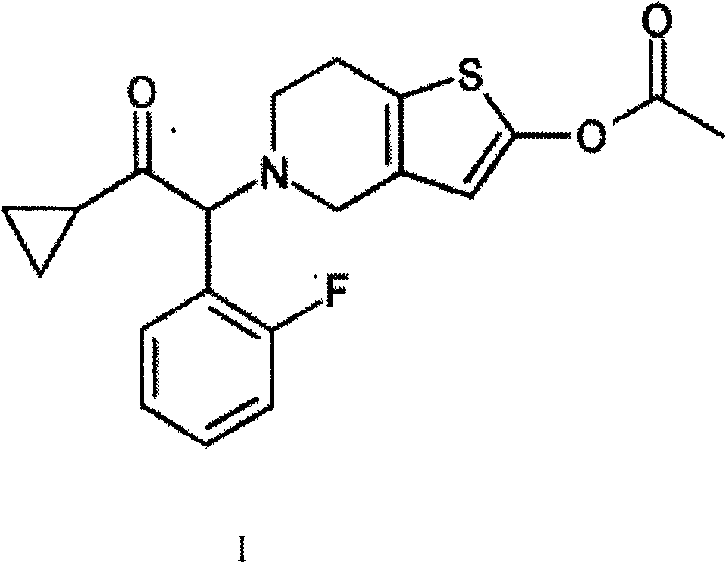
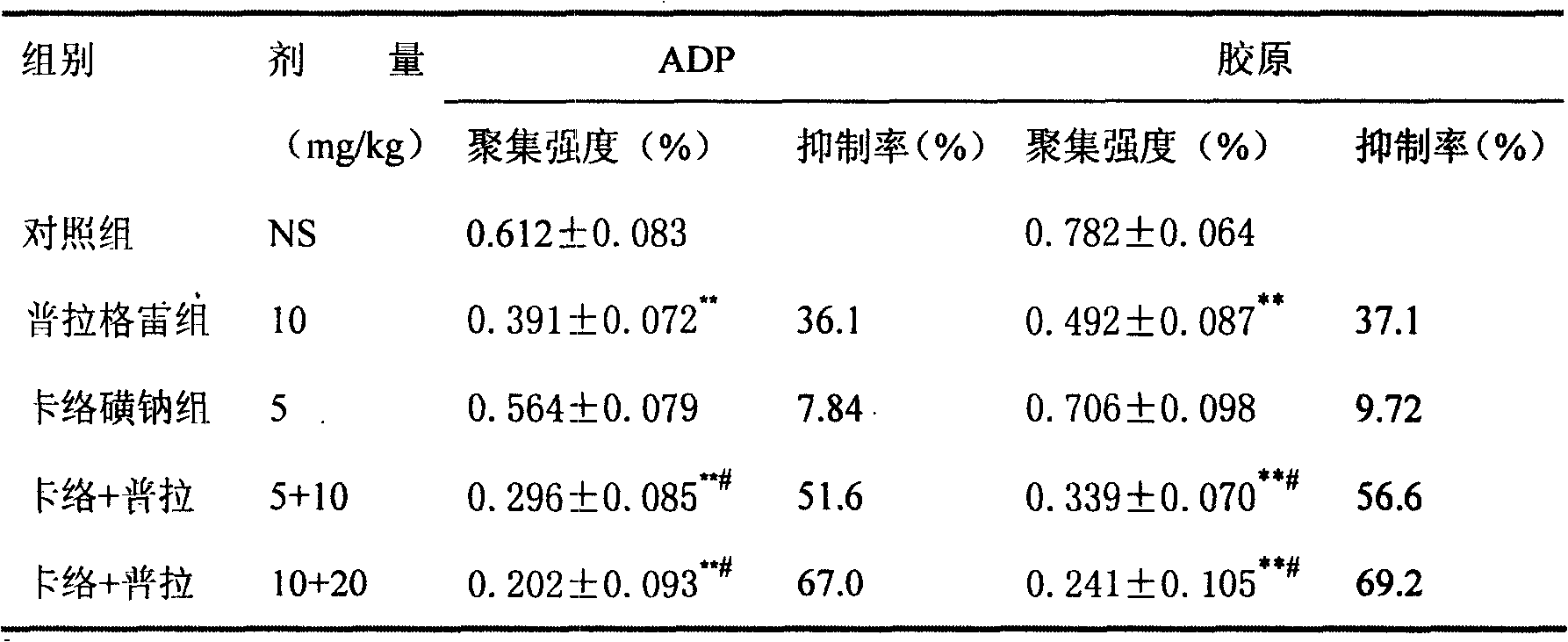
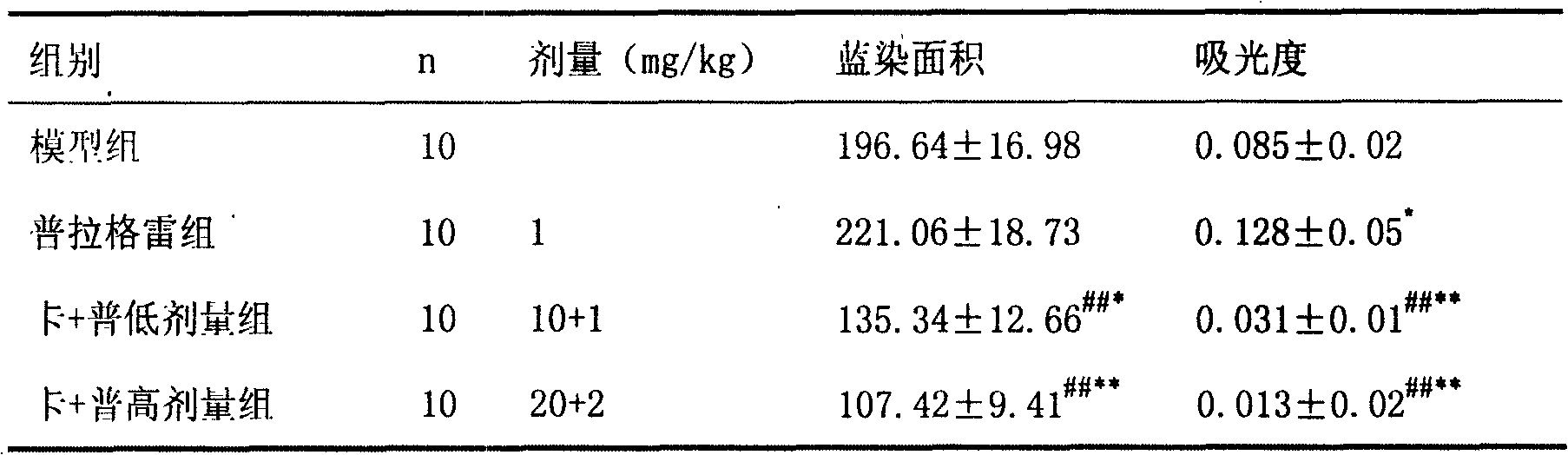
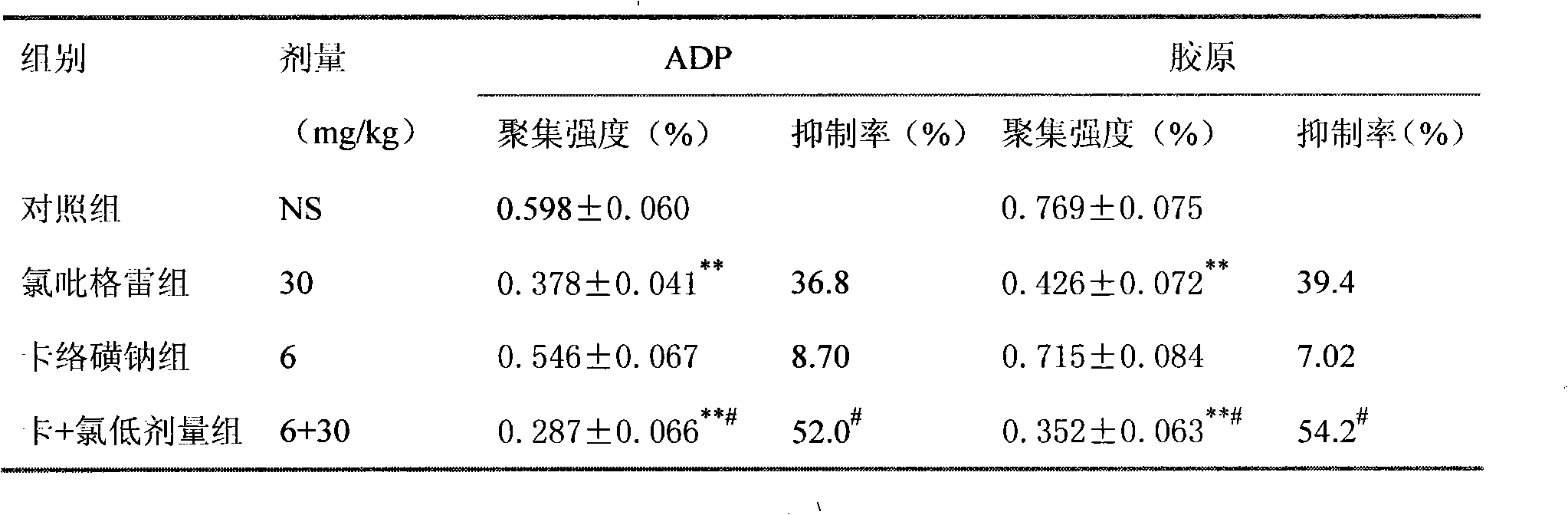
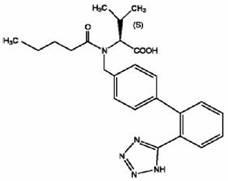
Pharmaceutical composition containing prasugrel and carbazochrome sodium sulfonate
ActiveCN101554377ADoes not affect anticoagulant activityInhibit aggregationOrganic active ingredientsBlood disorderDiseaseCarbazochrome Sodium Sulfonate
The invention provides a pharmaceutical composition containing active carbazochrome sodium sulfonate and prasugrel or the pharmacologically acceptable salt thereof. The invention aims to invent a more effective and low adverse-reaction method for curing thrombotic diseases by using the combined medication of the carbazochrome sodium sulfonate and the prasugrel. After conscientious trail of the patent applicant for many times, the effect of applying the carbazochrome sodium sulfonate to inhibit thrombosis in a combined manner is discovered unexpected in the process of applying the prasugrel to cure the thrombotic diseases, the anticoagulant effect of the prasugrel is not abated but increased, and the very good synergistic effects are achieved after the combination of the carbazochrome sodium sulfonate and the prasugrel, therefore, the risk of bleeding is greatly reduced when the advantages of good anticoagulant activity and fast effect of the prasugrel during the antiplatelet aggregation are fully exerted, the risk of bleeding caused by the prasugrel is effectively reduced and the compliance of patients is enhanced.
Owner:LUNAN PHARMA GROUP CORPORATION
Pharmaceutical composition containing clopidogrel and carbazochrome sodium sulfonate
ActiveCN101554375ADoes not affect anticoagulant activityInhibit aggregationOrganic active ingredientsBlood disorderDiseaseCarbazochrome Sodium Sulfonate
The invention provides a pharmaceutical composition containing active carbazochrome sodium sulfonate and clopidogrel or the pharmacologically acceptable salt thereof. The invention aims to invent a more effective and low adverse-reaction method for curing thrombotic diseases by using the combined medication of the carbazochrome sodium sulfonate and the clopidogrel. After conscientious trail of the patent applicant for many times, the effect of applying the carbazochrome sodium sulfonate to inhibit the thrombosis in a combined manner is discovered unexpected in the process of applying the clopidogrel to cure the thrombotic diseases, the anticoagulant effect of the clopidogrel is not abated but increased, and the very good synergistic effects are achieved after the combination of the carbazochrome sodium sulfonate and the clopidogrel, therefore, the risk of bleeding is greatly reduced when the advantages of good anticoagulant activity and fast effect of the clopidogrel during the antiplatelet aggregation are fully exerted, the risk of bleeding caused by the clopidogrel is greatly reduced and the compliance of patients is enhanced.
Owner:LUNAN PHARMA GROUP CORPORATION
Hemostatic injection of carbazochrome sodium sulfonate and method for preparing the same
ActiveCN1332660COrganic active ingredientsPharmaceutical delivery mechanismCarbazochrome Sodium SulfonateNitrogen gas
Disclosed is a hemostatic injection of carbazochrome sodium sulfonate and method for preparation, wherein the injection comprises carbazochrome sodium sulfonate 0.005-0.5 wt%, the optimum content being 0.008-0.2%, auxiliary solvent 0.001-0.5 wt%, the optimum content being 0.002-0.1%, osmoregulation agent 0.8-15 wt%, and water for injection 84.0-99.1%. The preparing process consists of boiling, filtering, removing charcoal, adjusting pH to 4.5-6.5, passing soup through a charcoal layer, loading into transfusion bottles, venting nitrogen, rolling the caps, and sterilizing.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD +1
Medicinal composition containing carbazochrome sodium sulfonate compound and preparation method thereof
ActiveCN102210656ASuitable for clinical usePowder deliveryOrganic active ingredientsSolubilityCarbazochrome Sodium Sulfonate
The invention provides a medicinal composition containing carbazochrome sodium sulfonate compound and a preparation method thereof. The medicinal composition containing carbazochrome sodium sulfonate compound consists of carbazochrome sodium sulfonate, mannitol, dextran and sodium hydroxide. The medicinal composition containing carbazochrome sodium sulfonate compound can solves the problems of low stability, low solubility and the like of the conventional carbazochrome sodium sulfonate powder injection and is more suitable for use in clinic.
Owner:福建康成医药有限公司
Pharmaceutical composition containing pentoxifylline and carbazochrome sodium sulfonate and application thereof
ActiveCN102125559AImprove microcirculationTreatment fitCardiovascular disorderHeterocyclic compound active ingredientsCarbazochrome Sodium SulfonateActive component
The invention belongs to the field of medicine, and relates to a pharmaceutical composition containing pentoxifylline and carbazochrome sodium sulfonate and application thereof. The pharmaceutical composition contains the active components pentoxifylline and carbazochrome sodium sulfonate, has the efficacies of diminishing inflammation, arresting bleeding, improving the vascular permeability and the like in treating hemorrhagic hemorrhoids, is obviously superior to the single drugs of pentoxifylline and carbazochrome sodium sulfonate in therapeutic action, and has a synergistic action.
Owner:LUNAN PHARMA GROUP CORPORATION
Carbazochrome sodium sulfonate and preparing method thereof
ActiveCN104926710AQuality improvementHigh purityOrganic chemistryCarbazochrome Sodium SulfonateUpper gastrointestinal
The invention relates to carbazochrome sodium sulfonate and a preparing method thereof. Particularly, on one aspect, the invention relates to carbazochrome sodium sulfonate which is prepared by the method with the following steps: raw material dissolving reaction, decoloration separation, crystallization and refining. The invention provides the carbazochrome sodium sulfonate with excellent properties, which can be used for bleeding of urinary systems, upper gastrointestinal tracts, respiratory tracts and obstetrical and gynecological diseases. The carbazochrome sodium sulfonate has an obvious effect for bleeding treatment of the urinary systems, can also be used for bleeding of wounds and operations. The carbazochrome sodium sulfonate prepared by the method has excellent physicochemical properties.
Owner:成都天台山制药股份有限公司
Valsartan sustained release tablet and preparation method thereof
InactiveCN102641253AImprove complianceThe effect of reducing blood pressure is stablePharmaceutical delivery mechanismHeterocyclic compound active ingredientsSustained Release TabletCarbazochrome Sodium Sulfonate
The invention provides a preparation method of a valsartan sustained release matrix tablet. Particular prescription components (g / g) of a carbazochrome sodium sulfonate trihydrate sustained release tablet is 20-50% of valsartan, 8-20% of framework materials, 35-75% of diluent, 0-5% of binding agent and 1-4% of lubricating agent. The preparation method adopts full powder direct compression or adopts a wet granulation to achieve compression after dryimng.
Owner:SHANDONG INST OF PHARMA IND
Carbazochrome sodium sulfonate freeze-dried powder injection and preparation method thereof
The invention discloses a carbazochrome sodium sulfonate freeze-dried powder injection and preparation method thereof, and the carbazochrome sodium sulfonate freeze-dried powder injection is composed of carbazochrome sodium sulfonate, mannitol, and buffer salts, wherein the solvent for preparation is injection water. Through a great number of tests, the problems of unstable pH value during the preparation of the carbazochrome sodium sulfonate freeze-dried powder injection and unqualified clarity during storage are solved only by adding buffer salts. The process of the prescription of the present invention overcomes the disadvantages of the prior arts, and provides a carbazochrome sodium sulfonate freeze-dried powder injection with the advantages of simple prescription, guaranteed quality and good stability; and is suitable for clinical applications. The present invention also achieves the advantages that: (1) the prescription is simple, wherein in addition to using carbazochrome sodium sulfonate as the main drug, the excipients in the prescription are only mannitol and buffer salts; (2) raw materials and excipients used in the prescription are all medicinal injection grade prescribed by the State Food and Drug Administration so as to eliminate the risk of unpredictable adverse reactions caused by non-pharmaceutical excipients; (3) the stability is good and the prescription is convenient for transportation and storage; (4) no hemolysis, agglutination, irritation, or allergic reactions are observed throughout special safety tests such as allergenicity, hemolytic activity andvascular irritation tests.
Owner:HAINAN LEVTEC PHARMA
Carbazochrome sodium sulfonate freeze-dried powder injection and preparation method thereof
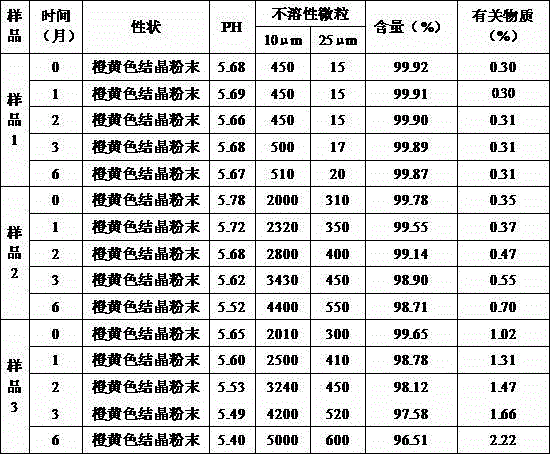
InactiveCN105434371ALow impurity contentStable contentPowder deliveryOrganic active ingredientsCarbazochrome Sodium SulfonateFreeze-drying
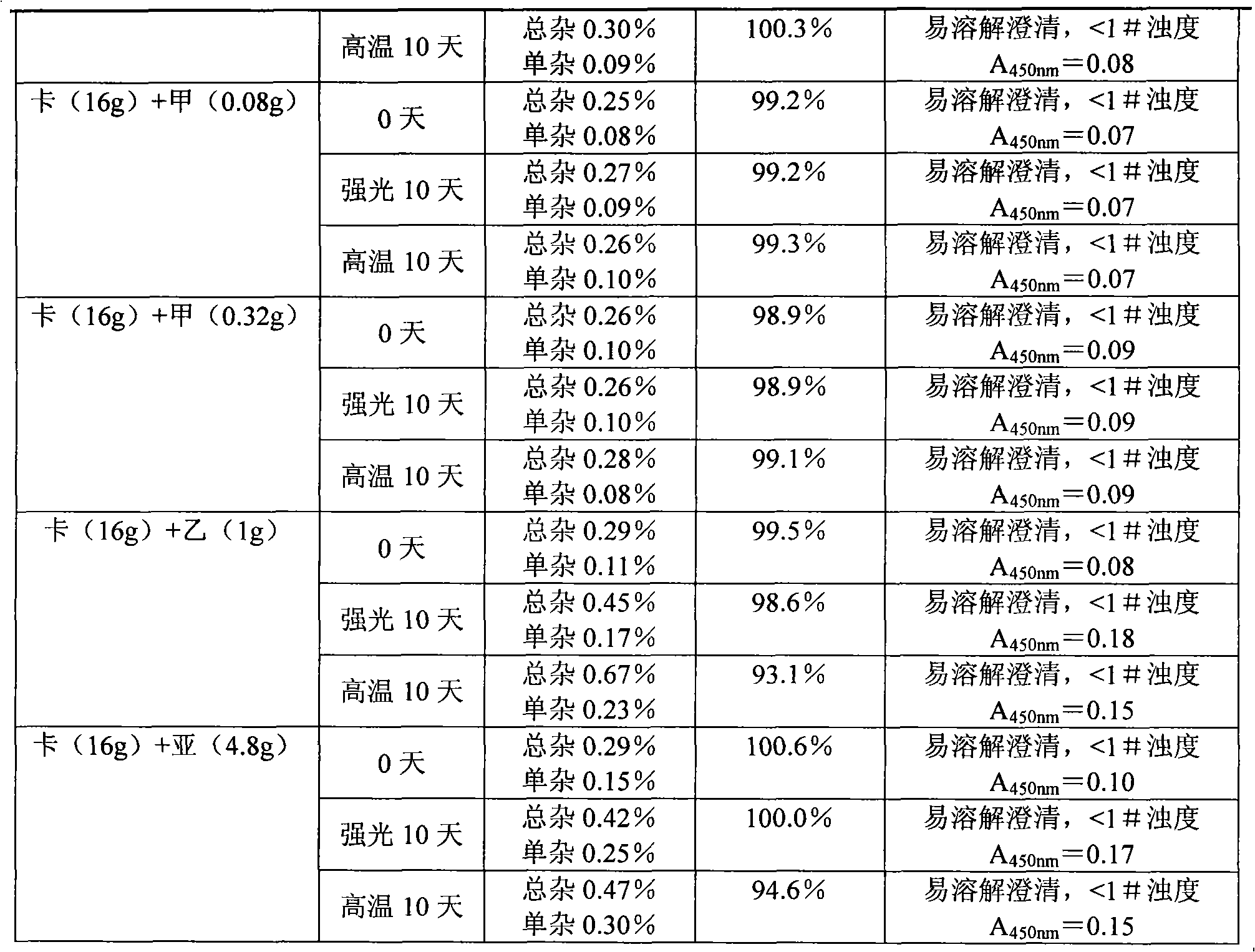
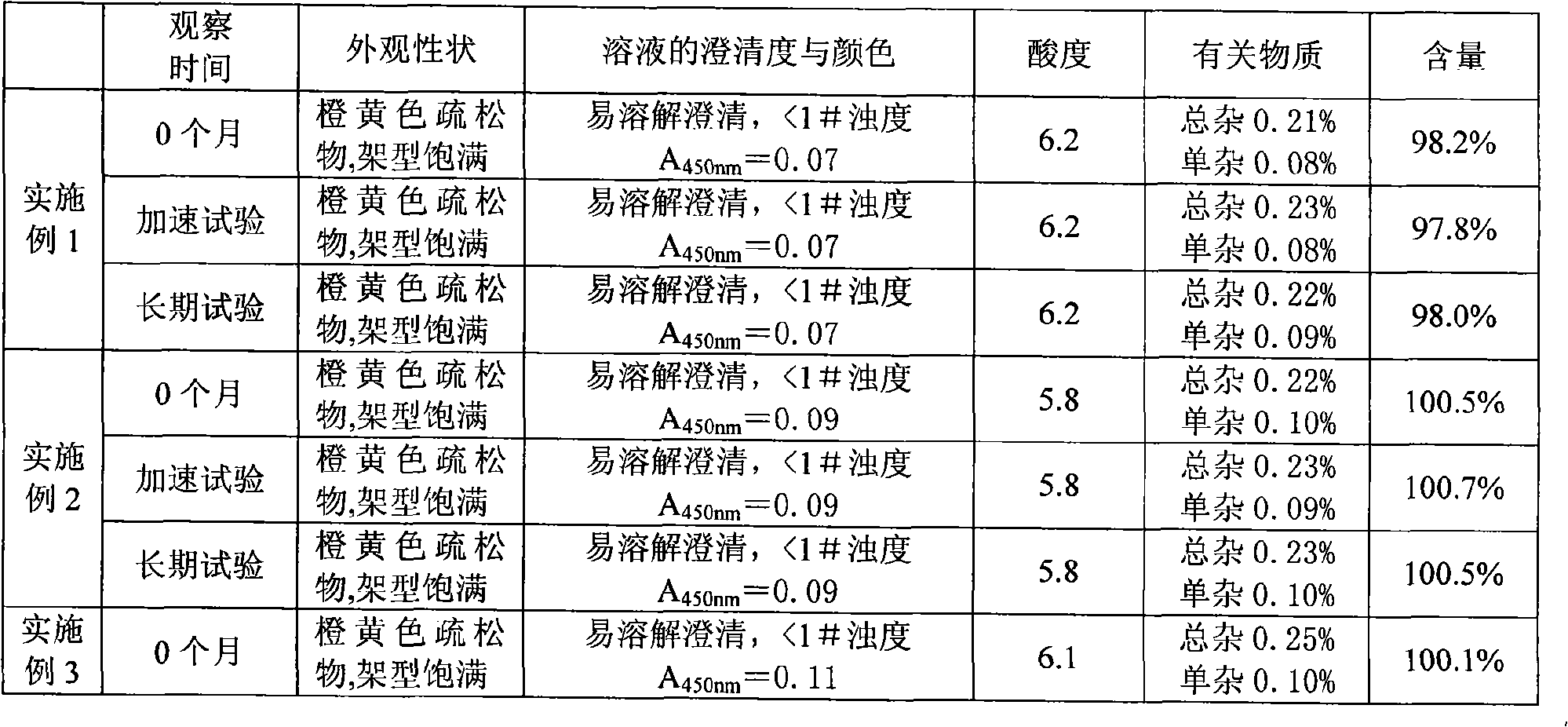
The invention belongs to the field of pharmaceutical preparations and particularly relates to a carbazochrome sodium sulfonate freeze-dried powder injection and a preparation method thereof. The carbazochrome sodium sulfonate freeze-dried powder injection comprises raw materials as follows: 10-50 parts of carbazochrome sodium sulfonate by weight and 1,800-2,200 parts of water for injection by volume. The freeze-dried powder injection has a good appearance property (orange-yellow freeze-dried cakes or powder) and has the lower impurity content, the change of the impurity content is slighter under high temperature and light conditions, the content of the carbazochrome sodium sulfonate is stable, and the quality stability is better. According to the preparation method of the carbazochrome sodium sulfonate freeze-dried powder injection, through the step that programmed temperature rise is adopted in freeze-drying treatment, the temperature rise speed is controlled, the carbazochrome sodium sulfonate is fully freeze-dried and formed in the stepped temperature change environment, and the good appearance property and better quality stability are obtained; besides, the preparation method only takes less than 17 h for freeze-drying, the freeze-drying time is greatly shortened, and industrial production is facilitated.
Owner:SUZHOU SIXTH PHARMA PLANT OF JIANGSU WUZHONG PHARMA GROUP
Carbazochrome sodium sulfonate oral disintegrating tablets and preparation method thereof
InactiveCN101797235AFlat surfaceBeautiful surfaceOrganic active ingredientsPill deliveryIrritationAdhesive
The invention discloses carbazochrome sodium sulfonate oral disintegrating tablets and a preparation method thereof. The carbazochrome sodium sulfonate oral disintegrating tablets are prepared from the following components in percentage by mass: 1-15% of carbazochrome sodium sulfonate, 50-90% of filler, 5-40% of disintegrant, 1-8% of adhesive, 0.1-5% of lubricant and 0.1-5% of flavoring agent. The oral disintegrating tablets have the advantages of smooth surface, attractive appearance, moderate hardness, quick absorption, quick effect taking, small liver first-pass effect, high bioavailability, little irritation to the gastrointestinal tract, favorable mouthfeel and convenient taking, can quickly disintegrate in the oral cavity without water, is particularly suitable for aged people, children, patients in bed or with dysphagia, and especially for bleeding patients inconvenient to drink water during or after operations. The oral disintegrating tablets are prepared in a wet-method pelletizing tabletting way. The preparation method has the advantages of simple production technology, stable and easily-controlled preparation quality, and low cost, and is suitable for industrial large-scale production.
Owner:SOUTHWEST UNIV
Injection carbazochrome sodium sulfonate suspension and preparation method thereof
ActiveCN102600074BImprove stabilityImprove solubilityOrganic active ingredientsSolution deliverySide effectPharmaceutical medicine
The invention relates to the technical field of medicine, and discloses a suspensoid of sodium carbosulfonate for injection and a preparation method thereof. Composition, the parts by weight of each component are: 1 part of sodium carbosulfonate; 1.5-8.5 parts of a pharmaceutically acceptable biological carrier; 0.1-1.8 parts of a stabilizer; and 2-5 parts of a freeze-drying protective agent. The present invention improves the stability and solubility of the sodium carbosulfonate, and there is no obvious change in various indicators detected after long-term storage, ensuring that the product quality is qualified within the validity period; it can be administered slowly for a long time, and the bioavailability is greatly improved. The pharmaceutically acceptable biological carrier, that is, the protein, is degraded in the body, has no toxicity and no immunogenicity, and can effectively improve the therapeutic index of the drug, reduce the toxicity of the drug and reduce the side effects of the drug.
Owner:SHANGHAI JINCHENG PHARMACEUTICAL CO LTD
A kind of sodium carbosulfonate semi-solid preparation and preparation method thereof
ActiveCN104473864BLow incidence of adverse reactionsImprove medication safetyOrganic active ingredientsAerosol deliveryCarbazochrome Sodium SulfonateDrug utilisation
The invention relates to the technical field of medicines and in particular relates to a carbazochrome sodium sulfonate semisolid preparation and a preparation method thereof. The preparation method comprises the following steps: with high-purity carbazochrome sodium sulfonate with the purity above 99.80 percent as an active ingredient, adding a medical matrix to prepare one of suppository, ointment and gel; wherein every10g of the suppository contains 20-80mg of carbazochrome sodium sulfonate; the ointment per 10g contains 40-80mg of the carbazochrome sodium sulfonate; every 5g of the gel contains 40-80mg of the carbazochrome sodium sulfonate. According to the carbazochrome sodium sulfonate semisolid preparation and the preparation method disclosed by the invention, the purity of the carbazochrome sodium sulfonate serving as the active ingredient is above 99.80 percent and by-products are controlled to be below 0.2 percent; when the carbazochrome sodium sulfonate semisolid preparation is used, the adverse reaction incidence in the medication process can be lowered and the medication safety can be improved; meanwhile, the prepared carbazochrome sodium sulfonate semisolid preparation can be delivered by virtue of body cavities so that the high-purity carbazochrome sodium sulfonate is capable of improving the medication safety while high bioavailability of a product is ensured.
Owner:贵州健瑞安药业有限公司
Preparation technology of carbazochrome sodium sulfonate for injection
InactiveCN102961330ALight inspection reject rate reducedLow costOrganic active ingredientsDigestive systemWater basedCarbazochrome Sodium Sulfonate
The invention discloses a preparation technology of carbazochrome sodium sulfonate for injection, solving the shortcoming of the conventional preparation technology of carbazochrome sodium sulfonate injection. The preparation technology is low in cost, high in yield, and high in stability of the quality of carbazochrome sodium sulfonate. The preparation technology comprises the following steps of: (1) adding mannitol to about 25% of infusion water based on preparation amount, heating to completely dissolve, then adding 0.2% (W / V) medical carbon based on the preparation amount, boiling for 30 minutes, and decarburizing and filtering; (2) getting 30% of injection water based on the preparation amount, cooling to reach temperature less than 40 DEG C, adding absolute carbazochrome sodium sulfonate, stirring to completely dissolve, and adding the solution to the mannitol filtrate to uniformly mix; and (3) adjusting the pH (Potential of Hydrogen) of the liquid medicine into 5.5 to 6.0 by 10% of sodium hydroxide solution, adding the injection water until reaching the preparation amount, stirring uniformly, rechecking the pH between 5.5 and 6.0, sequentially filtering by microfiltration membranes of 0.45 microns and 0.22 microns, delivering semi-finished products for inspection, and filling after passing the inspection. By adopting the preparation technology, the market competitiveness of the carbazochrome sodium sulfonate is improved, the convenience is provided for market sales of the carbazochrome sodium sulfonate, and the preparation technology is worthy for popularization.
Owner:HENAN FUREN HUAIQINGTANG PHARMA
Carbazochrome sodium sulfonate injection agent and preparation method thereof
ActiveCN113476399AImprove stabilityFew accessoriesOrganic active ingredientsInorganic non-active ingredientsCarbazochrome Sodium SulfonateGlycine
The invention belongs to the technical field of medicine, and particularly relates to a carbazochrome sodium sulfonate injection agent and a preparation method thereof. The carbazochrome sodium sulfonate injection agent is composed of carbazochrome sodium sulfonate, glycine, hydrochloric acid and water, wherein the weight of the glycine accounts for 5%-15% of the weight of the carbazochrome sodium sulfonate, the pH value is 5-6.5, and the concentration of the carbazochrome sodium sulfonate is 5-10 g / L. The carbazochrome sodium sulfonate injection agent is good in stability, few in auxiliary materials, few in adverse reactions, capable of effectively solving the problems of poor solubility and stability of the carbazochrome sodium sulfonate injection agent, low in impurity content generated at high temperature, simple in process and benefit for large-scale production.
Owner:KAMP PHARMA
Stabilized pharmaceutical composition
InactiveUS20100249413A1Good compatibilityAvoid timeOrganic active ingredientsOrganic chemistryCarbazochrome Sodium SulfonateGallic acid ester
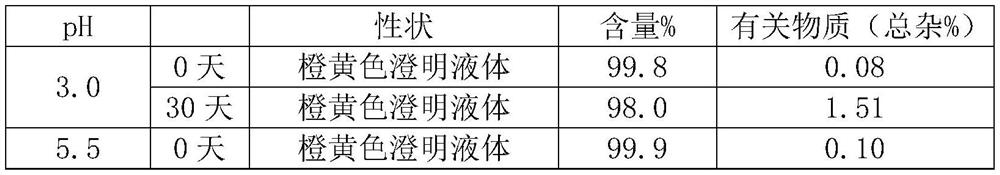
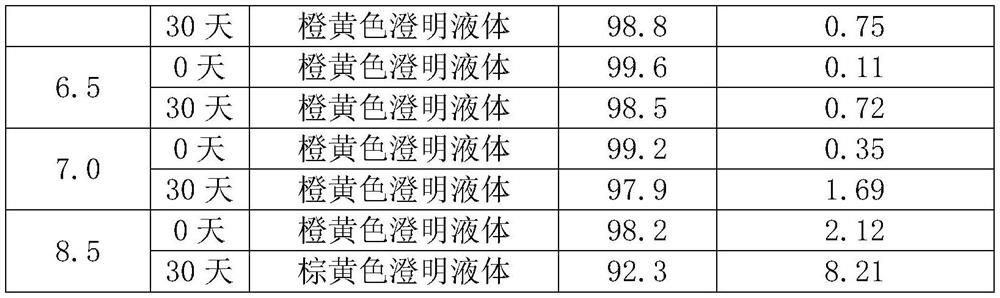
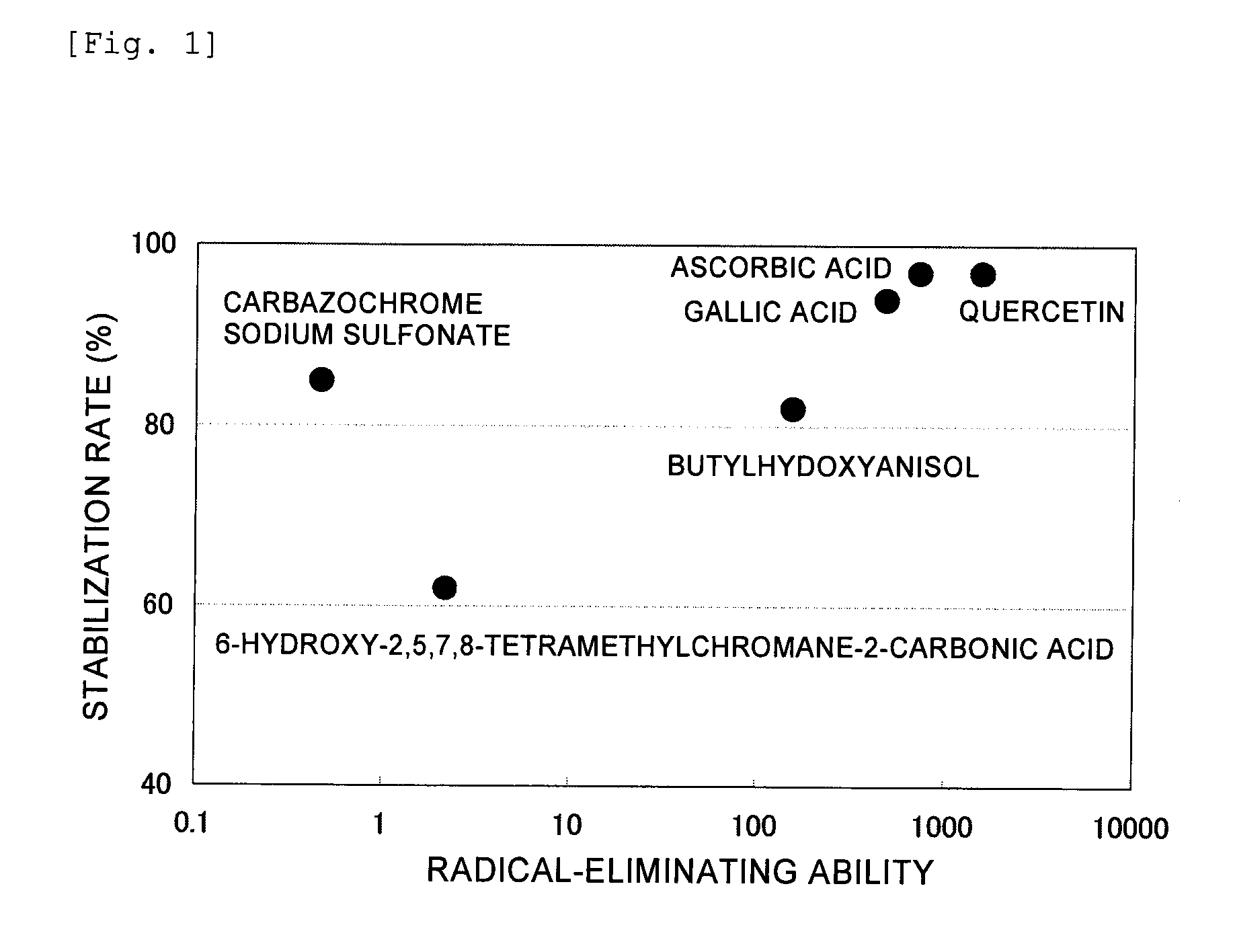
[Problem] A stable liquid-form pharmaceutical composition wherein the decomposition with time is prevented and a stabilization method thereof is provided, for providing a liquid-form pharmaceutical composition of 1-(2-methoxyethyl)-2-methyl-4,9-dioxo-3-(pyrazin-2-ylmethyl)-4,9-dihydro-1H-naphtho[2,3-d]imidazol-3-ium or a pharmaceutically acceptable salt thereof to a clinical field.[Means for Solution] Related are a stable pharmaceutical composition of 1-(2-methoxyethyl)-2-methyl-4,9-dioxo-3-(pyrazin-2-ylmethyl)-4,9-dihydro-1H-naphtho[2,3-d]imidazol-3-ium or a pharmaceutically acceptable salt thereof, containing one kind or two or more kinds selected from the group consisting of carbazochrome sodium sulfonate and derivatives thereof, vitamin C's, butylhydroxylanisol, vitamin E's, Vitamin P's, gallic acid, propyl gallate, alpha-thioglycerine and cysteine hydrochloride, as well as a lyophilized product thereof and a stabilizing method thereof.
Owner:ASTELLAS PHARMA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com