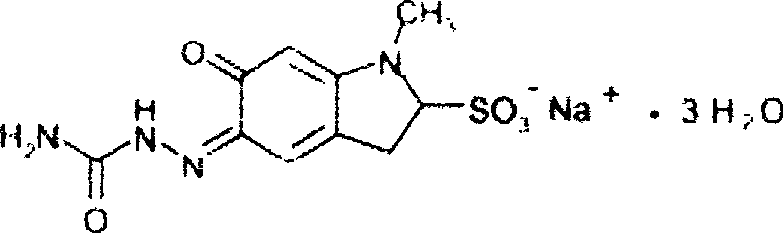
Hemostatic injection of carbazochrome sodium sulfonate and method for preparing the same
The technology of sodium carboxysulfonate and sodium carboxylate is applied in the field of hemostatic sodium carboxylate injection and its preparation, and can solve problems such as adverse consequences, influence on treatment effect, and decrease of sodium carboxylate content.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0009] Weigh raw materials according to the following formula
[0010] Sodium carbosulfate 2g
[0012] Acetamide 0.25g
[0013] 1N hydrochloric acid 0.14mg
[0014] Water for injection 1000ml
[0015] Take potassium chloride, add 600ml of water for injection, add 0.8g of activated carbon and boil for 15min, filter and decarbonize, add 200ml of water for injection, add sodium carbosulfate and acetamide, adjust the pH value to 4.5-6.5 with 1N hydrochloric acid, and replenish 200ml of water , After the solution passes through the carbon layer, it is filtered through a 0.22um microporous membrane and then filled into an infusion bottle, ventilated with nitrogen, crimped and sterilized.
Embodiment 2
[0017] Weigh raw materials according to the following formula
[0018] Sodium carbosulfonate 0.18g
[0019] Sodium chloride 9g
[0020] Sodium citrate 0.125g
[0021] 1N hydrochloric acid 0.12mg
[0022] Water for injection 1000ml
[0023] Weigh sodium chloride, add 700ml of water for injection, add 0.7g of activated carbon and boil for 15 minutes, filter and decarbonize, add 100ml of water for injection, add sodium carbosulfonate and sodium citrate, adjust the pH value to 4.5-6.5 with 1N hydrochloric acid , replenish 200ml of water, pass the solution through the carbon layer, filter it through a 0.22um microporous membrane, fill it, pass nitrogen, crimp the cap, and sterilize.
Embodiment 3
[0025] Weigh raw materials according to the following formula
[0026] Sodium carbosulfonate 0.8g
[0027] Sodium chloride 9g
[0028] Sodium citrate 0.1g
[0029] 1N hydrochloric acid 0.06mg
[0030] Water for injection 1000ml
[0031] The preparation process is the same as in Example 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com