Pharmaceutical composition containing pentoxifylline and carbazochrome sodium sulfonate and application thereof
A technology of pentoxifylline and sodium carboxylate, which is applied in the field of medicine to achieve the effects of significant anti-inflammatory effect, regulating activity and reducing toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The experiment of embodiment 1 rat croton oil hemorrhoid model
[0024] experimental method:
[0025] 50 SD rats (about 200 grams) are randomly divided into five groups according to body weight: model control group, pentoxifylline group (2% pentoxifylline glycerin solution, each administration 0.6ml), sodium carbosulfate group ( 1% sodium carbene sulfonate glycerin solution, each administration 0.6ml), composition group (glycerol solution of the composition, containing pentoxifylline 2%, carbene sulfonate sodium 1%, each administration 0.6ml), Mayinglong group (manufactured by Mayinglong Musk Hemorrhoid Ointment Wuhan Mayinglong Pharmaceutical Group Co., Ltd., Z42021920, batch number 080615, specification: 10g / tube), 10 in each group. Intrarectal administration to rats two days in advance, twice a day at 9:00 am and 21:00 pm, soaked with 10 mg cotton balls and stuffed into the rectum. There are cotton balls (if there are cotton balls, they should be removed). The las...
Embodiment 2
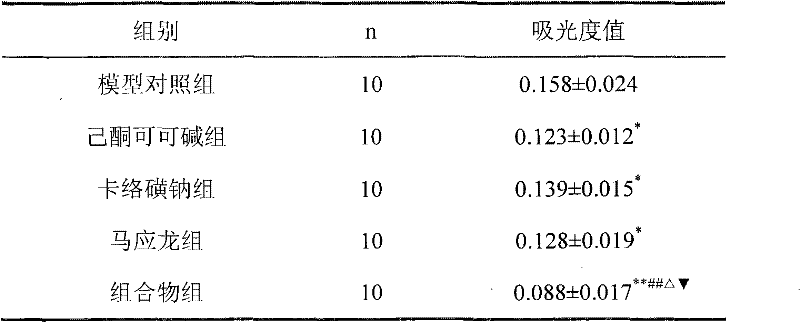
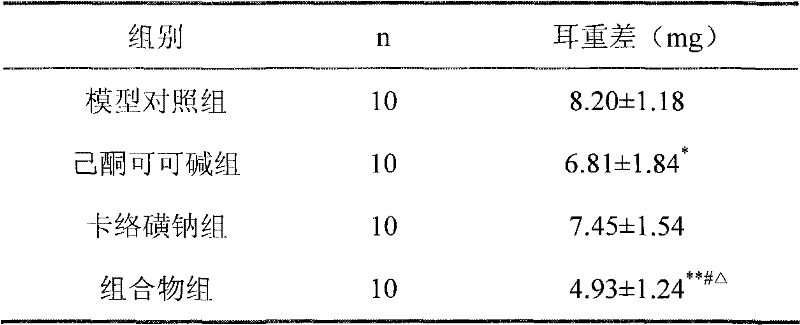
[0037] Example 2 Xylene Anti-inflammatory Experiment in Mice
[0038] Perianal pain caused by perianal inflammation is one of the symptoms of hemorrhoids, and detecting the anti-inflammatory effect of the composition can reflect the therapeutic effect of the composition. In the present invention, the anti-inflammatory effect of the medicine is detected by a mouse xylene anti-inflammatory experiment.
[0039] experimental method:
[0040] 40 KM mice, half male and half female, body weight 25-30 grams, were randomly divided into four groups according to body weight, i.e. model control group, pentoxifylline group (1.2% pentoxifylline glycerin solution), sodium carbosulfate group ( 2% sodium carbosulfate glycerin solution), composition group (glycerin solution of the composition, containing 1.2% pentoxifylline, 2% sodium carbosulfate), 10 rats in each group. Take 20 μl of xylene and apply it to the right ear of each mouse. After 30 minutes, take 0.03ml of each test drug and app...
Embodiment 3
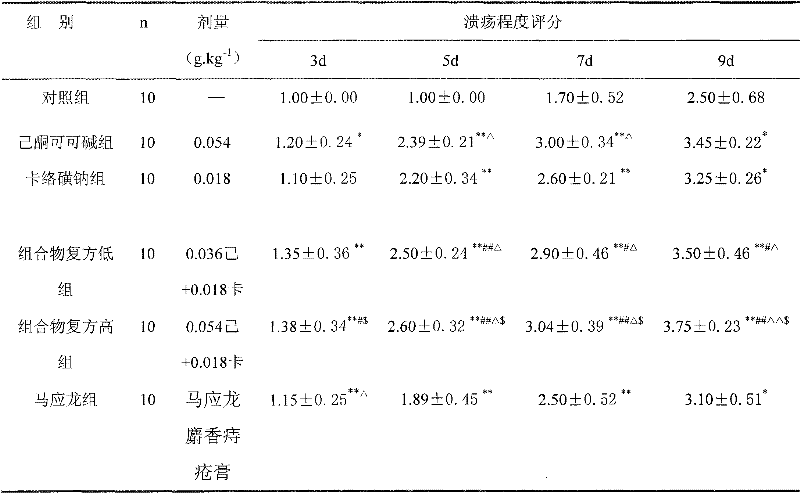
[0047] The effect of embodiment 3 composition on rat perianal ulcer caused by acetic acid
[0048] Take 60 rats, half male and half male, weighing 200-250 g. Take the filter paper and use a puncher with an inner diameter of 6mm to make filter paper pieces of equal size, put them into 99.0% acetic acid solution and soak them fully, put the soaked filter paper pieces around the anus, so that the filter paper pieces are in close contact with the perianal skin and mucous membranes, Use one piece of filter paper each time, change the filter paper after 1 min for each rat, and remove the filter paper after 0.5 min. On the 2nd day, the rats were randomly divided into 6 groups, 10 in each group, and the high and low dose groups of the composition were respectively given high concentration (composition glycerin solution, containing 1.8% pentoxifylline+0.6% sodium carbosulfonate), low concentration (composition glycerin solution, 1.2% pentoxifylline+0.4% carbosulfonate sodium solution)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com