Patents
Literature
122results about How to "Reduce first pass effect" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Medicinal composition for treating coronary heart disease
ActiveCN1714819AIncrease contentQuality improvementPill deliveryCardiovascular disorderAlcoholCoronary artery disease
The present invention relates to coronary heart disease treating medicine composition, and is especially one kind of medicine composition prepared with Chinese herbal medicine material and through special extraction process and other technological process. Specifically, the medicine composition is prepared with notoginseng, red sage and borneol, and through alkali extraction, concentration, alcohol precipitation, and adding supplementary material.
Owner:TIANJIN TASLY PHARMA CO LTD
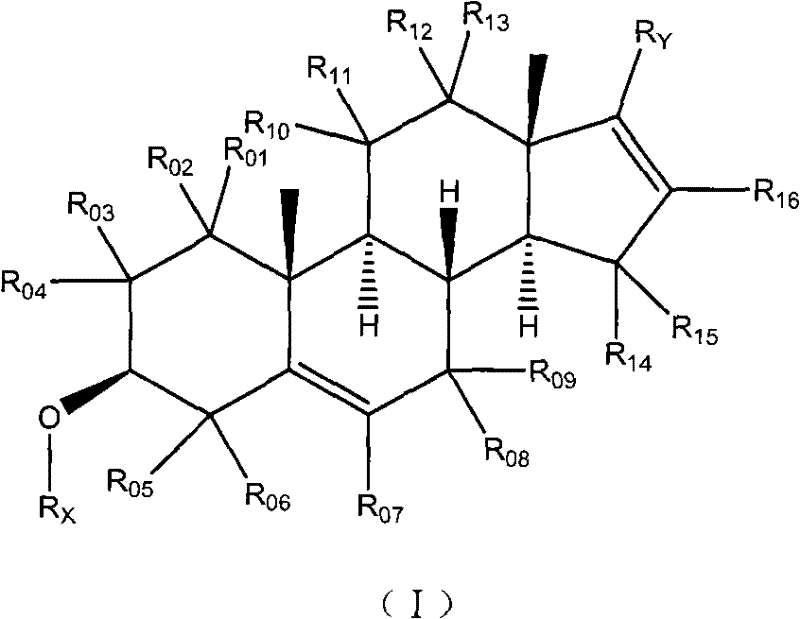
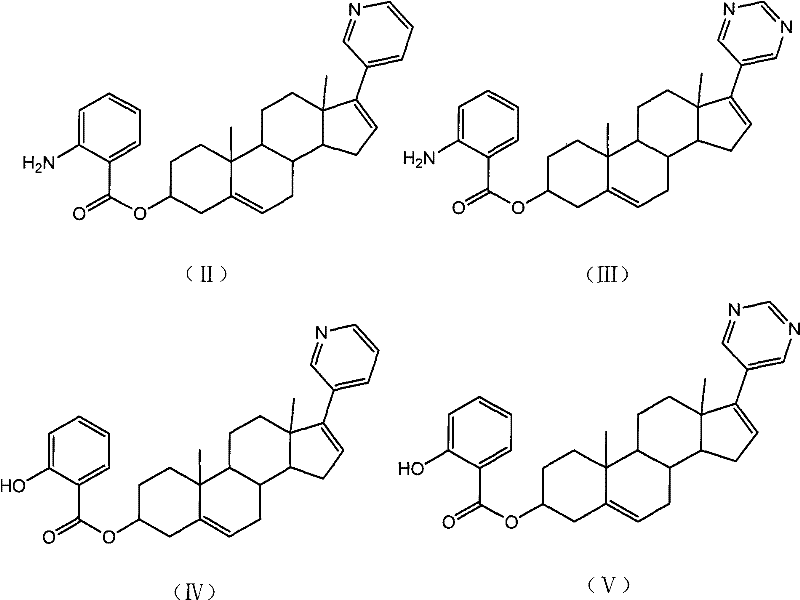
Pyridine androstane derivative and application thereof to preparing medicine for preventing and/or treating prostatic cancer
InactiveCN102477061AReduce first pass effectImprove effective bioavailabilityOrganic active ingredientsSteroidsTolerabilitySide effect
The invention relates to a novel pyridine androstane derivative and application thereof to preparing a medicine for preventing and / or treating prostatic cancer. The pyridine androstane derivative disclosed by the invention is an ideal selective cytochrome oxidase CYP450c17 inhibitor and can be used for treating or preventing various indications relative to male hormonal functions, in particular prostatic cancer. The pyridine androstane derivative has lower oxidative metabolism and higher effective bioavailability, therefore having the advantages of less application dosage, better tolerance and little side effect.
Owner:苏州波锐生物医药科技有限公司
Tripterine nano structure lipid carrier and preparation method and application thereof
InactiveCN102225205AReduce systemic side effectsImprove bioavailabilityOrganic active ingredientsAntipyreticNano structuringTreatment effect
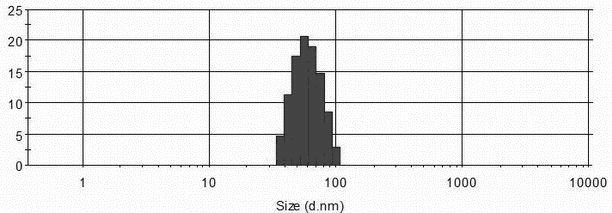
The invention relates to the field of Chinese medicine preparation, in particular to a method for preparing tripterine nano structure lipid carrier containing traditional Chinese medicine monomer and application of the tripterine nano structure lipid carrier in preparation of transdermal drugs used for treating psoriasis, rheumatoid arthritis, skin cancer and breast cancer. The tripterine nano structure lipid carrier is characterized by comprising the following components in parts by weight: 1 part of tripterine, 5-100 parts of mixed lipid, 0.5-10 parts of phospholipid, 0.1-15 parts of poloxamer-188 and 0.5-10 parts of vitamin E and tocopherol polyethylene glycol succinate, wherein the mixed lipid is composed of solid lipid monoglycerine and liquid lipid octylic acid / caprin according to the weight ratio of 1: 0.1-10: 1. Tripterine is prepared into the nano structure lipid carrier, the tripterine nano structure lipid carrier in a semi-solid or liquid preparation form is applied in a transdermal way, bioavailability of the tripterine can be improved, toxic response of tripterine can be reduced, and the nano structure lipid carrier provided by the invention has great clinical application value in the improvement of the treatment effect of tripterine.
Owner:JIANGSU PROVINCE INST OF TRADITIONAL CHINESE MEDICINE
Degradable esophagus tubular intervention support and preparation method thereof
InactiveCN101513367AReduce first pass effectPromote absorptionStentsProsthesisX-rayBiomedical engineering
The invention relates to a degradable esophagus tubular intervention support which is characterized in that the degradable esophagus tubular intervention support is a tubular intervention support with various patterns obtained by hollowing out outer wall of a hollow tube made of mixture of degradable macromolecular material and medicament or X-ray developer, external diameter of the hollow tube is 1-100 millimeters, thickness of the tube wall is 0.05-10 millimeters, and medicament film or X-ray developer is coated on the tube wall of the degradable tubular intervention support.
Owner:中国人民解放军总医院第二附属医院
Esomeprazole magnesium injection liquid
InactiveCN101513387AConvenient for clinical operationImprove bioavailabilityOrganic active ingredientsDigestive systemPatient needReflux
The invention discloses an esomeprazole magnesium injection liquid capable of treating gastroesophageal reflux disease. At present, esomeprazole magnesium in the market is only tablets, and the patient needs to take the esomeprazole magnesium for 1 to 3 times per day; and because the dosage taken by the patient per day is large and the frequency is high, side effects generated by the medicine are large, too. In order to solve the problems, the invention aims to provides the esomeprazole magnesium injection liquid with convenient clinical use, high bioavailability and low price, which can make the medicine quickly reach effective treatment concentration in vivo through direct intravenous injection or intramuscular injection, reduce first-pass effect of the medicament in liver and improve the bioavailability of the medicament in vivo. The esomeprazole magnesium injection liquid provided by the invention mainly comprises the esomeprazole magnesium or pharmaceutically acceptable salts thereof and solvent for injection; and 1 ml of the injection liquid comprises 10 to 200 mg of the esomeprazole magnesium or the pharmaceutically acceptable salts thereof, and the pH of the injection liquid is between 3.0 and 8.0.
Owner:李铁军
Nimodipine capsule containing semi-solid combination and preparation
InactiveCN101254180AHigh dissolution rateDissolution rate is fastOrganic active ingredientsNervous disorderHard CapsuleSemi solid
The invention discloses a nimodipine capsule with high dissolution and semi-solid composition as content and a preparation method thereof, and belongs to the pharmaceutical preparation field. The method comprises the following steps of mixing micronized nimodipine and a hydrophilic carrier, preparing into solid dispersion by melting extrusion method, cooling, pulverizing, mixing with semi-solid matrix, surfactant, melting point regulator under heating, and filling the content into hard capsule with a capsule filling machine. The invention combines the melting extrusion technique and semi-solid filling hard capsule technique, and after the prepared novel nimodipine capsule is administered into human body, the nimodipine quickly diffuses in molecular form and saturates metabolic enzymes, so as to improve bioavailability. The inventive nimodipine capsule solves the low dissolution and low utilization ratio problems of prior nimodipine preparation; and has the advantages of high dissolution, good stability, simple preparation method, and applicability to industrial production.
Owner:武汉星福海药业有限公司
Compound anti-aging quercetin nanoemulsion health care product
InactiveCN102631385ASignificant and long-lasting antioxidantVisible and long-lasting anti-aging effectOrganic active ingredientsAntinoxious agentsAcute hyperglycaemiaDisease
The invention discloses a compound anti-aging quercetin nanoemulsion health care product which comprises the following raw materials in mass percent: 1-15% of quercetin, 15-40% of surfactant, 1-15% of cosurfactant, 5-25% of oil, 1-15% of celery aqueous extract, 1-15% of mushroom aqueous extract, and the balance of distilled water; and the sum of mass percents of the above raw materials is 100%. The nanoemulsion is small in emulsion droplet, uniform in distribution, low in viscosity and good in liquidity. The compound anti-aging health care product takes fat soluble natural product quercetin with an efficient anti-aging effect as a main component, takes the celery aqueous extract and the mushroom aqueous extract as auxiliary components, has effects of keeping skin moisture, adjusting slumber, delaying senescence and adjusting immunologic function and has certain health care effect on diseases such as hypertension, hyperlipemia, hyperglycemia, heart disease and the like.
Owner:NORTHWEST A & F UNIV
Children amoxicillin-potassium clavulanate composition
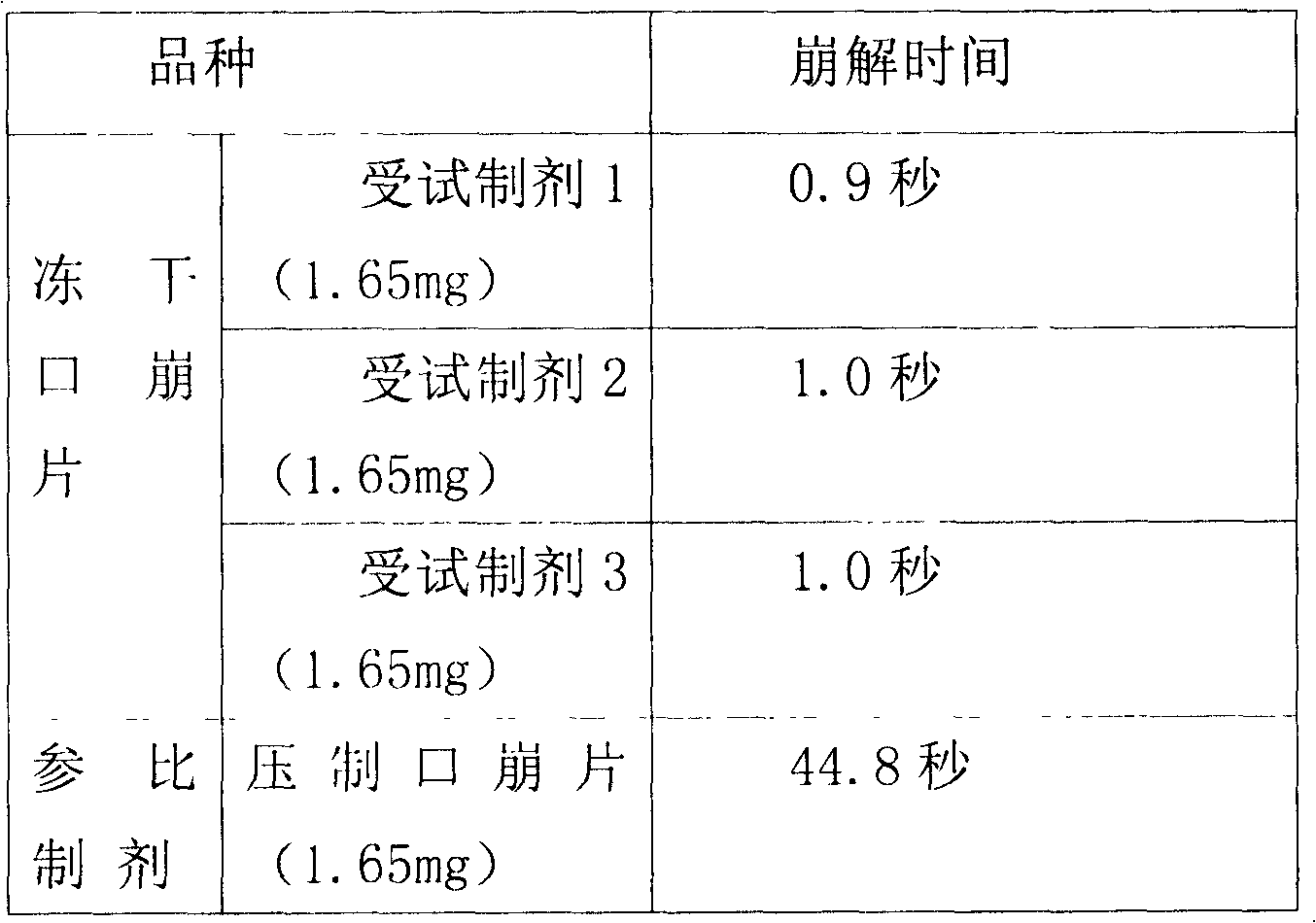
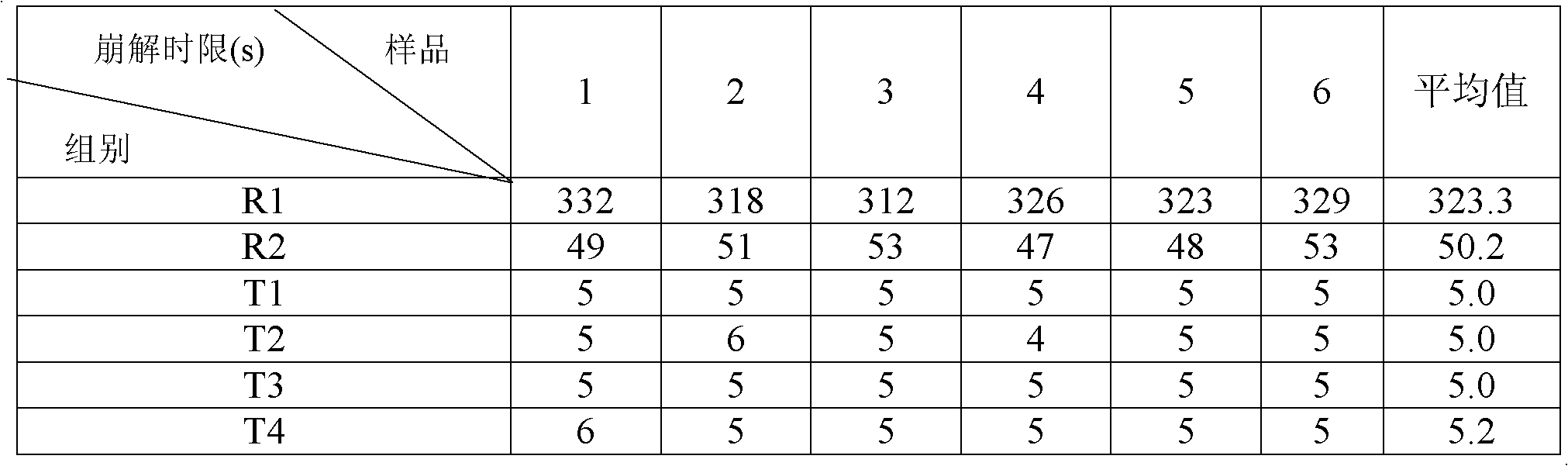
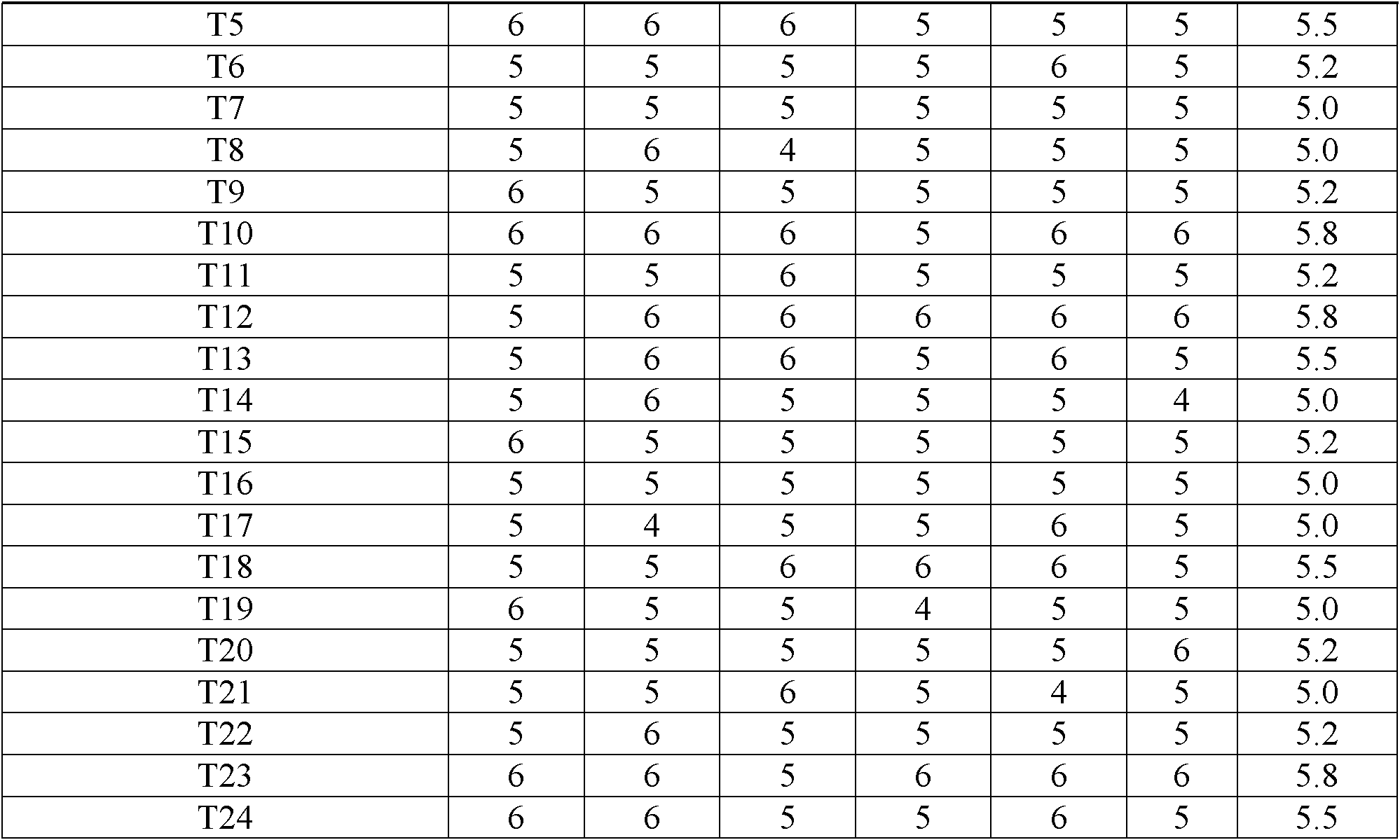
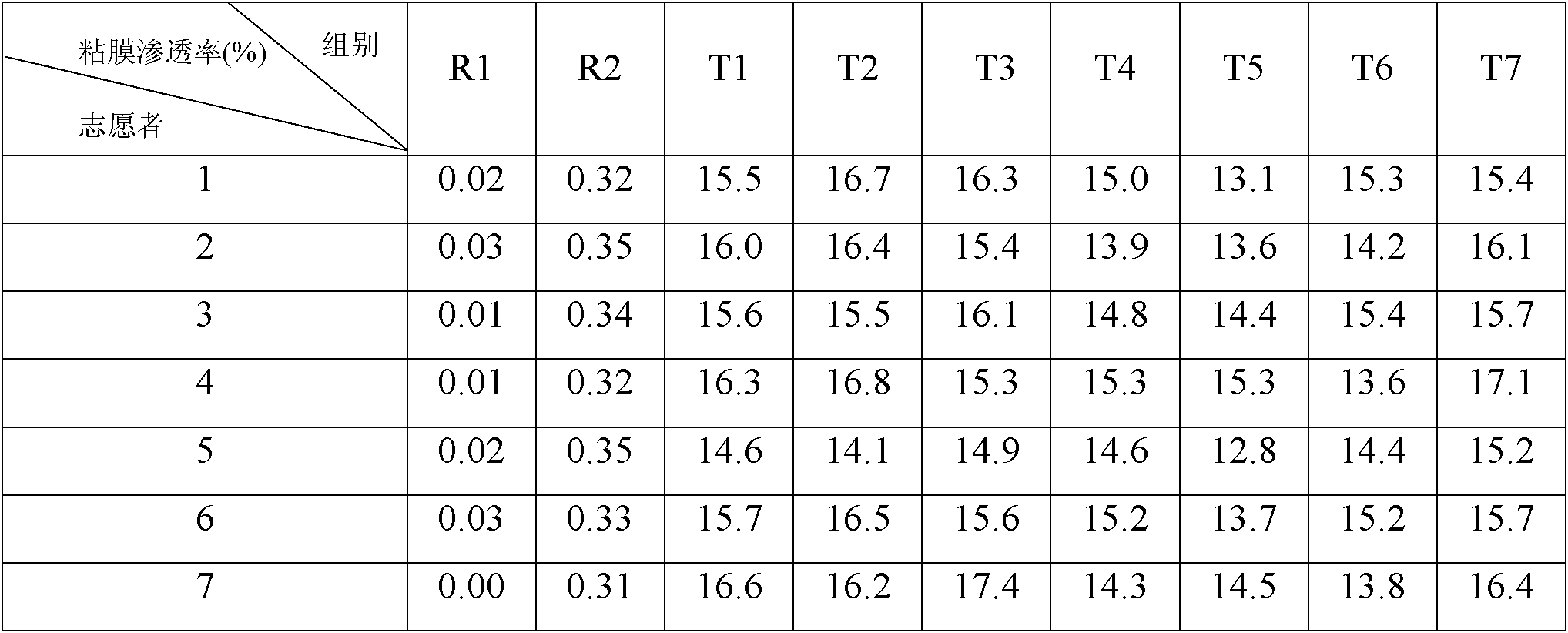
ActiveCN102525978ANon-irritatingNo grittinessAntibacterial agentsPill deliverySide effectOrally disintegrating tablet
The invention discloses a children amoxicillin-potassium clavulanate composition, relating to the technical field of medicinal preparations. The children amoxicillin-potassium clavulanate composition comprises the following components: 24wt% of amoxicillin-potassium clavulanate (7:1), 24-72wt% of mannitol, 2-4wt% of gelatin, 0.2-1.0wt% of xanthan gum and 0.1-0.15wt% of sucralose. The invention further discloses a freeze-dried orally disintegrating tablet of the children paracetamol prepared from the composition, wherein the freeze-dried orally disintegrating tablet comprises simple components, is taken without water and chewing, disintegrates in the oral cavity of a human body within 2 seconds, has the advantages of high effect-taking speed, fewer intestinal tract residue, absorption sufficiency, low side effect and good mouthfeel, and is particularly suitable for being taken by infants and babies. Furthermore, a freeze-drying preparation method is characterized in that a tertiary butanol-water cosolvent is used as a dissolvent, so that the sublimation period of a medicine can be quickened, a freeze-drying period is shortened, the dissolution of amoxicillin and the stability of medicine are enhanced, and the crystallization of the medicine is promoted.
Owner:HAINAN WEI KANG PHARMA QIANSHAN
Alprostadil nano granule formulation and preparation thereof
InactiveCN101322712AReduce chance of regroupingOvercoming chemistryPowder deliveryOrganic active ingredientsDiseasePulmonary vasculature
The invention relates to an alprostadil nanoparticle preparation and a preparation method thereof. The preparation is essentially used for transdermal administration and used for treating diseases such as diabetic ulcer, ischemia of extremity end, burn and the like, and belongs to the technical field of medicine. The preparation consists of the raw materials by the following weight ratio that alprostadil:lipid component: emulsifier: excipient matrix: water is 0.01-0.1:0.5-10:0.5-5:550-950:5. The alprostadil nanoparticle preparation has the advantages of overcoming the chemical and physiological instability of the alprostadil, strengthening in vitro stability, reducing degradation of the alprostadil caused by inactivation effect of in vivo pulmonary circulation, enhancing concentration of the preparation on the local affected parts, being more easy to aggregate at the affected part, sustained release and long effect, and reducing irritation of the preparation.
Owner:SHENYANG WANJIA INST OF BIOLOGICAL TECH RES
Loratadine lyophilized tablets and preparation method thereof
ActiveCN102451169AReduce dosageFast and fully dispersedOrganic active ingredientsPill deliveryOlder peopleMedicine
The invention relates to loratadine lyophilized tablets, and a lyophilization prescription and a technology thereof. The loratadine lyophilized tablets provided by the invention are prepared from a main medicine loratadine and medicinal auxiliary materials. When the tablets are taken, no water is used, and the tablets disintegrate rapidly in mouths. Therefore, the tablets are suitable for patients with swallowing difficulties such as old people and children. Also, the tablets are suitable to be used in trips with water source shortages. The tablets are advantaged in convenient administration, fast absorption, small first-pass effect, and small irritation to gastrointestinal mucous membranes. The market prospect of the tablets is wide. With the lyophilized tablets provided by the invention, side effects of loratadine can be substantially reduced. Also, the invention relates to a preparation method of the loratadine lyophilized tablets.
Owner:BEIJING QUANTUM HI TECH PHARMA TECHCO
Rotundine orally disintegrating tablets and preparation method thereof
InactiveCN102451165AGreat tasteSimple materialOrganic active ingredientsNervous disorderHigh absorptionSide effect
The invention relates to rotundine orally disintegrating tablets and a prescription and process for preparing the rotundine orally disintegrating tablets by adopting a freeze drying method. The rotundine orally disintegrating tablets are prepared from rotundine as a main medicine and pharmaceutic adjuvants, can be taken without water, are capable of rapidly disintegrating after entering the mouth, are suitable for taking by patients suffered from dysphagia, such as the old, children and the like, are simultaneously suitable for medications in the travel journey under the condition of being not easy to obtain a water source, has the advantages of convenience for taking, high absorption speed, small fast pass effect, little irritation to digestive tract mucosa, wide market application prospect and the like, and are capable of remarkably reducing side effects of rotundine. In addition, the invention also relates to a preparation method of the rotundine orally disintegrating tablets.
Owner:QUANTUM HI TECH BEIJING RES INST
Azithromycin composition freeze-dried orally disintegrating tablet and preparation method thereof
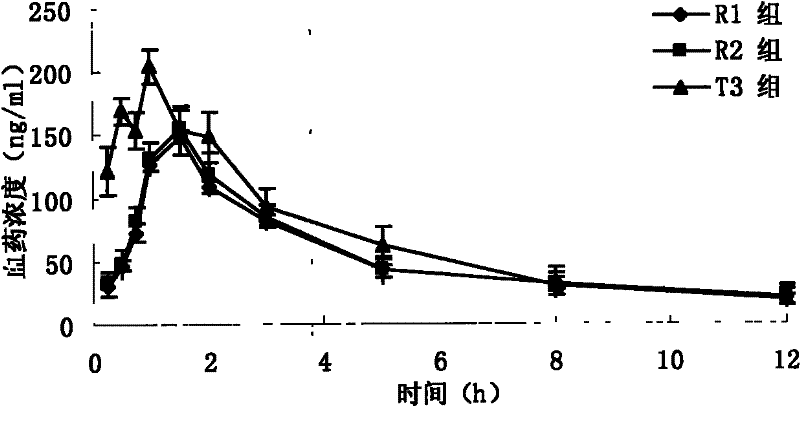
InactiveCN102772383ADisintegrates quicklyPromote dissolutionAntibacterial agentsOrganic active ingredientsAzithromycinSide effect
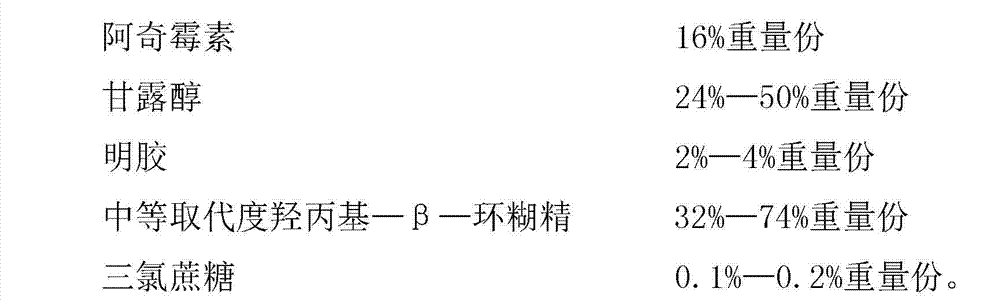
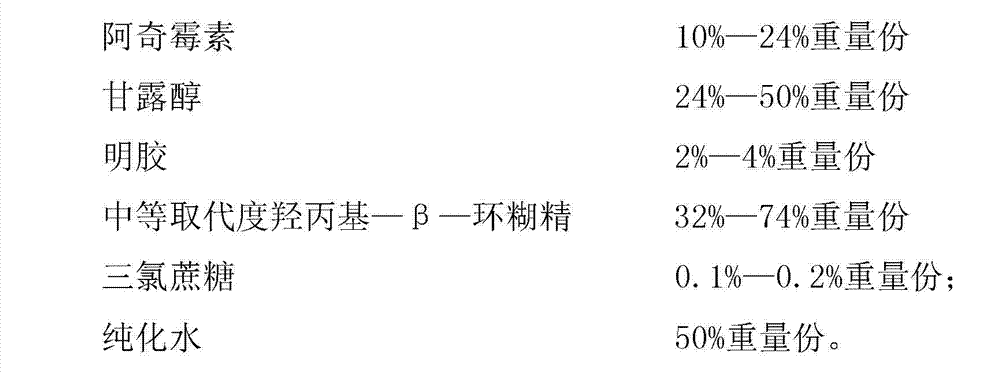
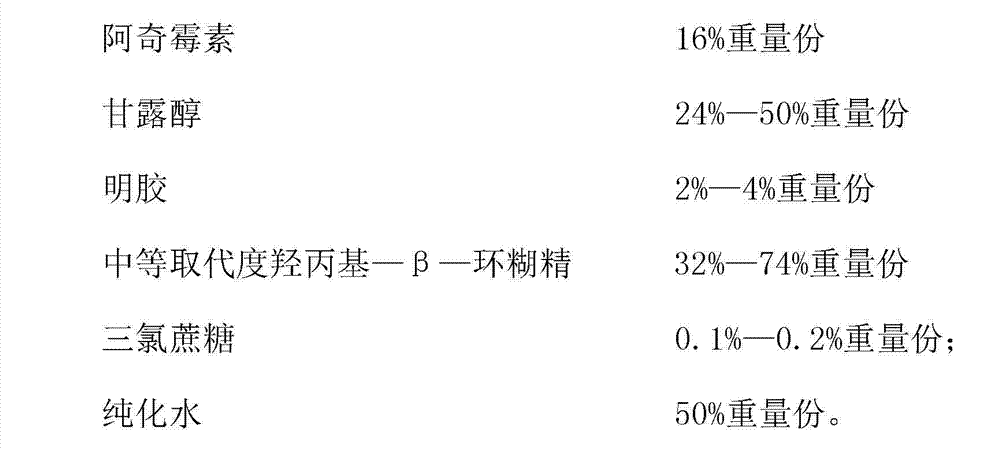
The invention discloses an azithromycin composition freeze-dried orally disintegrating tablet and a preparation method thereof, relates to the technical field of medicines and preparation methods thereof, and mainly solves the problems that the production process and the azithromycin preparation components are complicated, and the disintegrating tablet is low in disintegrating speed in the prior art. The azithromycin composition freeze-dried orally disintegrating tablet comprises the following components in percentage by weight: 10 to 24 percent of azithromycin, 24 to 50 percent of mannitol, 2 to 4 percent of gelatin, 32 to 74 percent of middle-substitution-degree hydroxypropyl-beta-cyclodextrin, 0.1 to 0.2 percent of sucralose and 50 percent of purified water. The preparation process of the azithromycin composition freeze-dried orally disintegrating tablet prepared by the components is simple, and the components are simple; and the azithromycin preparation can reduce the side effect of azithromycin, is convenient to take and tastes good.
Owner:HAINAN WEI KANG PHARMA QIANSHAN
Lapatinib injection and preparation method thereof
InactiveCN101444479AConvenient for clinical operationStable for clinical useOrganic active ingredientsPharmaceutical delivery mechanismIntramuscular injectionSolvent
The invention relates to the field of pharmaceutical technology and discloses a lapatinib injection which has convenient and stable clinical use, high bioavailability, and cheap price and can be used for treating solid tumors. The direct intravenous injection or the intramuscular injection can lead a drug to rapidly achieve the effective therapeutic concentration in vivo, reduce the first pass effect of the drug in liver and improve the bioavailability of the drug in vivo. In order to achieve the purposes, the lapatinib injection mainly contains lapatinib injection liquid or medicinal salt thereof and a solvent for injection; the injection liquid per1ml contains 10-500mg of the lapatinib injection liquid or the medicinal salt thereof, 200-300mg is preferential, and the pH of the injection liquid is 3.0-8.0.
Owner:李铁军
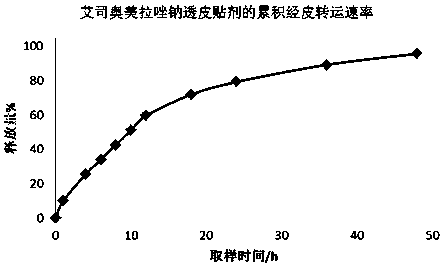
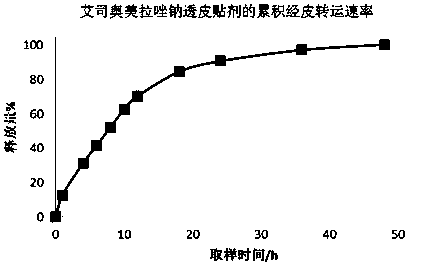
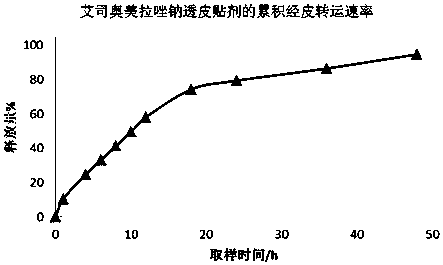
Transdermal patch containing proton pump inhibitor drug and preparation method of transdermal patch
InactiveCN107929268AAvoid oral gastrointestinalReduce the influence of gastrointestinal environmentOrganic active ingredientsDigestive systemTransdermal patchBlood concentration
The invention relates to a transdermal patch containing a proton pump inhibitor drug. The transdermal patch is characterized by mainly comprising a back lining layer, a drug library layer and an anti-sticking layer, wherein the drug library layer contains the following components in percentage by weight: 0.1%-40% of active components, 20%-90% of a drug library matrix, 1%-5% of a stabilizer, 1%-30%of a transdermal absorption enhancer and 0-20% of a dispersing agent. According to the transdermal patch, long-time continuous transdermal penetration of the drug can be effectively realized, a constant blood concentration can be maintained, and a preparation is high in transdermal absorption speed and transdermal absorbing capacity; and the transdermal patch has the characteristics of stability,high efficiency, relatively high pharmaceutical safety and the like.
Owner:ZHENGZHOU TAIFENG PHARMA CO LTD
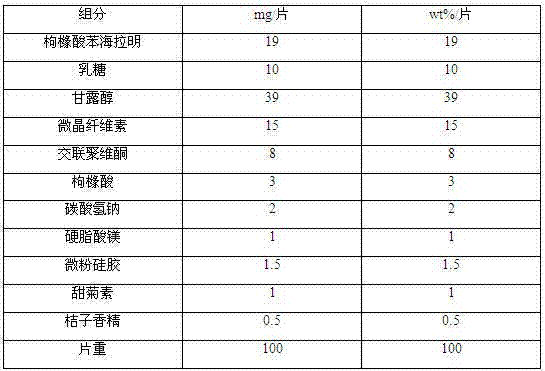
Diphenhydramine citrate orally disintegrating tablet and preparation method thereof
InactiveCN102440973AMeet quality requirementsReasonable prescription designOrganic active ingredientsNervous disorderSodium bicarbonateOrally disintegrating tablet
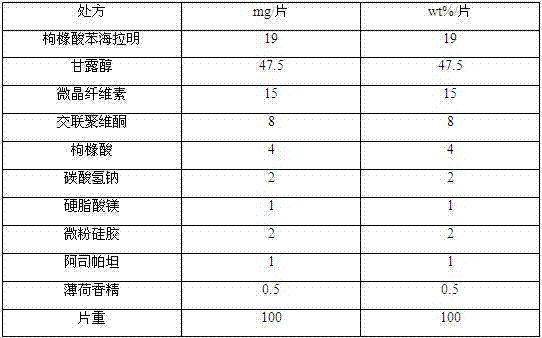
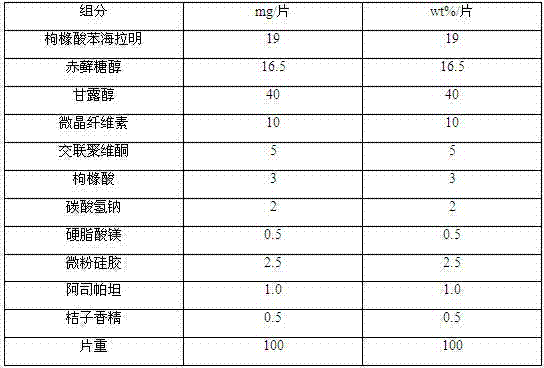
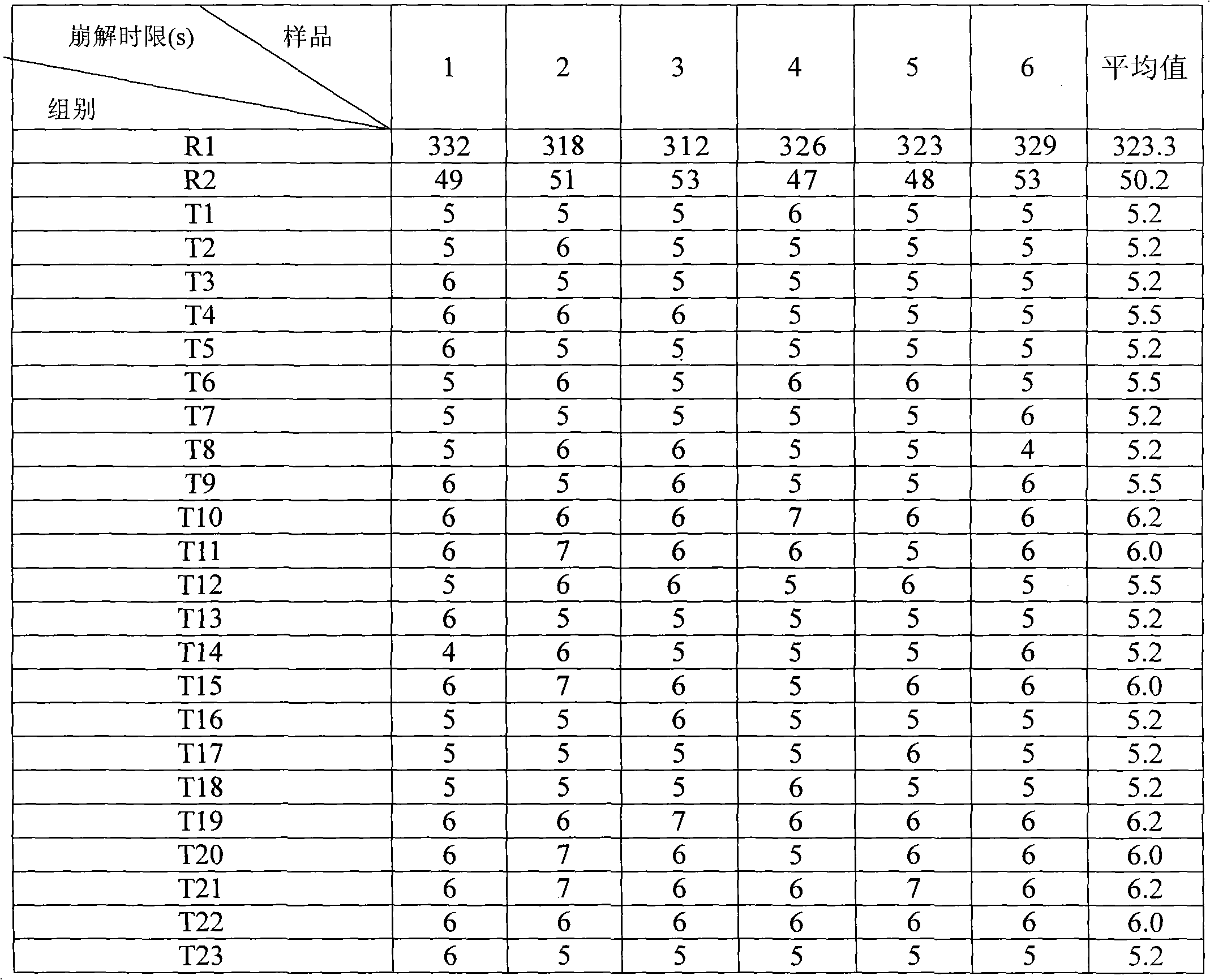
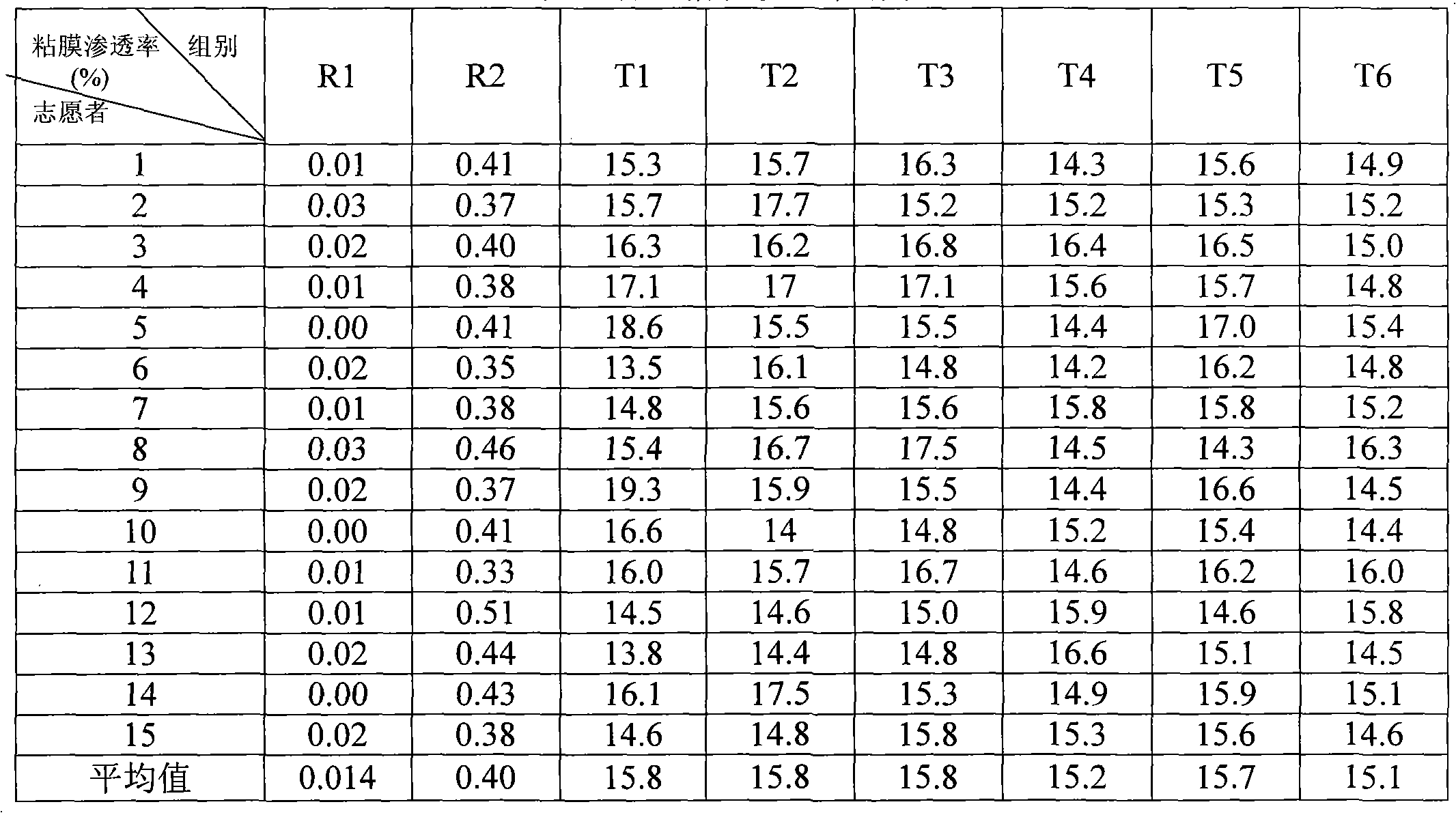
The invention discloses a diphenhydramine citrate orally disintegrating tablet, the prescription is composed by the following components in mass percent: 19% of diphenhydramine citrate, 45 swung dash 58% of filler, 15 swung dash 25% of disintegrating agent, 4 swung dash 8% of effervescent disintegrant, 0.5 swung dash 1% of lubricant, 1 swung dash 3% glidant, 1 swung dash 2% of sweetner, 0.2swung dash 0.6% aromatic and 0 swung dash 0.5% of surfactant, wherein the filler uses mannitol, or mannitol and lactose or erythritol, the disintegrating agent uses any one of polyplasdone, crosslinking sodium carboxy methyl cellulose and low substitution hydroxyl propyl cellulose together with microcrystalline cellulose, the effervescent disintegrant comprises citric acid and sodium bicarbonate which have the mass ratio of 1 swung dash 2 / 1, the lubricant is magnesium stearate, the glidant is aerosil or talcum powder, the sweetner is aspartame or stevia rebaudianum, the aromatic is the pharmaceutically acceptable essence, and the surfactant is lauryl sodium sulfate; and the invention further discloses a preparation method of the orally disintegrating tablet, which is simple to operate, the cost is low, the obtained product is in accordance with the quality requirement of the orally disintegrating tablet, and has attractive appearance, good taste and stable quality.
Owner:SOUTHWEST UNIV
Orally disintegrating tablet of 5-HT receptor agonist and preparation method thereof
ActiveCN102552191AGreat tasteSimple materialOrganic active ingredientsNervous disorderUse medicationSide effect
An orally disintegrating tablet of 5-HT receptor agonist and a preparation method thereof are disclosed. The invention relates to an orally disintegrating tablet of 5-HT receptor agonist as well as a prescription and a process for preparing the orally disintegrating tablet of 5-HT receptor agonist by a freeze-drying method. The orally disintegrating tablet of 5-HT receptor agonist disclosed by the invention is prepared from main medicines and pharmaceutical excipients, without the need of water while administration, capable of being rapidly disintegrated in mouth, and suitable for the administration of patients with difficulty in swallowing, such as the old and children; simultaneously, the orally disintegrating tablet of 5-HT receptor agonist is suitable for administration in a condition that water is not easy to obtain during travelling; and the orally disintegrating tablet of 5-HT receptor agonist has the advantages of being convenient to take, fast in absorption, small in first pass effect, small in irritation on digestive tract mucosa and the like, as well as has wide market application prospect. Moreover, the orally disintegrating tablet of 5-HT receptor agonist disclosed by the invention can obviously reduce the side effects of 5-HT receptor agonist. Additionally, the invention further relates to a preparation method for the orally disintegrating tablet of 5-HT receptor agonist.
Owner:BEIJING QUANTUM HI TECH PHARMA TECHCO
Propofol ester derivative containing amino carboxylic acid amide structure, its preparation method and its purpose
ActiveCN102382005AOvercome the disadvantage of poor water solubilitySmall toxicityOrganic active ingredientsNervous disorderAlkaline earth metalSide effect
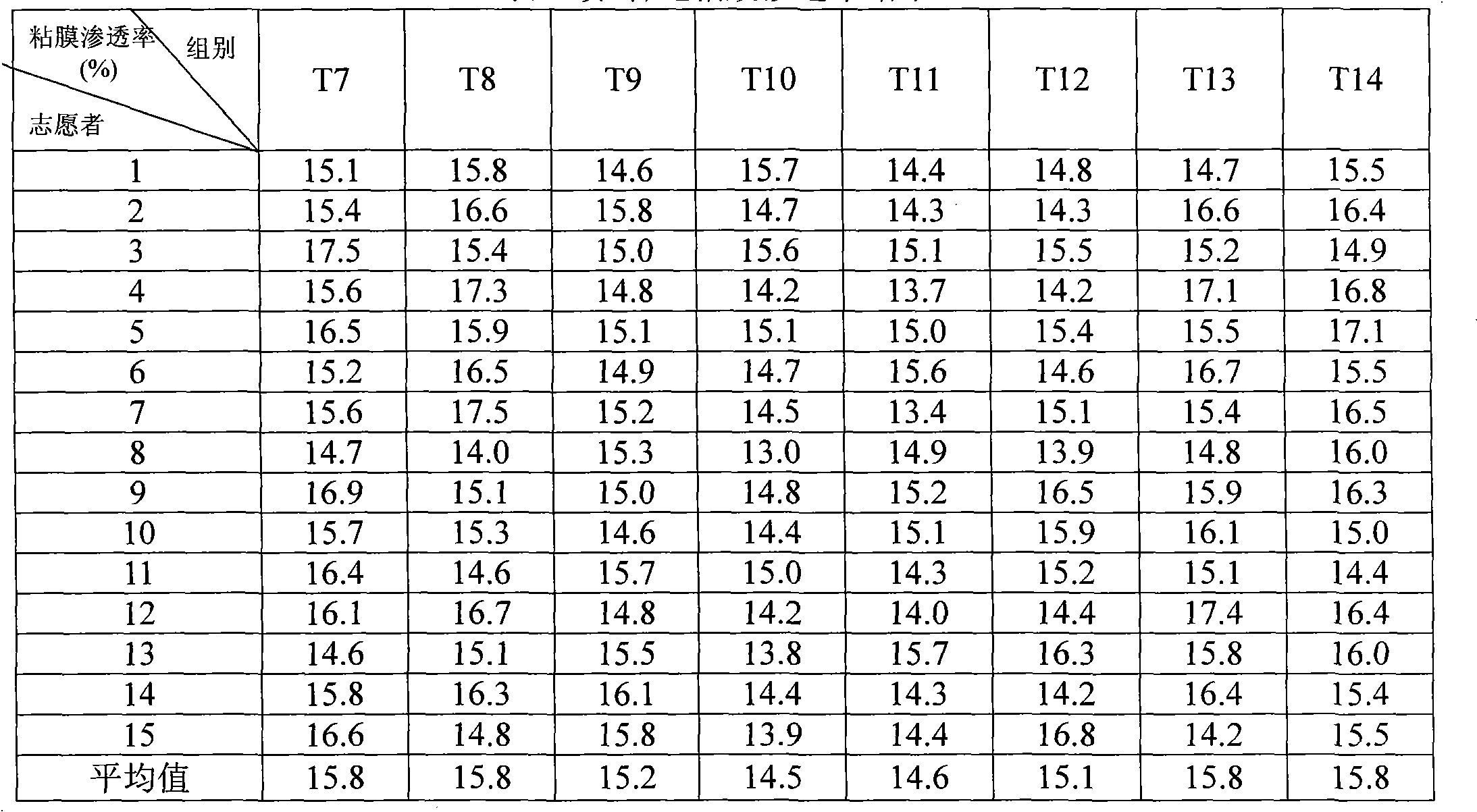
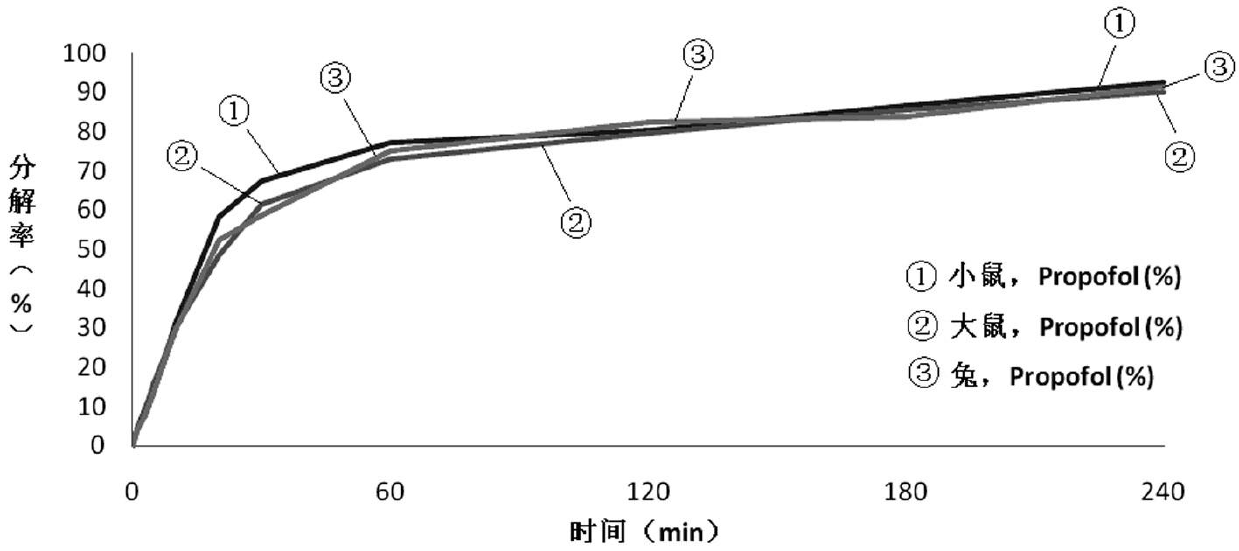
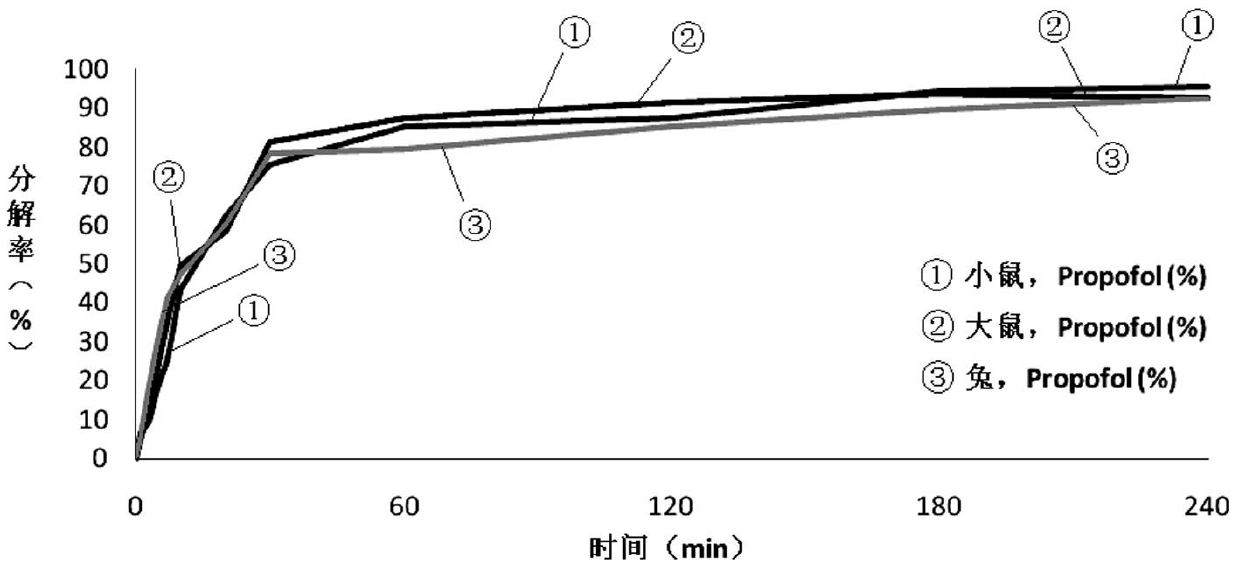
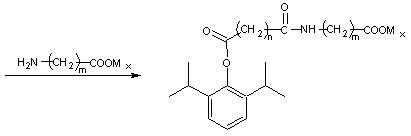
The invention relates to a propofol ester derivative containing an amino carboxylic acid amide structure, its preparation method and its purpose. The derivative has a following formula (I), in the formula, M is H, NH4 or pharmaceutically acceptable alkali metal element or alkali earth metal element, n=1-3, m=1-7, x=1 / 2-1. 2,6-diisopropyl phenol (II) is taken as a raw material, reacted with diacid or the corresponding anhydride to obtain a 2,6-diisopropyl phenol monoester intermediate, and continuously reacted with amino carboxylic acid to obtain the corresponding product (I). The propofol ester derivative can be used for preparing injection type central inhibition medicaments for generating effects of calmness, hypnosis and / or anaesthesia on animals and human, and is capable of improving the water-solubility of propofol, reducing the toxic and side effects of original drug and analogous prodrug, increasing the stability of profrug in vitro and increasing the application scope. The preparation method is simple which has industrial application value.
Owner:SICHUAN UNIV
Veterinary-use intestinal targeted antibacterial pellet and preparation method thereof
ActiveCN102600184AReduce first pass effectImprove bioavailabilityAntibacterial agentsTetracycline active ingredientsFormularyChlortetracycline Hydrochloride
The invention discloses a veterinary-use intestinal targeted antibacterial pellet. The veterinary-use intestinal targeted antibacterial pellet comprises a main drug and auxiliary drugs, wherein the main drug is composed of valnemulin hydrochloride and chlortetracycline hydrochloride; the mass of the valnemulin hydrochloride is 1-5% of that of the pellet; the mass of the chlortetracycline hydrochloride is 5-16 times of that of the valnemulin hydrochloride; the pellet sequentially comprises a valnemulin hydrochloride core, an intestinal targeted coating layer, a chlortetracycline hydrochloride drug layer and an outer coating layer from inside to outside. The invention further discloses a preparation method of the veterinary-use intestinal targeted antibacterial pellet. Through the combination of the method and the formula, the valnemulin hydrochloride and the chlortetracycline hydrochloride are adopted for preparing the pellet; the pellet is released in a gradient form in an animal digestion system; the chlortetracycline hydrochloride is firstly released and absorbed by the stomach and the intestine; the valnemulin hydrochloride is rapidly released and absorbed by the intestinal tract with the pH value larger than or equal to 6.8, so that an excellent intestinal tract sterilization effect is achieved, the drug absorbed by the colon can be prevented from suffering from the first-pass effect of the liver and the intestine, the haemoconcentration and bioavailability of the valnemulin hydrochloride can be improved, and an excellent treatment effect for systemic diseases is obtained.
Owner:山东胜利生物工程有限公司
Anti-dementia medicinal orally disintegrating tablet and preparation method thereof
ActiveCN102525970AGreat tasteSimple materialNervous disorderPharmaceutical product form changeDigestive canalUse medication
The invention discloses an anti-dementia medicinal orally disintegrating tablet and a preparation method thereof, and relates to an anti-dementia medicinal orally disintegrating tablet and a prescription and a process for preparing the anti-dementia medicinal orally disintegrating tablet with a freeze-drying method. The anti-dementia medicinal orally disintegrating tablet disclosed by the invention is prepared from a main medicament and medicinal auxiliary materials, can be taken without water, is quickly disintegrated after being put into mouth, and is suitable for patients suffering from dysphagia such as the aged, children and the like; meanwhile, the anti-dementia medicinal orally disintegrating tablet is suitable for taking under the condition that a water source is difficult to obtain during traveling; the anti-dementia medicinal orally disintegrating tablet has the advantages of convenience for taking, quick absorption, small first pass effect, small irritation on digestive canal mucosa and the like, and has a wide market application prospect; and moreover, the anti-dementia medicinal orally disintegrating tablet can be used for remarkably reducing the side effects of an anti-dementia medicament. Moreover, the invention further relates to a preparation method of the anti-dementia medicinal orally disintegrating tablet.
Owner:BEIJING QUANTUM HI TECH PHARMA TECHCO
Thiazoleamide compound and use thereof for the preparation of anti-malignant tumor medicines
InactiveCN101948467AReduce first pass effectImprove bioavailabilityOrganic active ingredientsOrganic chemistryMyeloid leukemiaSide effect
The invention relates to a thiazoleamide compound and a use thereof for the preparation of anti-malignant tumor medicines. As an ideal Bcr-Abl and Src protein tyrosine kinase inhibitor, the thiazoleamide compound according to the invention shows outstanding effect when being used for chronic myeloid leukemia, acute myeloid leukemia, acute lymphoblastic leukemia, myeloproliferative syndrome, lung cancer, ovarian cancer, prostatic cancer, soft tissue sarcoma, malignant glioma and other malignant tumors, and simultaneously has the advantages of small dose and side effect.
Owner:苏州得普医药科技有限公司
Calcium-ion antagonist orally disintegrating tablet and preparation method thereof
ActiveCN102525972AGreat tasteSimple materialOrganic active ingredientsPill deliveryOld patientsSide effect
The invention discloses a calcium-ion antagonist orally disintegrating tablet and a preparation method thereof, and relates to a calcium-ion antagonist orally disintegrating tablet as well as a formula and technology for preparing the calcium-ion antagonist orally disintegrating tablet by use of a freeze drying method. The calcium-ion antagonist orally disintegrating tablet disclosed by the invention is mainly prepared from major medicines and medicinal accessories; the calcium-ion antagonist orally disintegrating tablet is taken without water and can be quickly disintegrated in mouth, thus being convenient to take; the compliance of the patient taking the medicine for a long time can be improved, the curative effect of the medicine is enhanced, and the orally disintegrating tablet is suitable for old patients, patients with difficulty in swallowing and the like and also applicable when water sources are not easily available during travel; and the orally disintegrating tablet is convenient to take, has the advantages of low first pass effect, low irritation to alimentary canal mucosa, wide market application prospect and the like, and can be used for obviously reducing the side effect of the calcium-ion antagonist. Moreover, the invention also relates to a preparation method of the calcium-ion antagonist orally disintegrating tablet.
Owner:BEIJING QUANTUM HI TECH PHARMA TECHCO
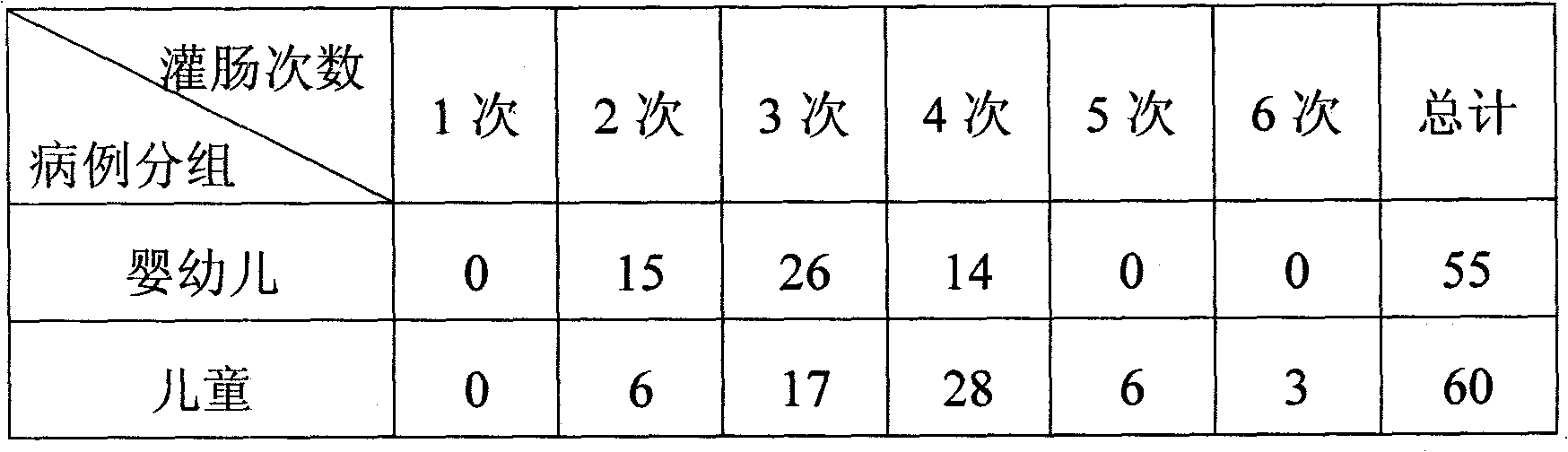
Enema liquid for treating hyperpyrexia and cough and asthma
InactiveCN101766787AEffective treatmentPrevention of cough and asthmaAnthropod material medical ingredientsAntipyreticDiseaseSide effect
The invention discloses an enema liquid for treating hyperpyrexia and cough and asthma, which is prepared by using stiff silkworm, curcuma longa, cicada slough, wine-processed rhubarb, raw ephedra herb, cassia twig, gypsum, almond, earthworm, red paeony root, tangerine peel, mix-fried tatarian aster root and mix-fried liquoric root as the raw material through the following steps: firstly adding water into gypsum to decoct; after decocting, adding the decocted gypsum in other raw material medicaments which are marinate in advance to decoct; and after decocting the raw material medicaments, filtering out a medicinal liquid, i.e. the enema liquid for treating hyperpyrexia and cough and asthma. The product of the invention mainly has the effects of expelling wind and cold pathogens externally, hot and suffocating of upper, middle and lower warmer internally, clearing and descending lung qi, purging fu-organs and relieving flatulence, relieving cough and asthma and relieving dizziness and convulsion, and is suitable to treat high fever in children, cough and dyspnea and tachypnea or the diseases with symptoms of aversion to cold, lassitude, syncope with convulsion, clonic convulsion and the like. The medicament formula of the product of the invention directly guides and is applied clinically according to the principles of Chinese medicine. The clinical curative effect shows that the product of the invention has the advantages of quick response, recurrence prevention, no any toxic or side effects, obvious treating effect and valuable popularization and application.
Owner:武月萍
Chinese medicine composition for treating prostatitis and preparation thereof
InactiveCN1679734ASignificant effectEasy to useUnknown materialsSolution deliverySafflowersCarthamus
A Chinese medicine for treating chronic prostatitis is prepared from 6 Chinese-medicinal materials including motherwort, common knot-grass, safflower, phellodendron bark, etc.
Owner:GANSU HEXI PHARMA
Vitamin B6 composition freeze-drying orally disintegrating tablets and preparation method thereof
InactiveCN102940610ADisintegrates quicklyPromote dissolutionOrganic active ingredientsMetabolism disorderFreeze-dryingOrally disintegrating tablet
The invention provides vitamin B6 composition freeze-drying orally disintegrating tablets and a preparation method thereof, relating to the technical field of medicines and preparation method of medicines, mainly solving the problem that vitamin B6 preparations have bad curative effect, are not suitable for children and patients who have difficulty in swallowing in the prior art. The vitamin B6 composition freeze-drying orally disintegrating tablets comprise 0.5-5wt% of vitamin B6, 30-70wt% of mannitol, 2-4wt% of gelatin, 0.1-0.2wt% of sucralose, and 36-67wt% of purified water. According to the invention, the preparation process is simple, the components are simple, the prepared orally disintegrating tablets are suitable for children and patients who have difficulty in swallowing, and have the advantages of fast disintegration, fast effect, full medicine absorption, convenient administration, good mouthfeel, and first pass effect avoidance to the liver.
Owner:HAINAN WEI KANG PHARMA QIANSHAN
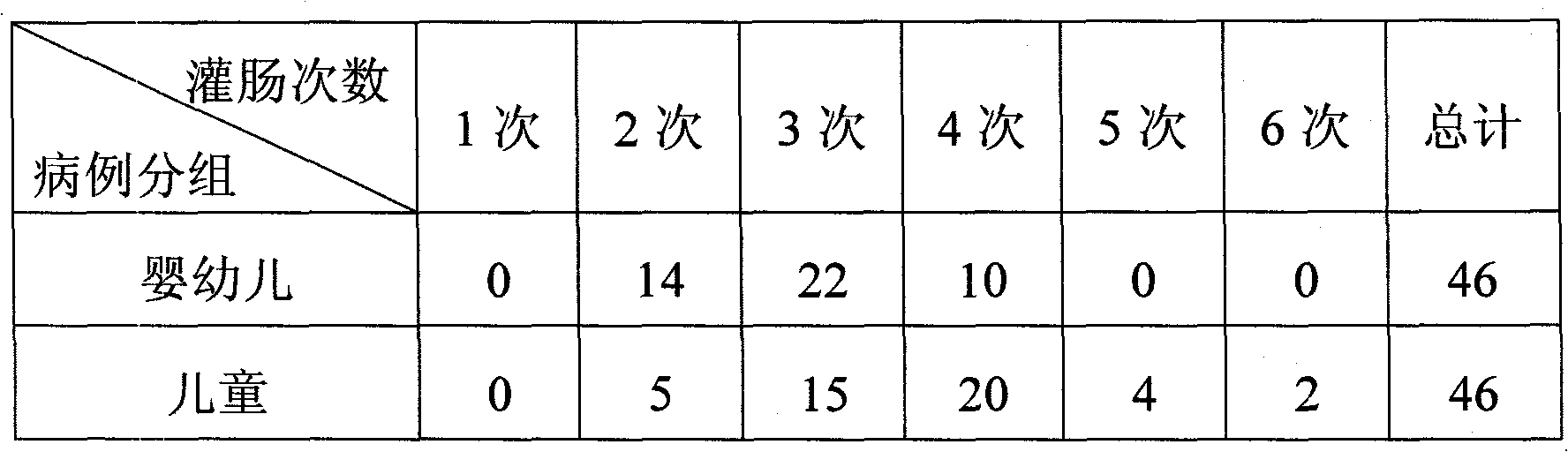
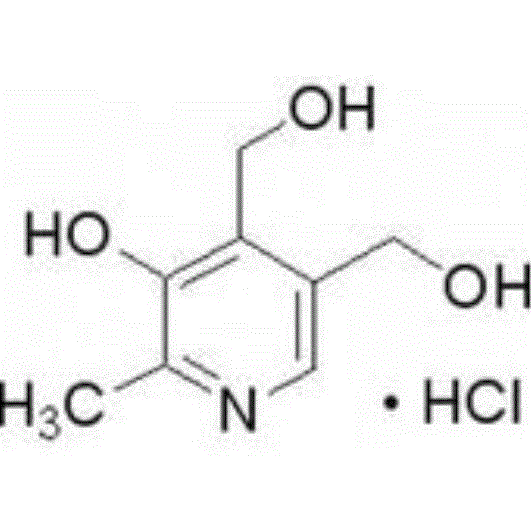
Pediatric compound anisodamine hydrobromide and chlorpheniramine maleate freeze-dried orally disintegrating tablets and manufacturing method thereof
ActiveCN102600153ADisintegrates quicklyPromote dissolutionNervous disorderAntipyreticSide effectFreeze-drying
The invention discloses pediatric compound anisodamine hydrobromide and chlorpheniramine maleate freeze-dried orally disintegrating tablets and a manufacturing method of the pediatric compound anisodamine hydrobromide and chlorpheniramine maleate freeze-dried orally disintegrating tablets, relating to the technical field of pharmaceuticals and pharmaceuticals manufacturing methods. The pediatric compound anisodamine hydrobromide and chlorpheniramine maleate freeze-dried orally disintegrating tablets comprise the following components by weight: 1.47-2% anisodamine hydrobromide, 0.14-0.2% chlorpheniramine maleate, 64-90% mannitol, 6-10% gelatin and 0.2-0.3% sucralose. The manufacturing method adopts the above components to manufacture the pediatric compound anisodamine hydrobromide and chlorpheniramine maleate freeze-dried orally disintegrating tablets. The freeze-dried orally disintegrating tablets have simple components, need no water for administration, do not need to be chewed, havea disintegration time of less than 2 minutes in an oral cavity of a human body, have the advantages of rapid absorption, high bioavailability, low intestinal tract residues, low hepatic first pass effect, less side effects and good mouthfeel, and are especially suitable for infant patients.
Owner:HAINAN WEI KANG PHARMA QIANSHAN
Non-steroidal anti-inflammatory drug orally disintegrating tablet and producing method thereof
ActiveCN102579376AGreat tasteSimple materialOrganic active ingredientsAntipyreticSide effectOrally disintegrating tablet
The invention discloses a non-steroidal anti-inflammatory drug orally disintegrating tablet and a producing method thereof, and relates to the non-steroidal anti-inflammatory drug orally disintegrating tablet and a prescription and a process utilizing the freeze-drying method to produce the non-steroidal anti-inflammatory drug orally disintegrating tablet. The non-steroidal anti-inflammatory drug orally disintegrating tablet is produced by main drug and pharmaceutic adjuvant, no water is needed while users take the tablet, and the tablet disintegrates rapidly after being delivered to the mouth, thereby the tablet is suitable for dysphagia patients such as the old, children and the like; the tablet is suitable for traveling under the condition that water is not easy to get; the non-steroidal anti-inflammatory drug orally disintegrating tablet has the advantages of convenience in taking, rapid absorption, small first pass effect, small irritation to digestive tract mucosa and the like, the tablet has a broad application prospect in the market, and the non-steroidal anti-inflammatory drug orally disintegrating tablet is capable of effectively reducing side effects of non-steroidal anti-inflammatory drugs. In addition, the invention further relates to the non-steroidal anti-inflammatory drug orally disintegrating tablet producing method.
Owner:BEIJING QUANTUM HI TECH PHARMA TECHCO
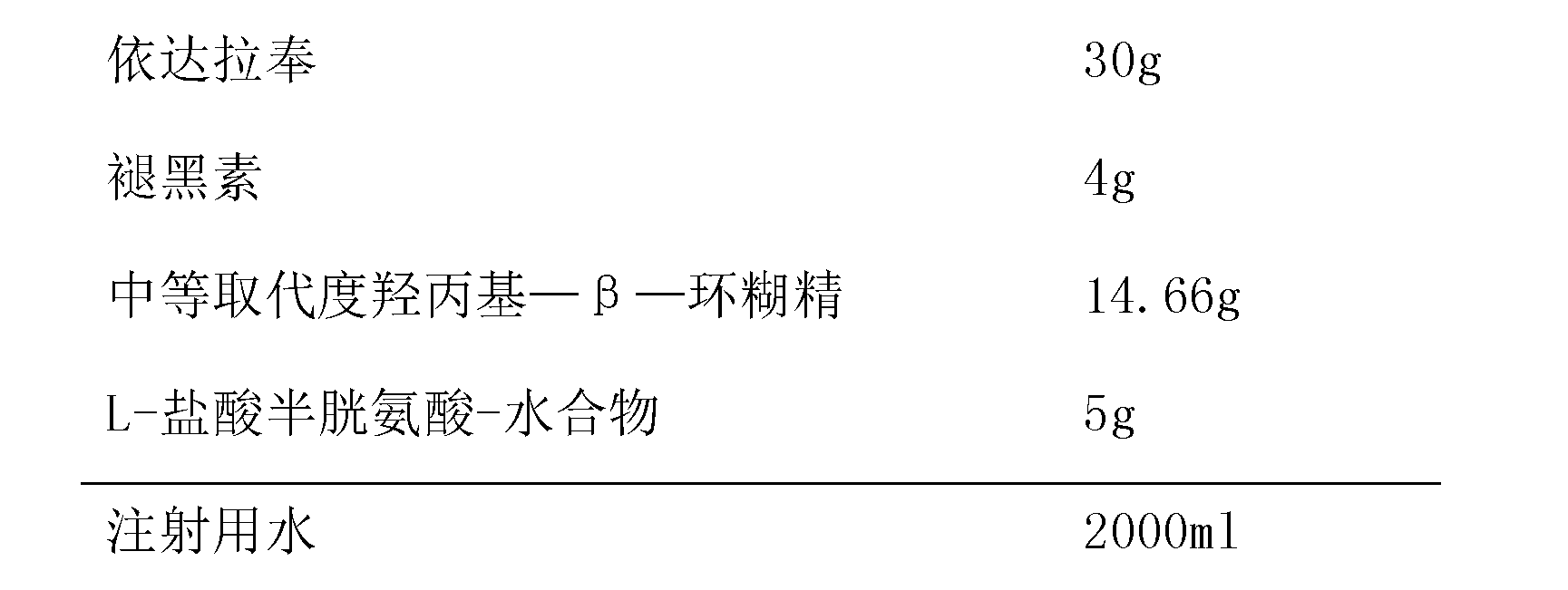
Edaravone composition for injection
InactiveCN103301119AGood treatment effectReduce dosePowder deliveryOrganic active ingredientsSide effectHalf-life
The invention provides an edaravone composition for injection, and relates to the technical field of medicine manufacturing. The main medicine of the composition comprises edaravone and melatonin, wherein the melatonin comprises a quick release part and a cyclodextrin-included slow release part. According to the edaravone composition for injection provided by the invention, the therapeutic effect of edaravone is improved, instability caused by oral administration of MT (Melatonin) is avoided and MT is quick to distribute and eliminate and the like, and the first pass effect of MT is reduced. The dosage of edaravone is reduced, and the side effect of edaravone is reduced. The design of dosage combining quick release and slow release is in accordance with secretion characteristic of MT, so that the problem of half-life period of MT is solved and the bioavailability of a product is improved. The melatonin combined with edaravone acted on nerve cells has the synergistic effect of preventing encephaledema, reducing the infarct volume and improving the neurological function.
Owner:HAINAN WEI KANG PHARMA QIANSHAN
Natural progesterone proliposome preparation and preparation method and using method thereof
InactiveCN102652733AInhibit metabolismPromote absorptionOrganic active ingredientsCapsule deliveryGastrointestinal absorptionProgesterones
The invention discloses a natural progesterone proliposome preparation and a preparation method and a using method thereof. The natural progesterone proliposome preparation contains 1 to 30 percent of progesterone in percentage by weight, and is prepared from progesterone, phosphatide, 1,2-propanediol or / and poly(ethylene glycol)-400 (PEG-400). The natural progesterone proliposome preparation is diluted with water before being taken, or is orally taken into a body, and contacts body fluid, and the phosphatide is hydrated and self-assembled to form liposome. The natural progesterone proliposome preparation avoids metabolizing of progesterone in gastrointestinal tracts and reduces the first pass effect by using a wrapping function of the liposome, and cellular barriers can be changed by using the liposome so as to promote transmembrane transport and inhibit P-glycoprotein to realize slow releasing; and the natural progesterone proliposome preparation has the functions of good cytocompatibility, promotion of lymphatic transport and the like so as to promote gastrointestinal absorption of natural progesterone. Meanwhile, the natural progesterone proliposome preparation can be further modified by chitosan which promotes absorption and has strong adhesivity to further improve the bioavailability of the progesterone. Therefore, the natural progesterone proliposome preparation is a novel preparation which improves the absorption and the bioavailability of the progesterone after oral administration from a variety of mechanism and a plurality of angles.
Owner:新疆维吾尔自治区包虫病临床研究所 +3
Etoposide freeze-dry powder preparation for injection and preparation method thereof
InactiveCN101422439AConvenient for clinical operationImprove bioavailabilityPowder deliveryOrganic active ingredientsVeinIntramuscular injection
The invention relates to the technical field of medicament and discloses an etoposide freeze-dried powder preparation used for injection, which is convenient to be used in clinic, is high in bioavailability, is low in cost and can cure solid tumors. The medicament can quickly achieve effective curing concentration in a body when being directly injected through vein or muscle; moreover, the first-pass effect of the medicament to a liver is reduced, the bioavailability of the medicament in the body is improved, and the freeze-dried powder preparation used for injection is more convenient to be transported and can be stored for a longer time. The etoposide freeze-dried powder preparation used for injection contains the etoposide or the pharmaceutically acceptable salt thereof and pharmaceutically acceptable accessories used as active components; wherein, the weight percentage of the etoposide or the pharmaceutically acceptable salt thereof can be 1 to 80 percent, preferably be 30 to 70 percent and more preferably be 40 to 60 percent; the content range thereof in the preparation is generally 1 to 1000mg and preferably be 10 to 500mg.
Owner:李铁军
Pyrrolo[2,3-d]pyrimidine derivative and application in preparation of anti-malignant tumor medicament thereof
InactiveCN102643278AHas inhibitory activityReduce first pass effectOrganic active ingredientsOrganic chemistryPancreas CancersSide effect
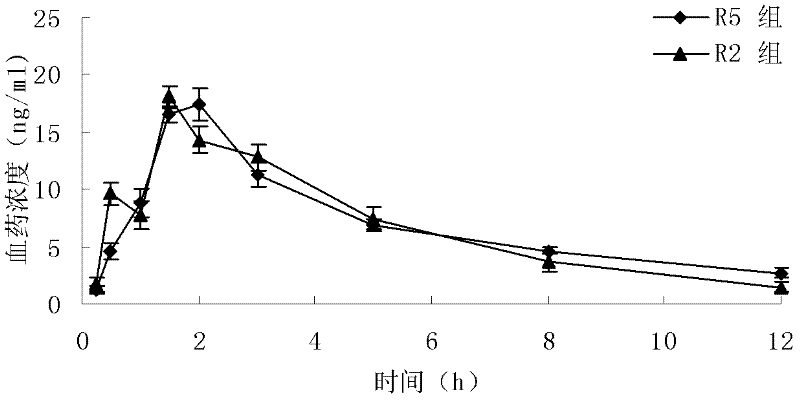
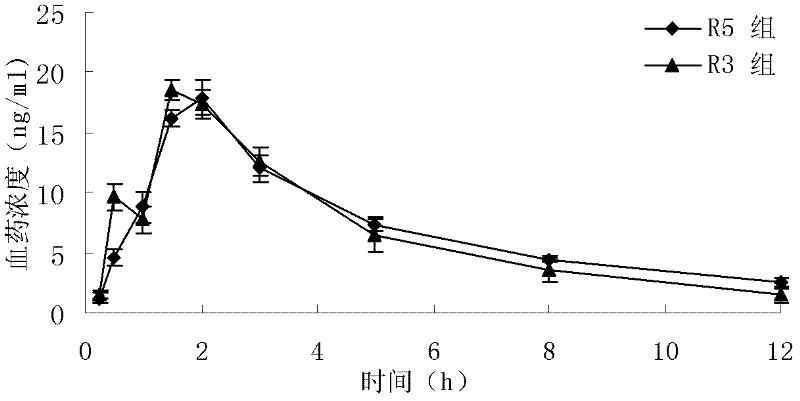
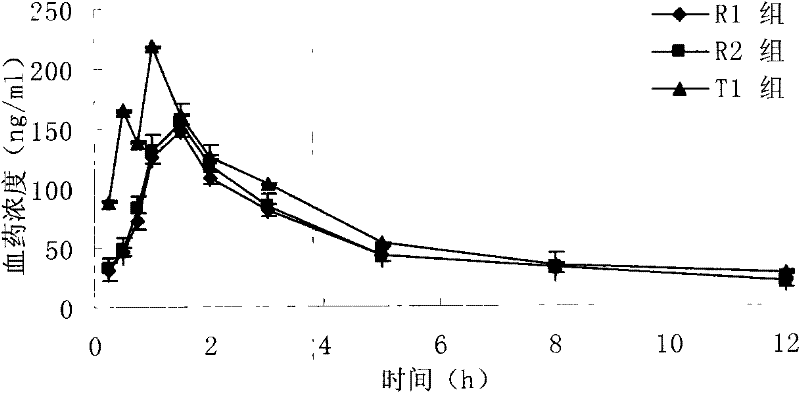
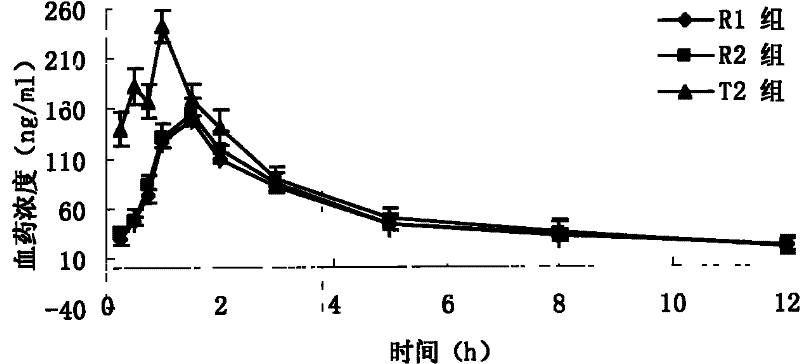
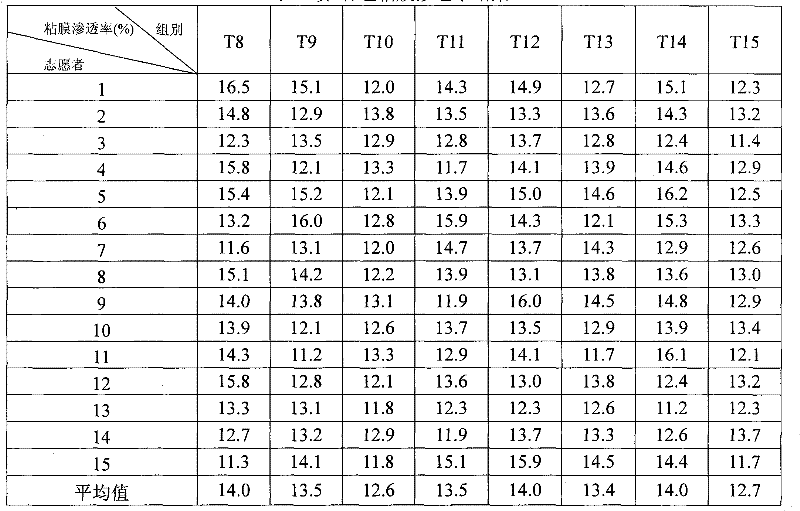
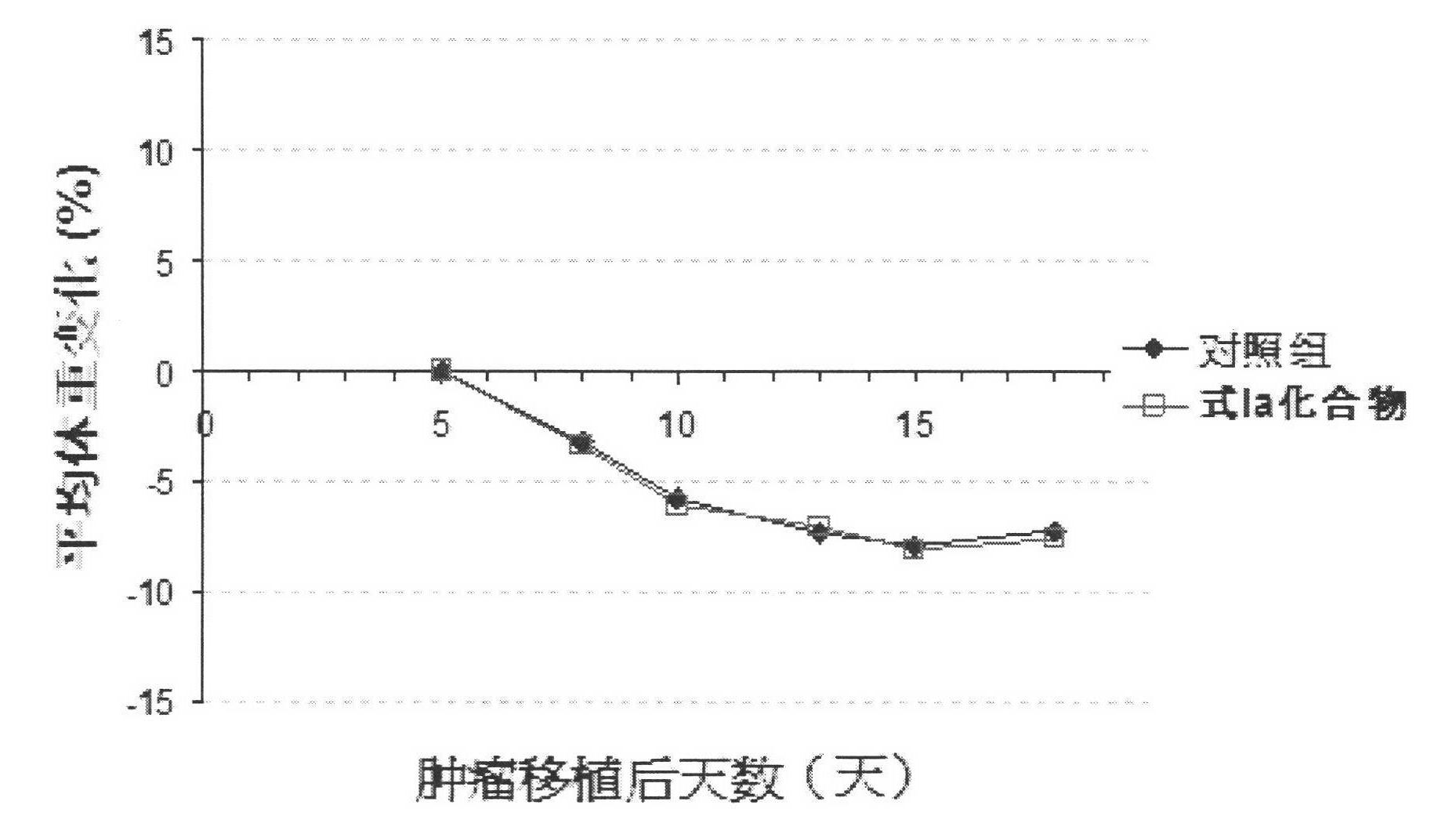
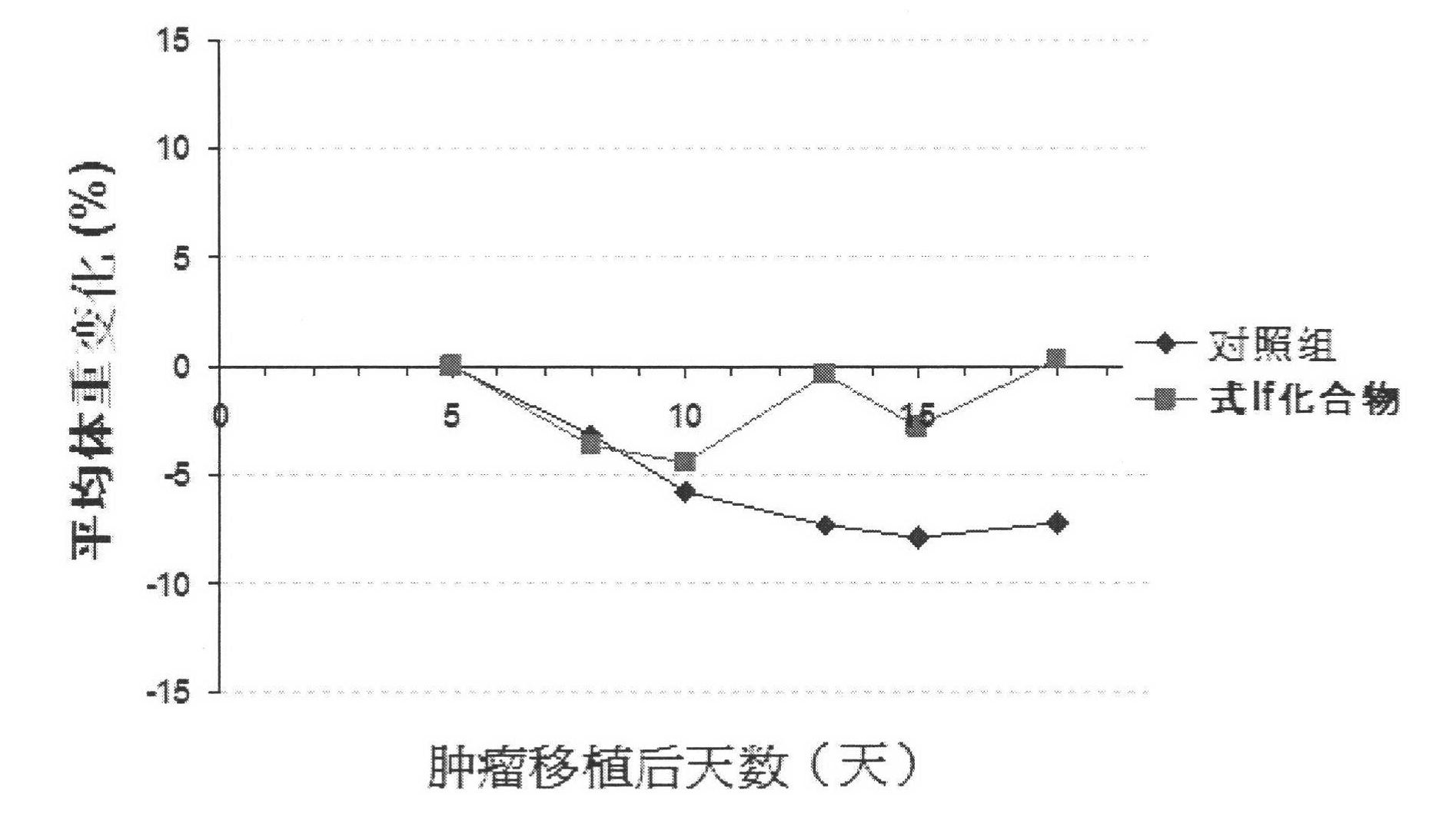
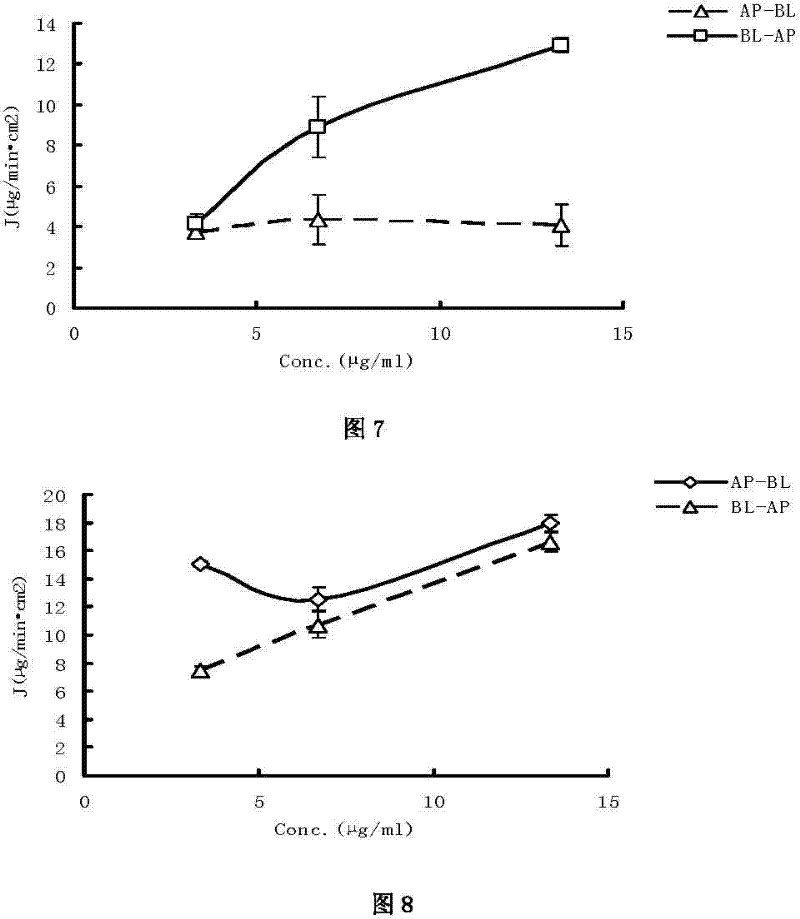
The invention relates to a novel pyrrolo[2,3-d]pyrimidine derivative and an application in preparation of anti-malignant tumor medicaments thereof. The pyrrolo-pyrimidine compound of the invention is an ideal folic acid-dependent enzyme inhibitor, and has the advantages of less first-pass effect, high effective bioavailability, few dosage, less side effect, and the like when used for the prevention or treatment of various tumors such as leukemia, breast cancers, lung cancers, pancreas cancers, bladder cancers, stomach cancers, mesothelial cancers, colorectal cancers, and the like.
Owner:苏州波锐生物医药科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
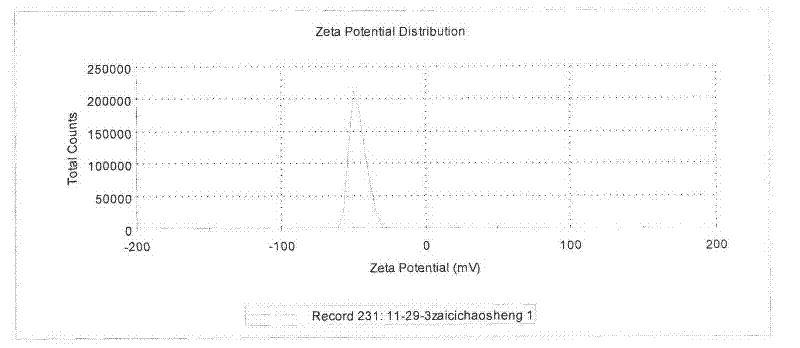
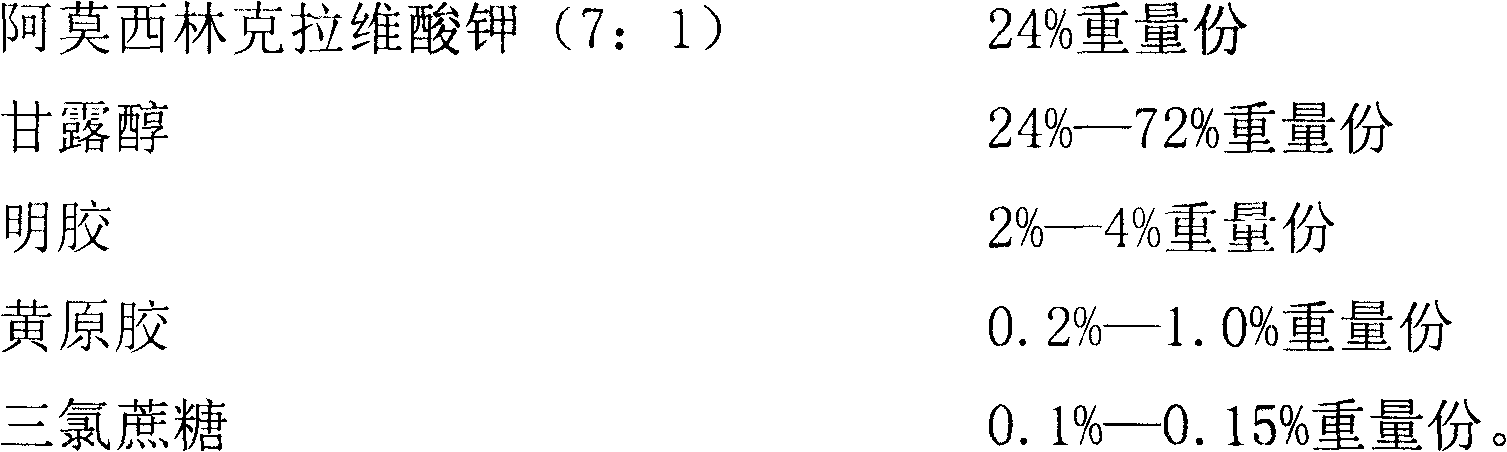
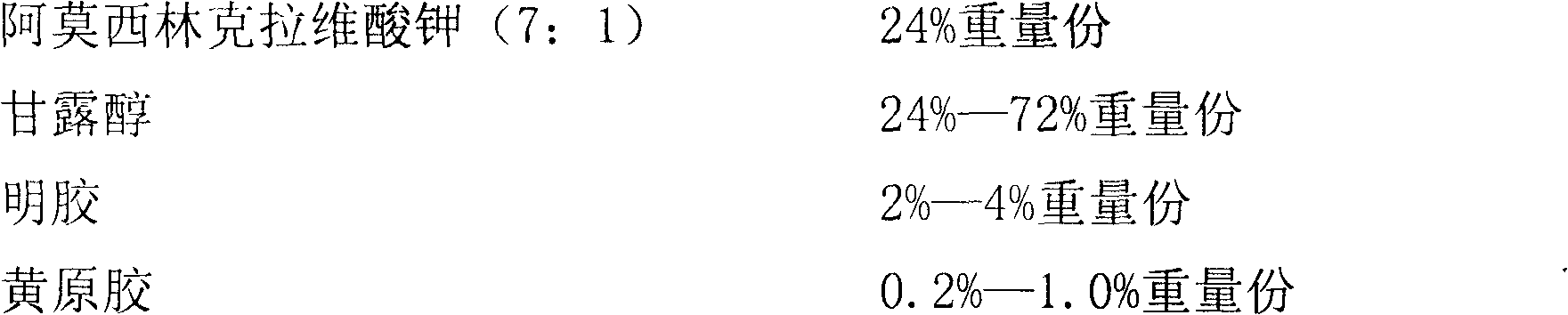


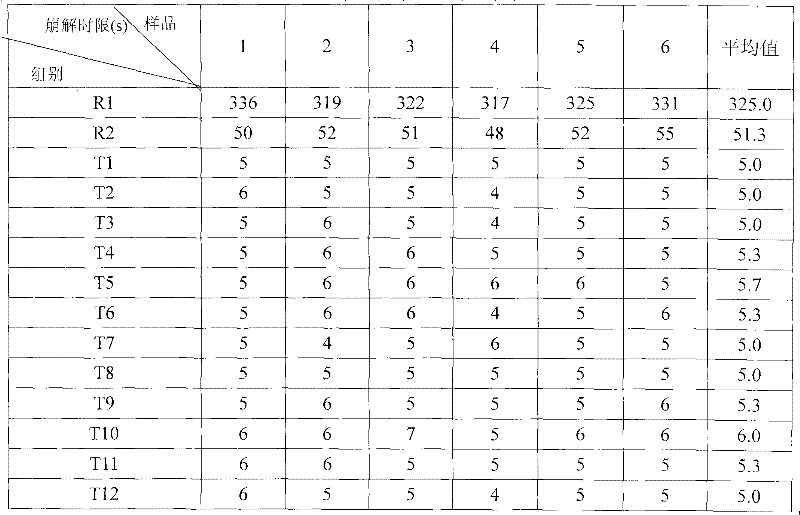


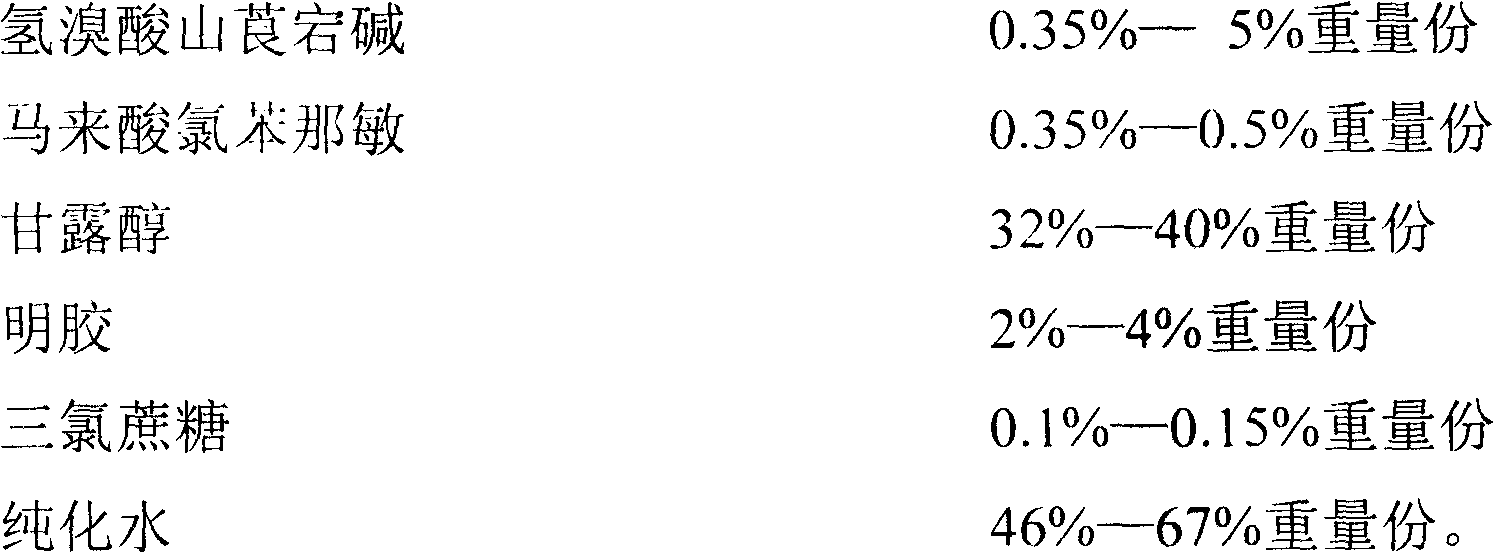
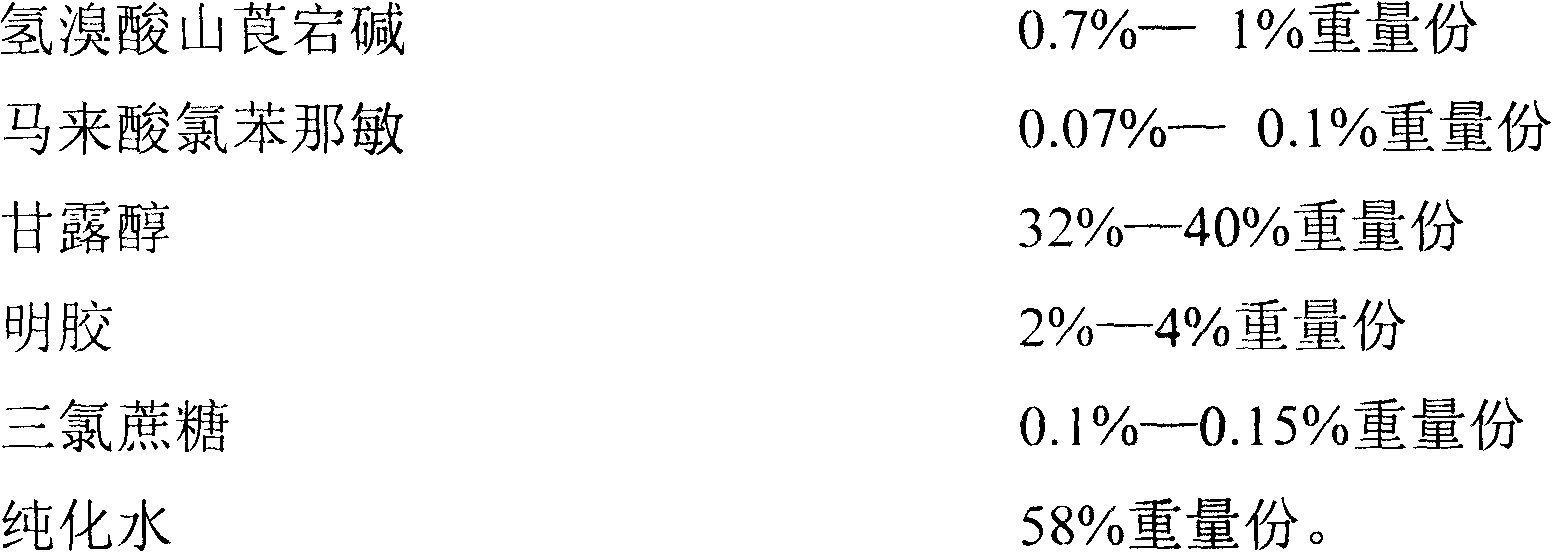

![Pyrrolo[2,3-d]pyrimidine derivative and application in preparation of anti-malignant tumor medicament thereof Pyrrolo[2,3-d]pyrimidine derivative and application in preparation of anti-malignant tumor medicament thereof](https://images-eureka.patsnap.com/patent_img/7098208b-e9d2-4062-a43a-2c3ce820f9e8/BSA00000435860900011.PNG)
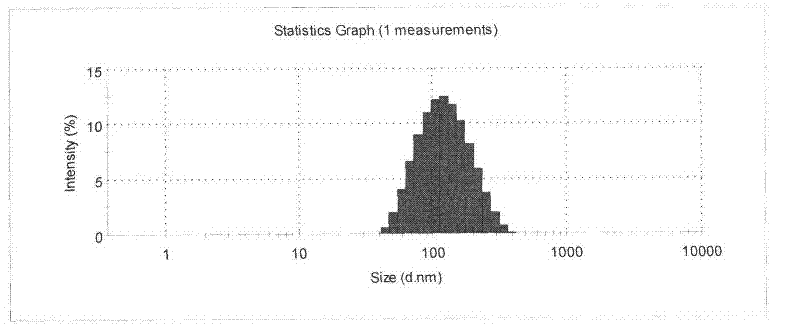
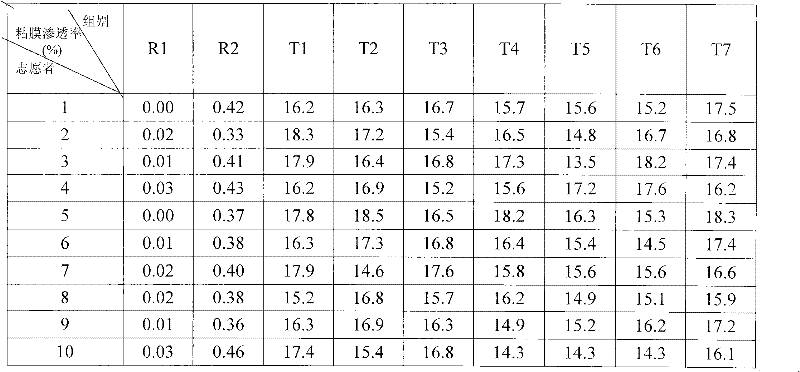
![Pyrrolo[2,3-d]pyrimidine derivative and application in preparation of anti-malignant tumor medicament thereof Pyrrolo[2,3-d]pyrimidine derivative and application in preparation of anti-malignant tumor medicament thereof](https://images-eureka.patsnap.com/patent_img/7098208b-e9d2-4062-a43a-2c3ce820f9e8/BSA00000435860900021.PNG)
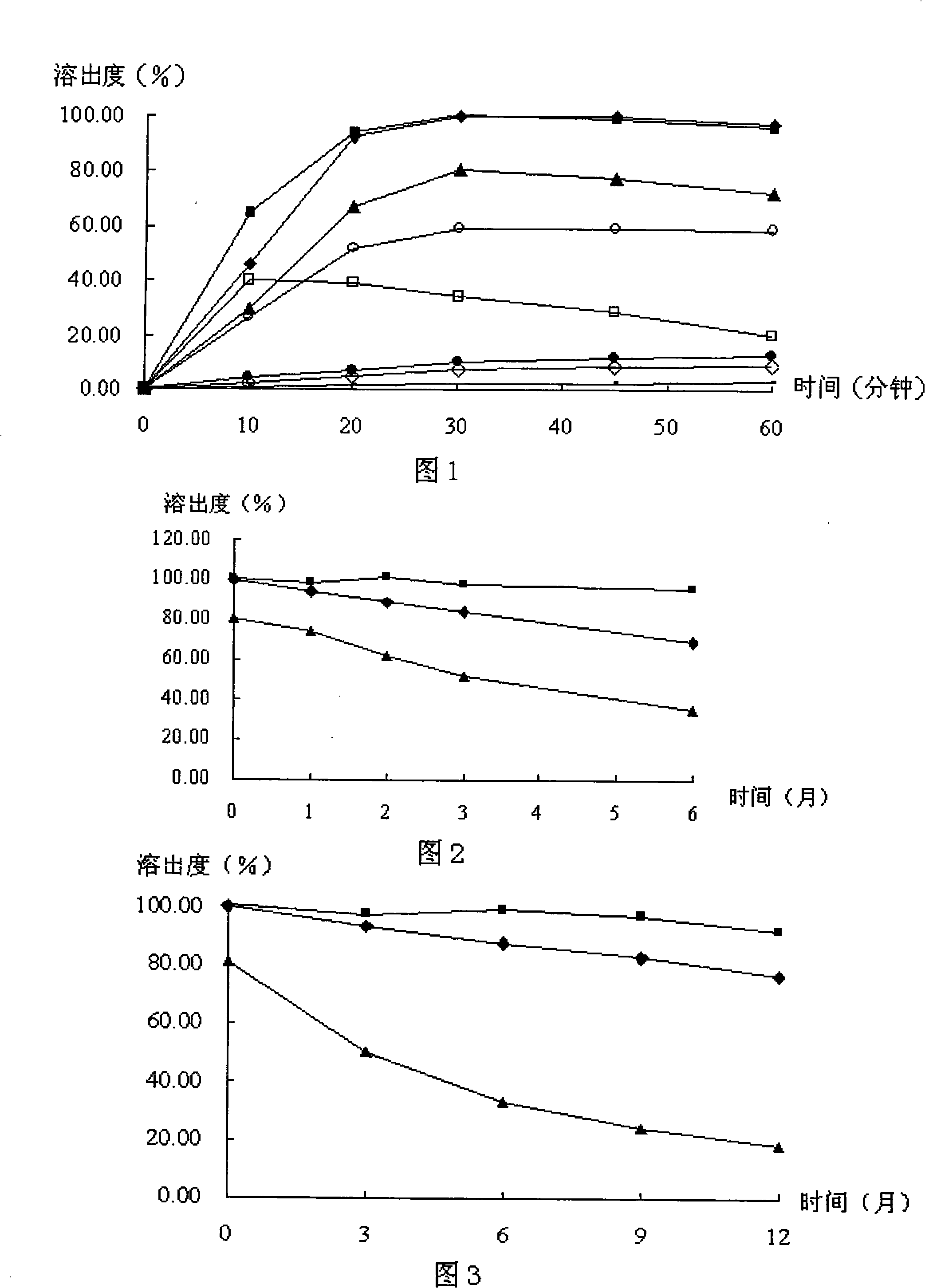
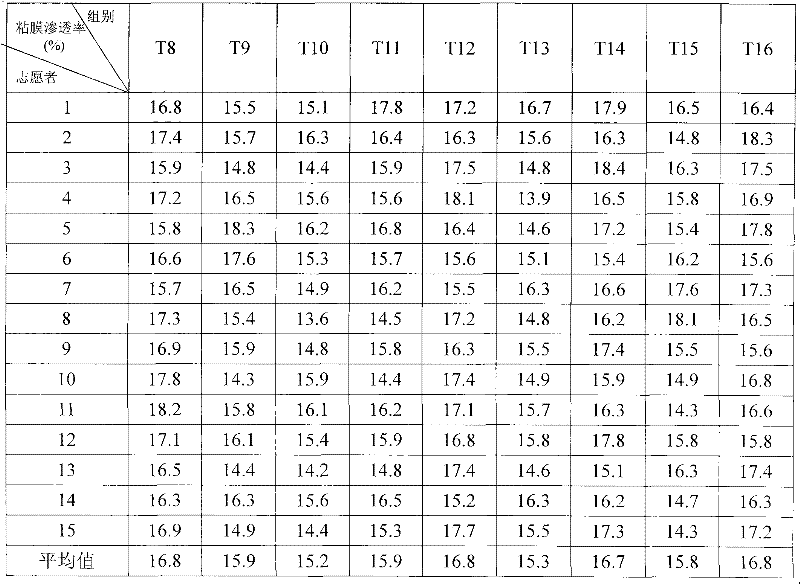
![Pyrrolo[2,3-d]pyrimidine derivative and application in preparation of anti-malignant tumor medicament thereof Pyrrolo[2,3-d]pyrimidine derivative and application in preparation of anti-malignant tumor medicament thereof](https://images-eureka.patsnap.com/patent_img/7098208b-e9d2-4062-a43a-2c3ce820f9e8/BSA00000435860900022.PNG)