Diphenhydramine citrate orally disintegrating tablet and preparation method thereof
A technology of diphenhydramine citrate and orally disintegrating tablets is applied in pharmaceutical formulations, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc., and can solve the problems of small first-pass effect, etc. High bioavailability, easy operation and low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
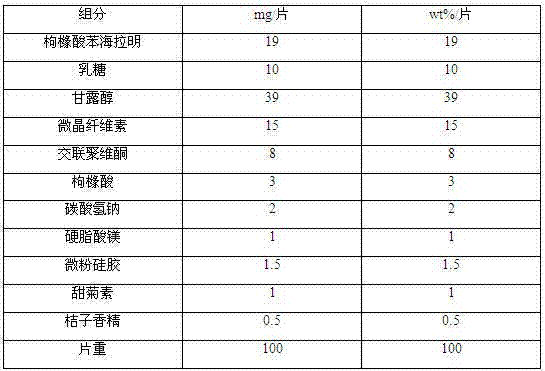
[0025] prescription:
[0026]
[0027] Preparation:
[0028] Crush each component in the prescription into a fine powder passing through a 100-mesh sieve, take the prescribed amount of diphenhydramine citrate and all the excipients except magnesium stearate, mix them evenly, and then add the prescribed amount of magnesium stearate , mix evenly, control the tablet hardness to be 7±2N and compress the tablet to obtain diphenhydramine citrate orally disintegrating tablets, each containing 19 mg of diphenhydramine citrate.
Embodiment 2
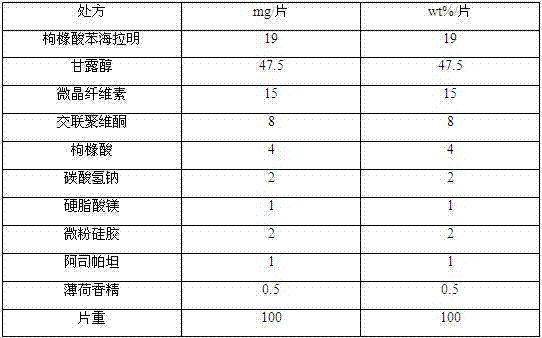
[0030] prescription:
[0031]
[0032] Preparation:
[0033] Grind each component in the prescription into fine powder passing through a 100-mesh sieve, take the prescribed amount of diphenhydramine citrate and stevioside, mix them evenly, and then add the prescribed amount of mannitol, lactose, microcrystalline cellulose and cross-linked Lipovidone, mix evenly, use ethanol solution with a volume fraction of 50% as a wetting agent to make a soft material, granulate with a 30-mesh sieve, ventilate and dry at 50-55°C, and granulate with a 30-mesh sieve. Add acid, sodium bicarbonate, orange essence and micropowder silica gel to the dry granules, mix evenly, then add the prescribed amount of magnesium stearate, mix evenly, control the tablet hardness to 7±2N, and press the tablet to obtain benzene citrate Diphenhydramine orally disintegrating tablets, each containing 19mg of diphenhydramine citrate.
Embodiment 3
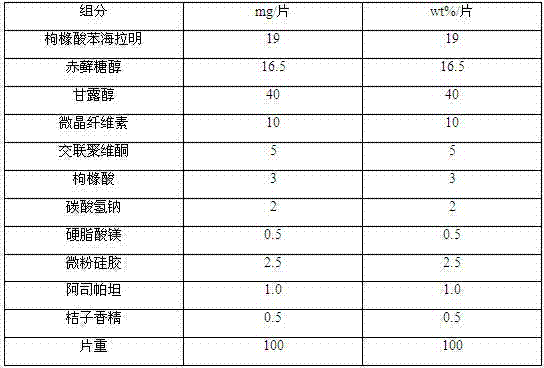
[0035] prescription:
[0036]
[0037] Preparation:
[0038] Grind each component in the prescription into fine powder passing through a 100-mesh sieve, take the prescribed amount of diphenhydramine citrate and aspartame, mix them evenly, then add the prescribed amount of mannitol and erythritol, and two Mix microcrystalline cellulose and cross-linked povidone one-half of the prescription amount evenly, use ethanol solution with a volume fraction of 50% as a wetting agent to make a soft material, granulate with a 30-mesh sieve, and ventilate and dry at 50-55°C. Sieve the 30-mesh granule, add the remaining prescription amount of microcrystalline cellulose and crospovidone, and the prescription amount of citric acid, sodium bicarbonate, lemon essence and talcum powder into the dry granules, mix well, and then add the prescription amount of magnesium stearate, mixed uniformly, and controlled tablet hardness is 7 ± 2N tabletting, promptly obtains diphenhydramine citrate orally...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com