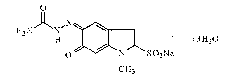
Carbazochrome sodium sulfonate oral disintegrating tablets and preparation method thereof
An orally disintegrating tablet and sodium carboxylate technology, applied in the field of pharmaceutical preparations, can solve the problems such as no oral disintegrating tablet on the market, no research report, etc., and achieve stable and easy-to-control formulation quality, high bioavailability, and ease of use. accepted effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] prescription
[0044]
[0045] The preparation method is to crush all the components in the prescription except sodium carbosulfonate into fine powder with a fineness of more than 100 mesh; dry the starch at 100-105°C to keep the water content below 8%; Sodium sulfonate and dry starch are mixed, pulverized, and passed through an 80-mesh sieve; then mixed with prescription quantities of mannitol, microcrystalline cellulose, hydroxypropyl methylcellulose and menthol and 1 / 2 prescription quantities of crospovidone Mix and pass through a 80-mesh sieve; then add an appropriate amount of ethanol water solution with a volume fraction of 60% to make soft materials, granulate with a 24-mesh sieve, dry at 60±5°C, pass the dry granules through a 24-mesh sieve for granulation; then add the remaining Crospovidone and the prescribed amount of magnesium stearate were mixed evenly and tabletted to make 1000 orally disintegrating tablets of sodium carbosulfonate, each containing 10 ...
Embodiment 2
[0048] prescription
[0049]
[0050] The preparation method is to crush all the components in the prescription except sodium carbosulfonate into fine powder with a fineness of more than 100 mesh; dry the starch at 100-105°C to keep the water content below 8%; Sodium sulfonate and dry starch are mixed, pulverized, and passed through an 80-mesh sieve; then cross-linked with mannitol, microcrystalline cellulose, citric acid, hydroxypropyl methylcellulose and menthol and 1 / 2 of the prescription amount Mix povidone and pass through a 80-mesh sieve; then add an appropriate amount of ethanol aqueous solution with a volume fraction of 60% to make soft materials, granulate with a 24-mesh sieve, dry at 60±5°C, and pass the dry granules through a 24-mesh sieve for granulation; Add the sodium bicarbonate and magnesium stearate of remaining crospovidone and prescription quantity in, mix, tabletting, make 1000 tablets of orally disintegrating sodium carbosulfonate altogether, each cont...
Embodiment 3
[0053] prescription
[0054]
[0055] The preparation method is to crush all the components in the prescription except sodium carbosulfonate into fine powder with a fineness of more than 100 mesh; dry the starch at 100-105°C to keep the water content below 8%; Sodium sulfonate and dry starch are mixed, pulverized, and passed through an 80-mesh sieve; then mixed with prescription quantities of mannitol, microcrystalline cellulose, hydroxypropyl methylcellulose and menthol and 1 / 2 prescription quantities of crospovidone Mix and pass through a 80-mesh sieve; then add an appropriate amount of ethanol water solution with a volume fraction of 60% to make soft materials, granulate with a 24-mesh sieve, dry at 60±5°C, pass the dry granules through a 24-mesh sieve for granulation; then add the remaining Cross-linked povidone, magnesium stearate and aspartame in the prescribed amount were mixed evenly, and tabletted to make 1000 orally disintegrating tablets of sodium carbosulfonate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com