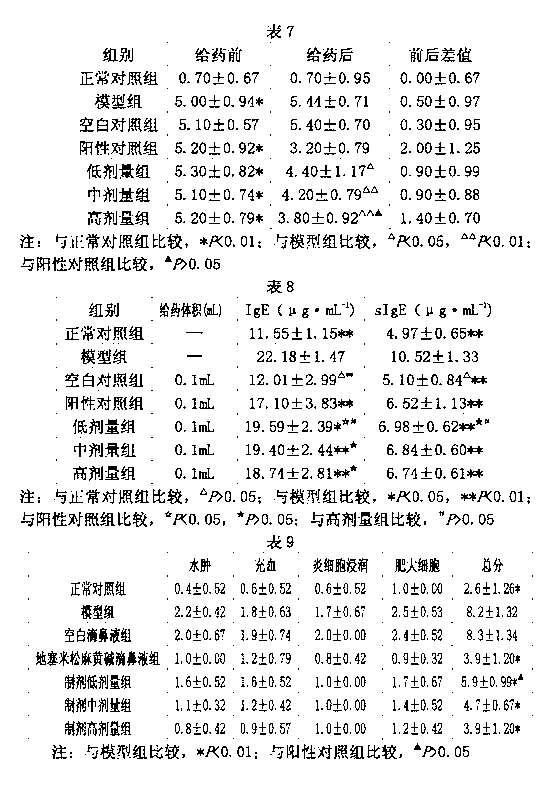
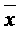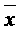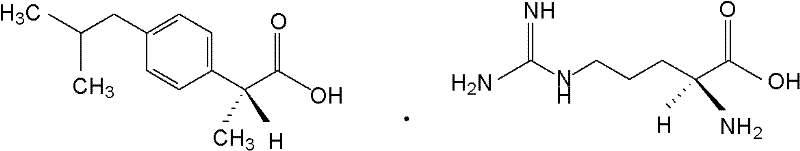Patents
Literature
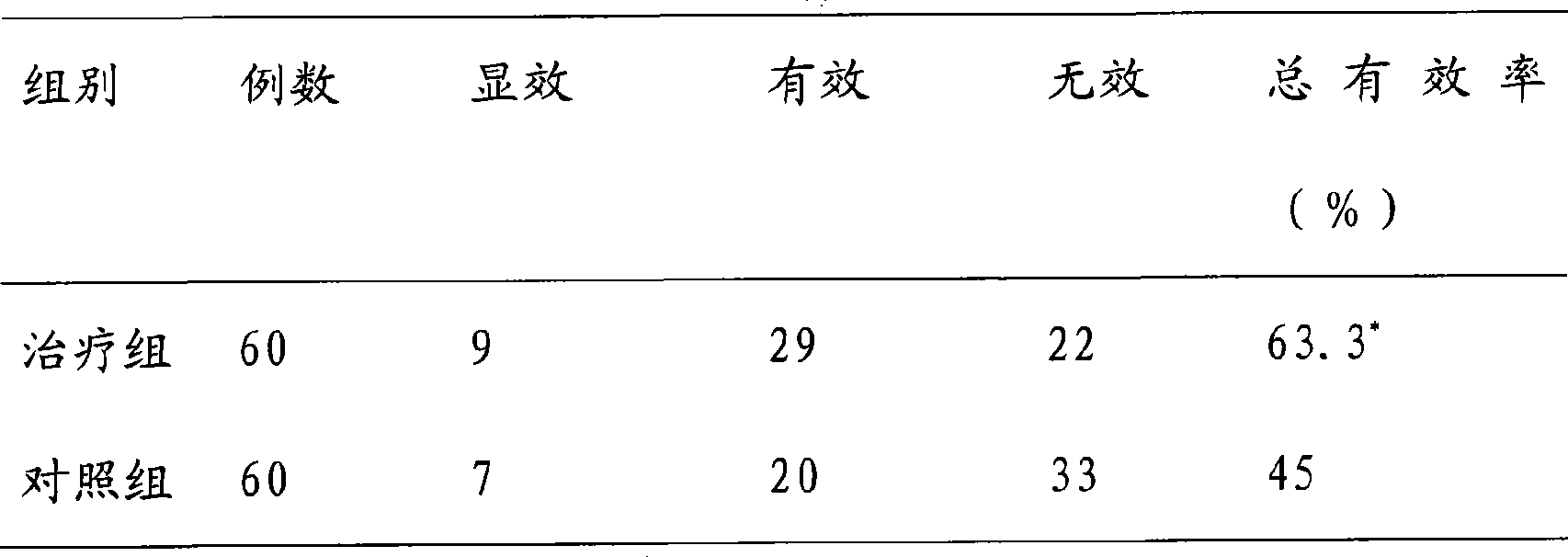
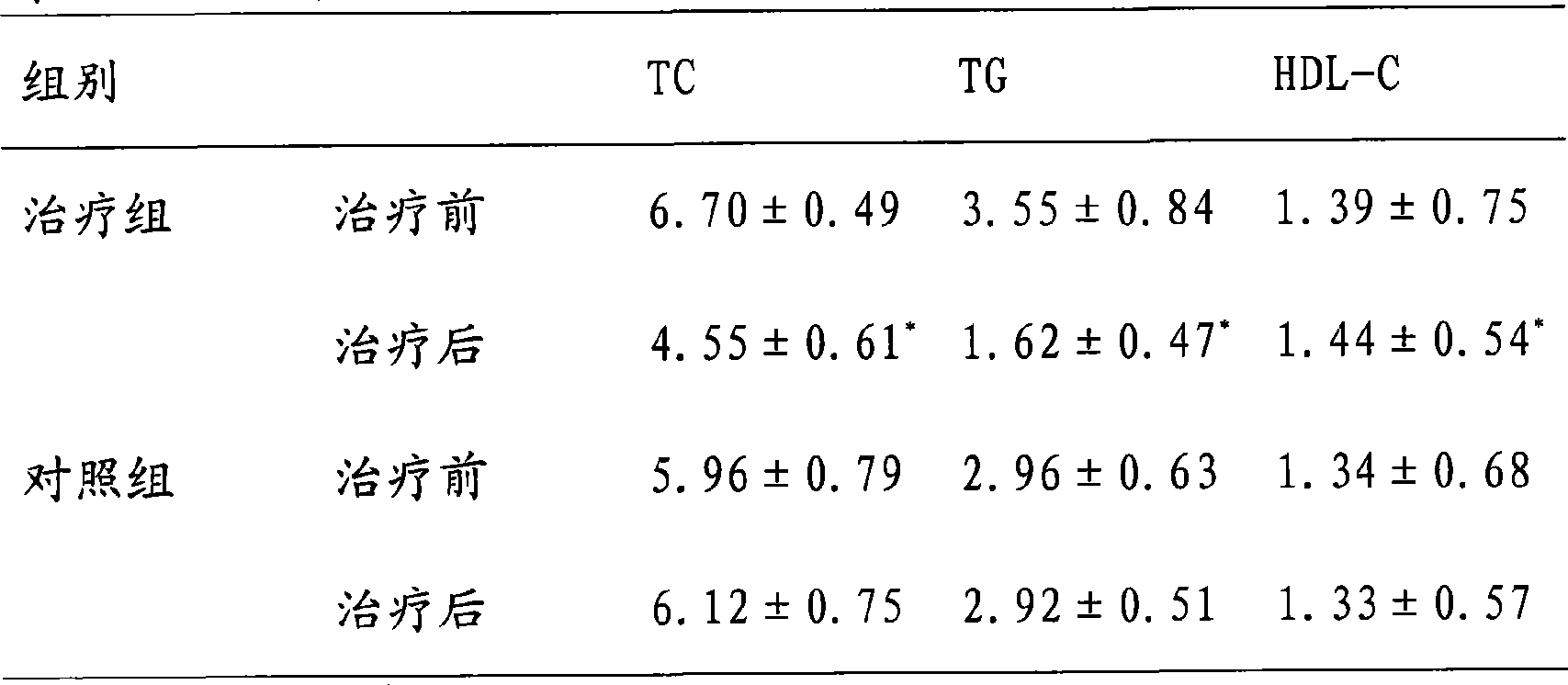
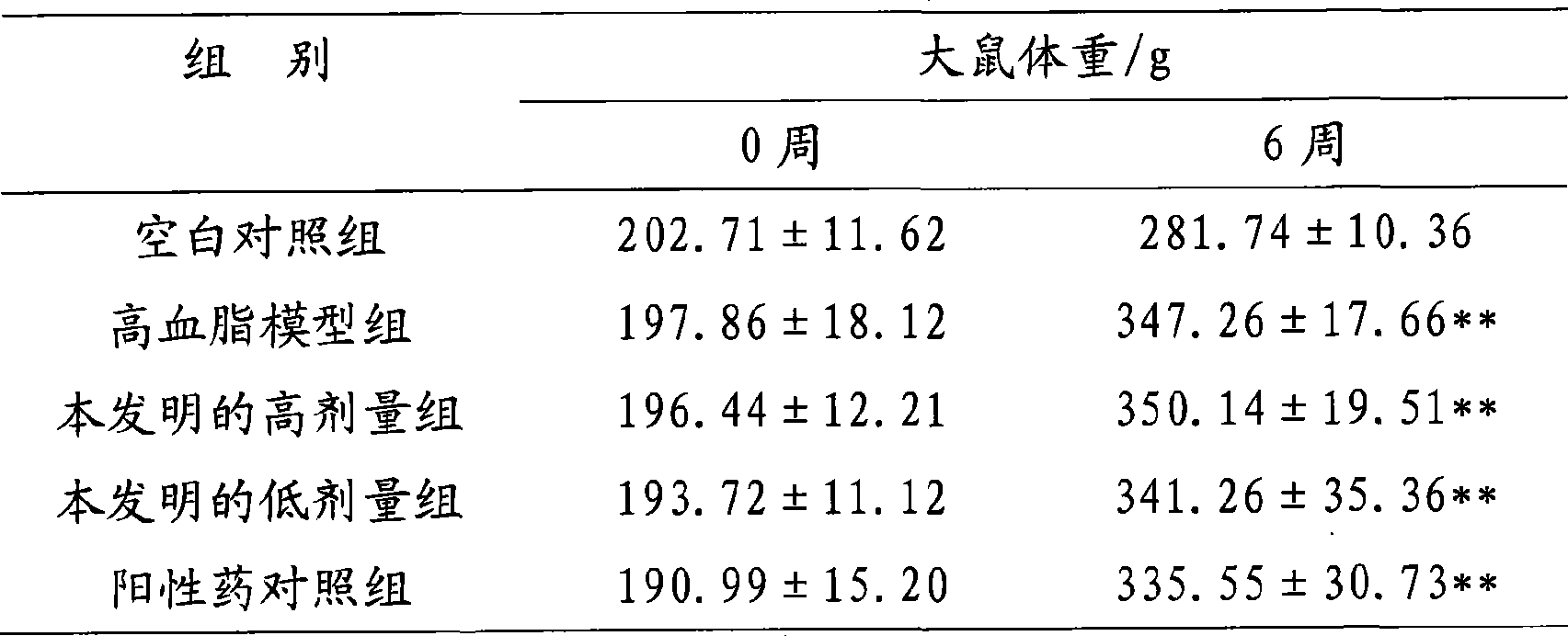
173results about How to "Product quality is stable and controllable" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
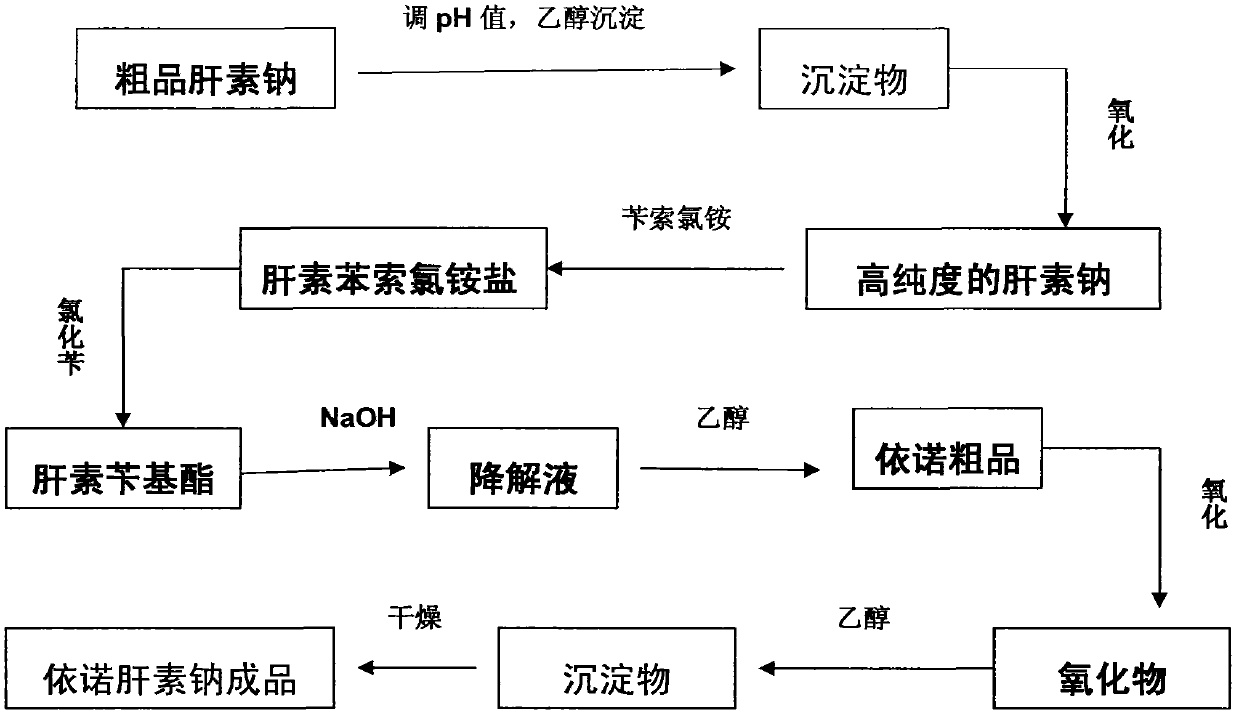
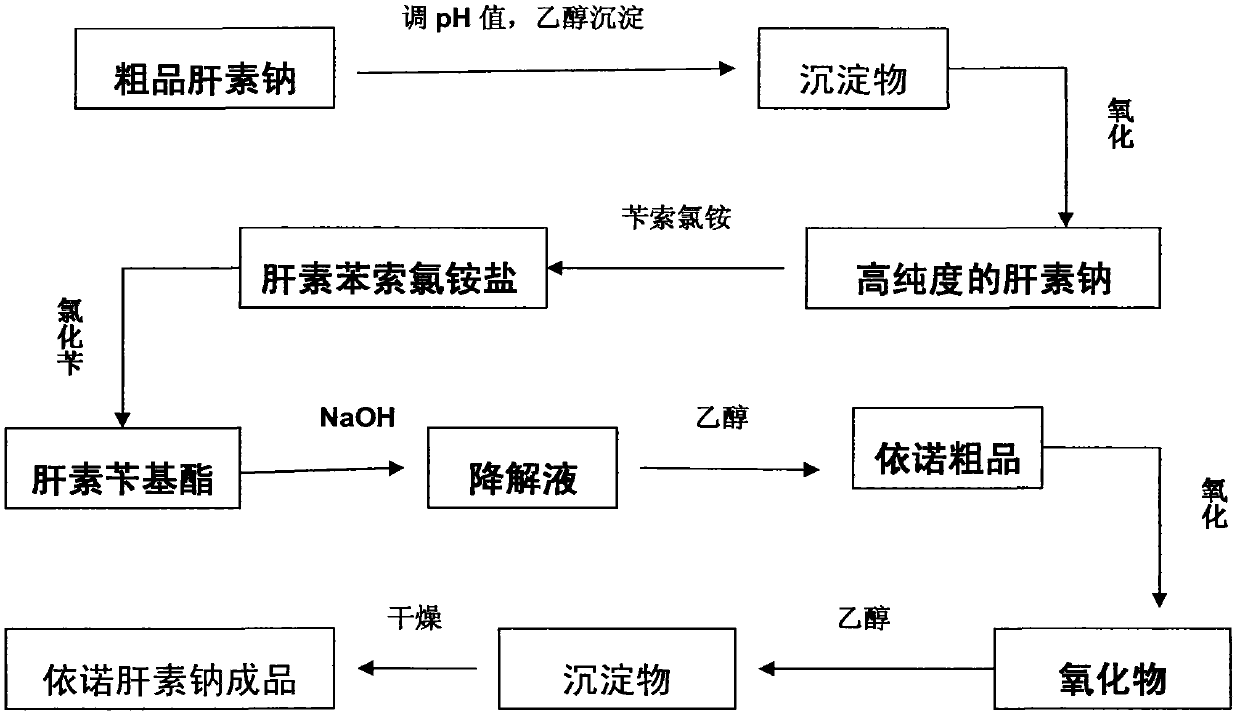
Method for directly producing enoxaparin sodium from crude product heparin sodium
ActiveCN102603925AControl impurity contentReduce intermediate environmentOrganic solventDepolymerization
The invention relates to a preparation method for directly producing enoxaparin sodium from crude product heparin sodium. The preparation method comprises the following steps of: taking the crude product heparin sodium as a raw material, performing fractionated precipitation through an organic solvent to remove most of impurities in the crude product heparin sodium, and then removing part of residual impurity proteins, pigments and other impurities by oxidation through hydrogen peroxide so as to get the high-purity heparin sodium which is in line with the production requirements of the enoxaparin sodium; and taking the high-purity heparin sodium as an intermediate product, preparing a heparin quaternary ammonium salt, preparing heparin benzyl ester, performing alkaline depolymerization on the heparin benzyl ester, neutralizing with an acid, performing alcohol precipitation, refining, decoloring, dehydrating and drying to get an enoxaparin sodium finished product. By adopting the method disclosed by the invention, the use of the organic solvent is greatly reduced, the production efficiency is improved, the influences on the environment are reduced, the enoxaparin sodium finished product which achieves or is better than European Pharmacopoeia 7.0 version is obtained, and the method is simple to operate and can realize industrialized production.
Owner:DONGYING TIANDONG PHARM CO LTD
Jerusalem artichoke pickle produced by direct-vat-set lactobacillus brevis leavening agent, and process of same
ActiveCN102613518AIncrease productivityShort fermentation cycleFood preparationNutritionFermentation starter
The utility model relates to jerusalem artichoke pickle produced by direct-vat-set lactobacillus brevis leavening agent and a process of same, and belongs to the technical field of deep processing of vegetable and food bioscience. Lactobacillus brevis is adopted to be developed into high-density lactobacillus brevis leavening agent (the total number of bacterial colonies reaches 109 / ml); and the jerusalem artichoke pickle is produced by utilizing a direct-vat-set leavening process, so that a modern bioprocess technology of producing high-quality jerusalem artichoke pickle quickly is formed and achieved. The invention has a simple technological process and is easy to operate; compared with a conventional leavening process, the direct-vat-set leavening process has the advantages that the operation procedure is simplified, the production cost is reduced, and the nutritional ingredient and flavor substance of the jerusalem artichoke can be kept furthest at the same time; the jerusalem artichoke pickle is crispy, tasty, refreshing, and nourishing, and is a probiotic health product applicable to all ages. The invention is a green natural, safe and healthful production process and technology.
Owner:东台海滨科技创业园管理有限公司
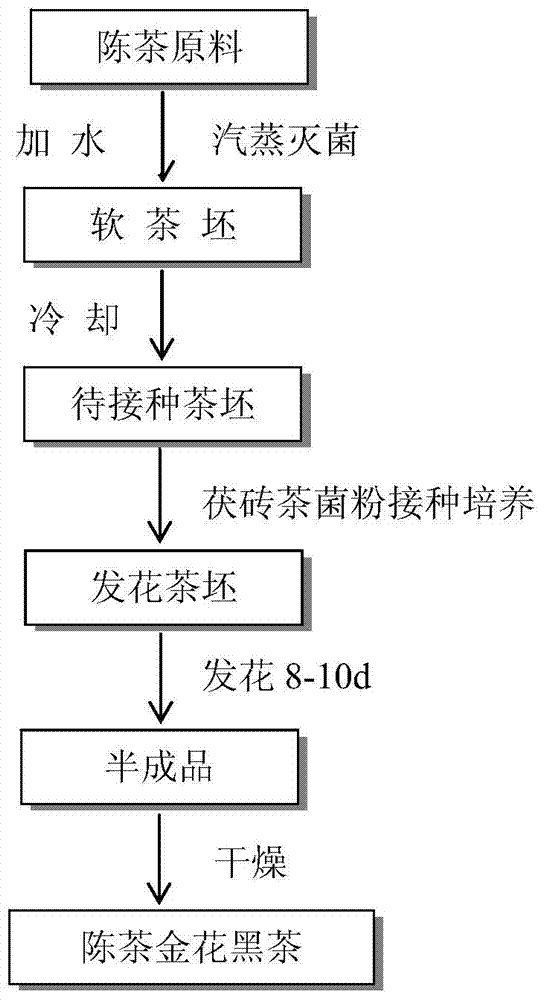
Aged golden-flower dark green tea and processing technic thereof
ActiveCN103859081ASimple processing technologyProduct quality is stable and controllablePre-extraction tea treatmentFlavorMicrobial transformation
The invention provides a novel aged golden-flower dark green tea processing technic, comprising the steps of taking aged tea of various tea leaves as raw material, carrying out humidification and germicidal treatment, adding Fuzhuan tea golden-flower fungus power to the obtained tea base, and then fermenting and cultivating, and drying after fermenting, thus obtaining the aged golden-flower dark green tea finished product. The novel aged golden-flower dark green tea processing technic provided by the invention, which is an import innovation of tea process technology, takes various aged teas as the raw material, fuses the key process of Fuzhuan tea, 'fungus growing', to reprocess, and obtains the aged golden-flower dark green tea through fermenting and drying; the obtained aged golden-flower dark green tea has the advantages that golden-flower is full, fungus potpourri is thick, taste is mellow and smooth, liquor color is bright, and the Fu tea quality flavor is obvious. The processing technic not only inherits part flavour characteristics of primary tea, simultaneously, the unique fungus potpourri caused by microbe fermenting is fused, a large variety of dark green tea products can be produced according to different raw material; meanwhile, aged tea and 'golden-flower' fungus are combined organically, the effective components of tea are converted through microbes, bioavailability is improved, and health care efficacy of tea is enhanced.
Owner:HUNAN AGRICULTURAL UNIV
Method for producing maotai-flavor flavoring wine
The invention belongs to the liquor production field, in particular to a preparation method of Maotai-flavor liquor. The method of the invention comprises the following steps: a. fermented grains or double bottom fermented grains which are not extracted wine in the production process of common Maotai-flavor liquor are added to Maotai-flavor liquor yeast powder and mature hull for uniformly blending, and then adding fluid is added to acquire Maotai-flavor flavoring wine fermented grains; b. the fermented grains produced by common Maotai-flavor liquor is cellared, and the Maotai-flavor flavoring wine fermented grains acquired from step a is placed on the top; c. cellar mud is used for sealing the cellar, the Maotai-flavor flavoring wine fermented grains is taken out after fermentation to distill and extract wine, thus acquiring the wine. The method of the invention has simple steps and low cost, the product manufactured by the method has intense and mellow Maotai-flavor style, and the taste is better than the Maotai-flavor liquor prepared by the existing method, thereby having great application prospect.
Owner:四川郎酒股份有限公司
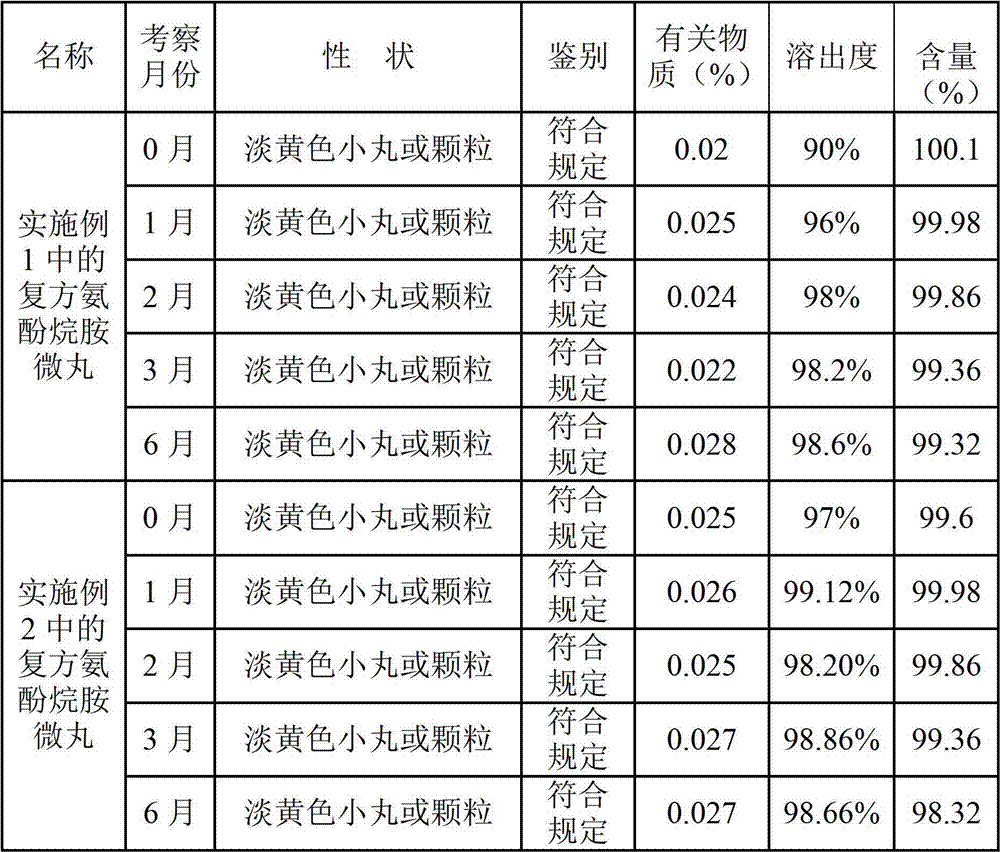
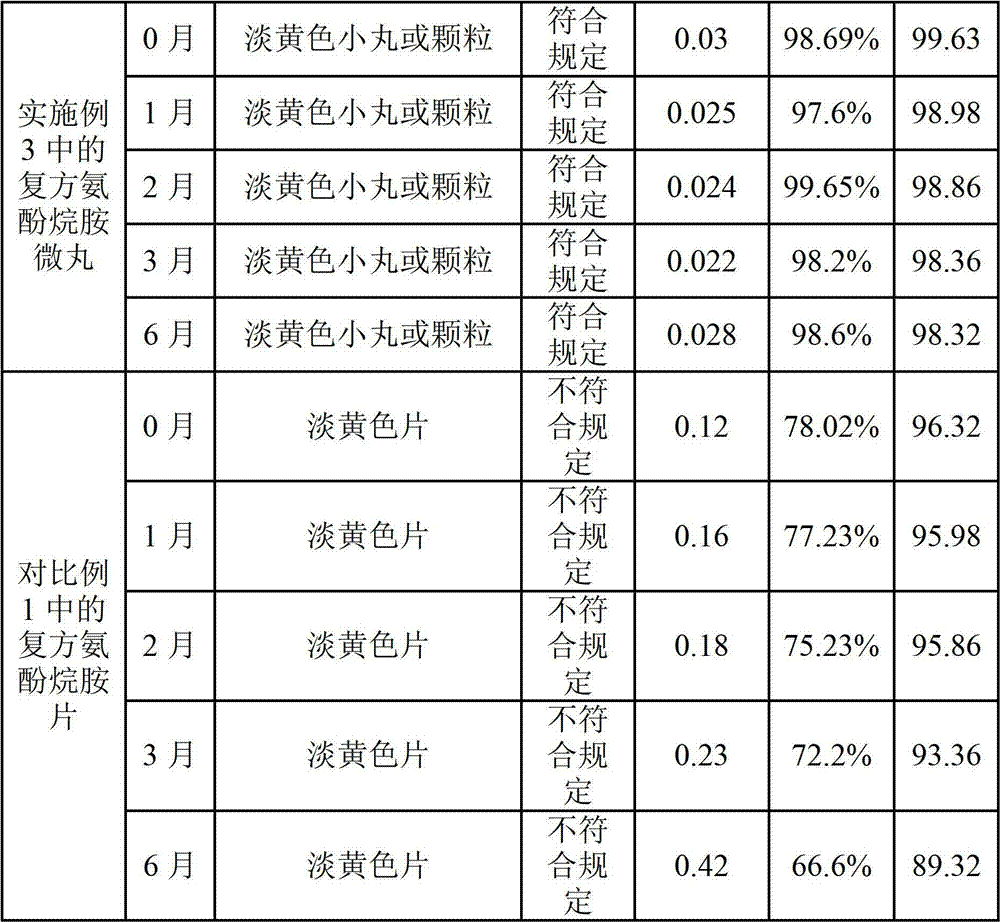
Preparation method of compound paracetamol and amantadine pellets
ActiveCN102861106AEvenly distributedEasy to fillNervous disorderAntipyreticDissolutionCalculus bovis
The invention discloses a preparation method of compound paracetamol and amantadine pellets. The compound paracetamol and amantadine pellet is mainly prepared from the following raw materials: chlorpheniramine maleate, calculus bovis factitious, caffeine, amantadine hydrochloride, acetaminophen and dextrin, and the mass ratio of the above raw materials is 1 to 5 to 7.5 to 50 to 125 to (4-5); the preparation method comprises the following steps: adding the chlorpheniramine maleate and the caffeine in the form of solution into a dextrin aqueous solution, spraying and packing the mixed solution on the mixed masterbatches consisting of the acetaminophen, the amantadine hydrochloride and the calculus bovis factitious, and then spraying the rest of dextrin and the rest of mixed powder to obtain the compound paracetamol and amantadine pellet. The method is simple to operate, liable to control and suitable for industrial production, and the obtained pellet has the advantages of low related substances, good content uniformity and dissolution rate and the like.
Owner:HAINAN HULUWA PHARMA GRP CO LTD
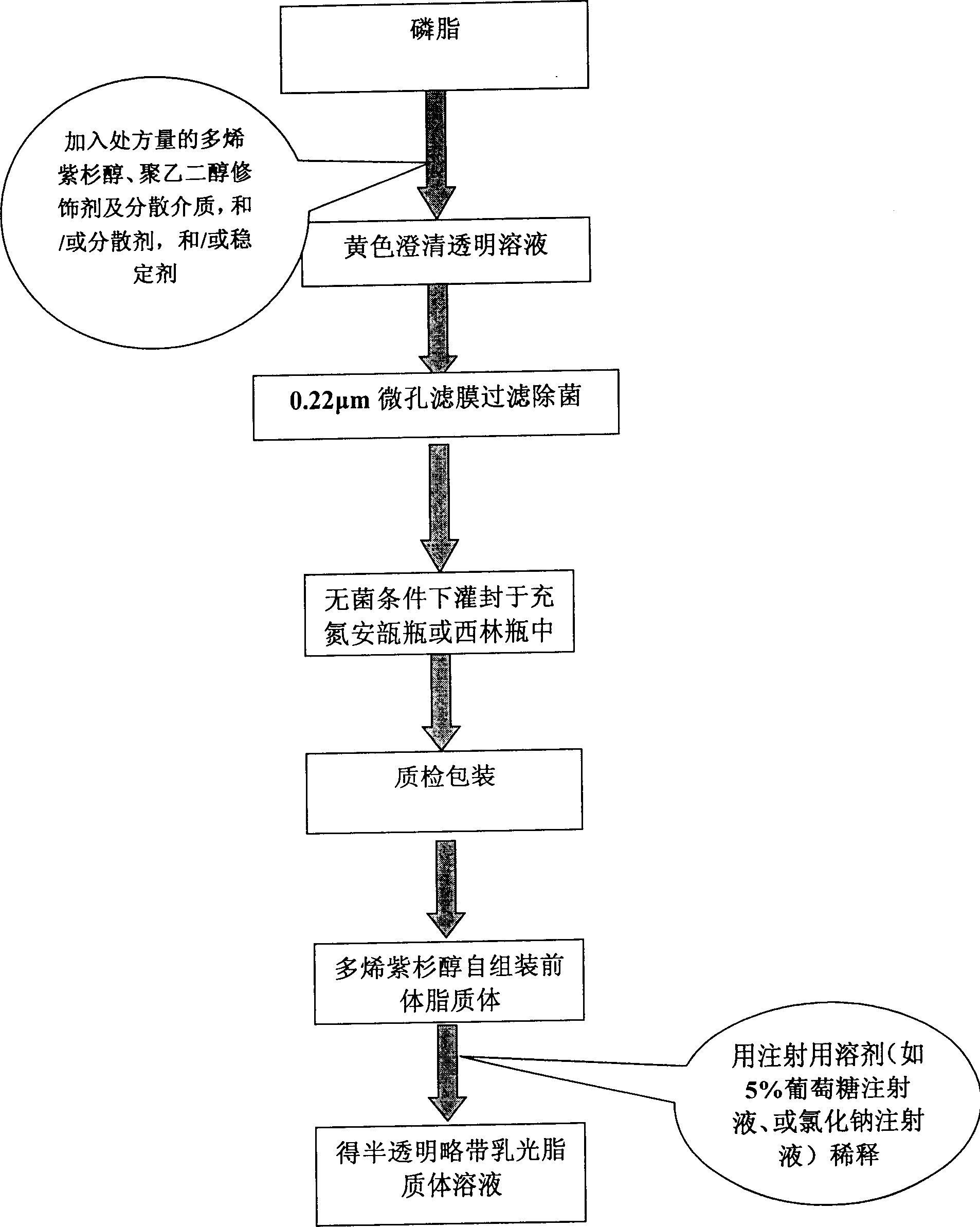
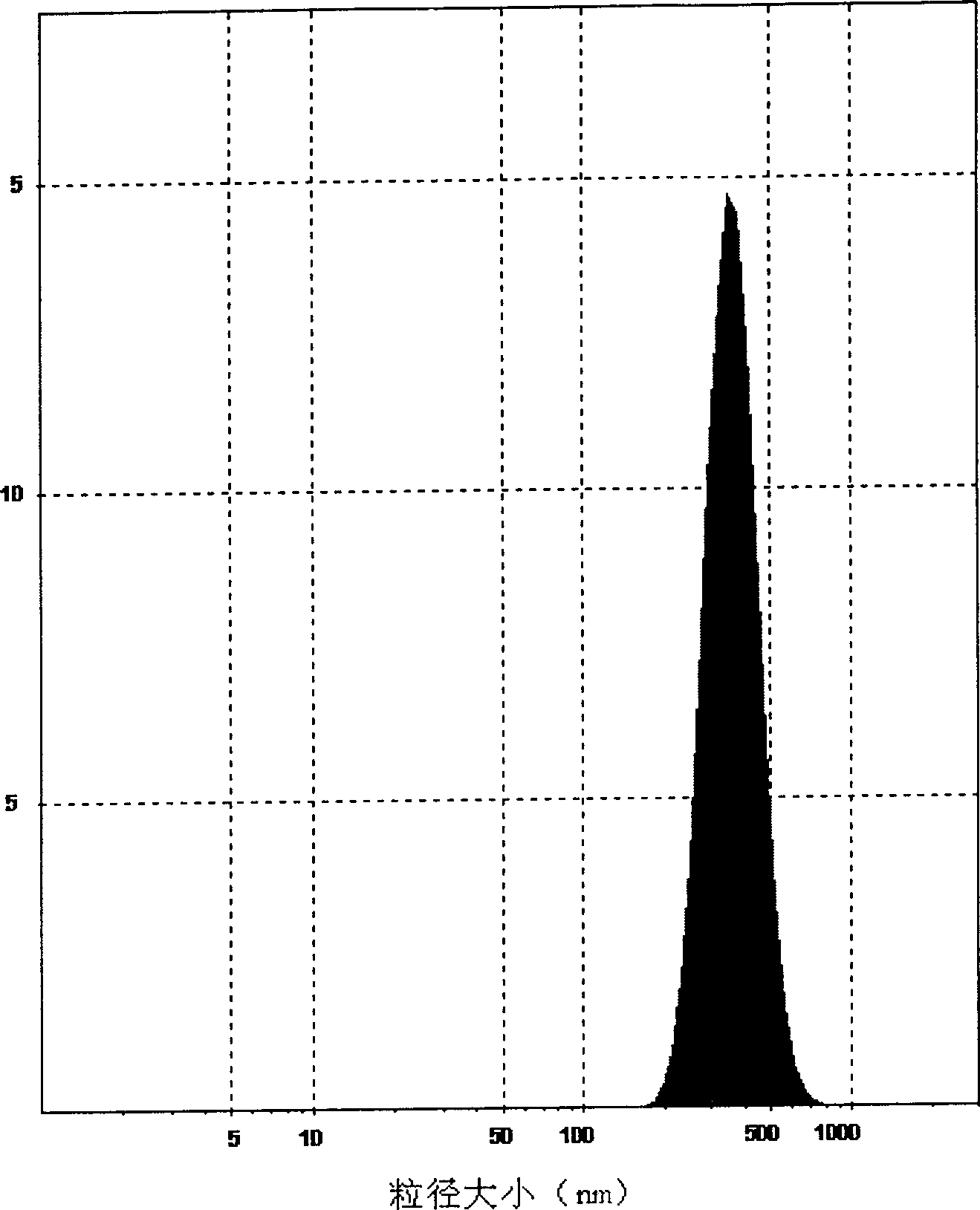
Poly olefinic taxadol self assembled precusor liposome and its preparation method
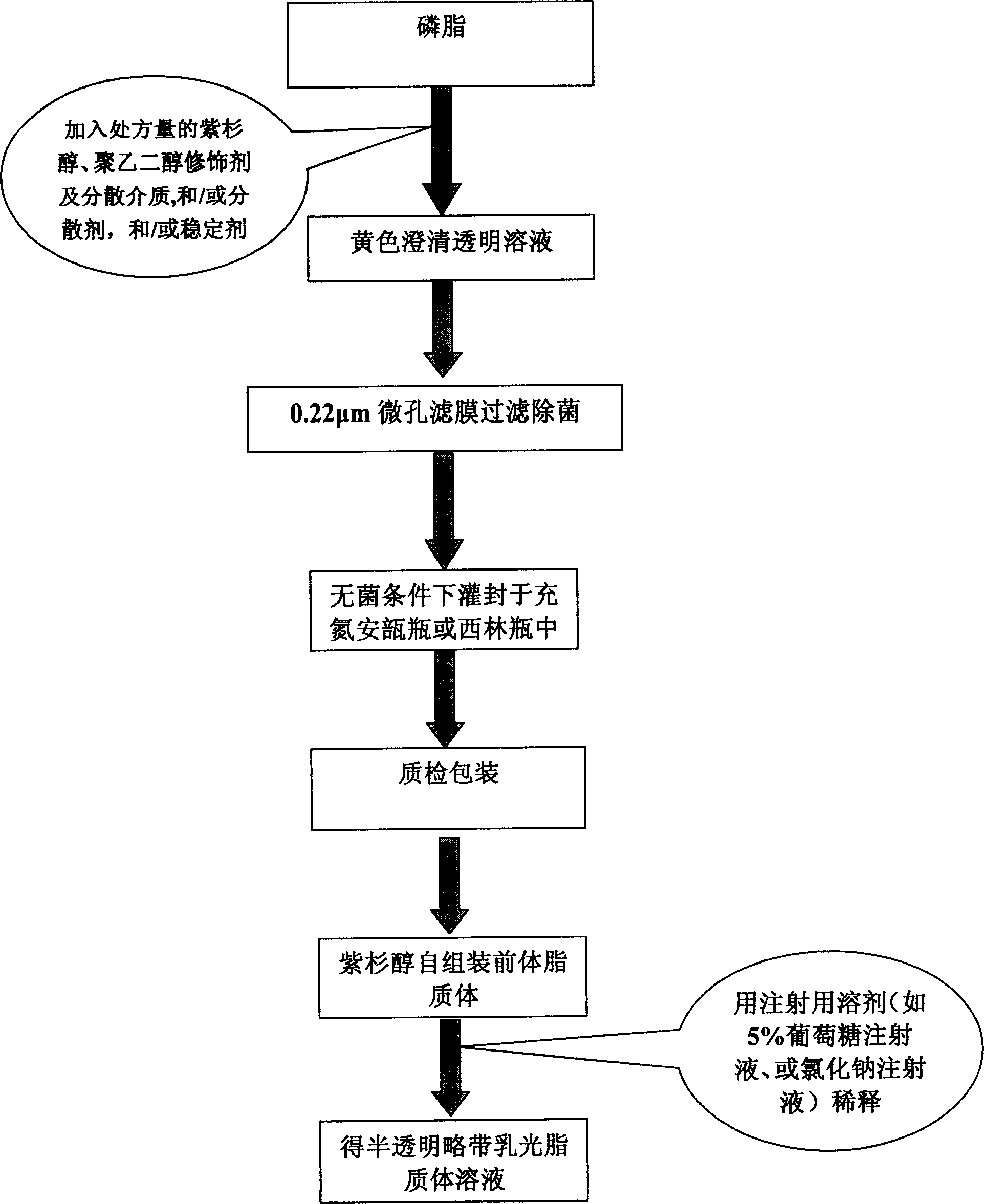
ActiveCN1823732AImprove product qualityHigh encapsulation efficiencyOrganic active ingredientsPharmaceutical non-active ingredientsPhospholipinPolymer science
A self-assembled precursor liposome of polyenic taxusol is proportionally prepared from polyenic taxusol, phosphatide, polyethylene glycol and dispersing medium through mixing, dispersing, press-filtering by millipore film, and pouring it in a container full of N2.
Owner:CHINA PHARM UNIV +1
Preparation method, quality control method and application for Chinese medicinal compound indigowoad leaf preparation
ActiveCN101708223AReduce contentMeet the requirements of healthy livingComponent separationAntiviralsAlcohol contentChlorogenic acid
The invention relates to a preparation method and a quality control method for a compound indigowoad leaf preparation. The preparation method comprises a step of preparing a basic remedy and a step of preparing a corresponding preparation. The basic remedy in the basic remedy preparation comprises the following compositions in part by weight: 360 to 440 parts of indigowoad leaves, 180 to 220 parts of lonicera confusa or honeysuckle flower, 90 to 110 parts of incised notopterygium rhizome, 90 to 110 parts of bistort rhizome, and 90 to 110 parts of rhubarb; the medicinal materials are decocted twice with conventional amount of water for 1 hour each time; the decoctions are mixed and filtered; the filtrate is concentrated to the relative density of between 1.08 and 1.32 at 60 DEG C; ethanol is added into the filtrate to ensure that alcohol content reaches 50 to 60 percent, the mixture is stood and is filtered, the filtrate is subjected to ethanol reclamation and is concentrated to an extract with the relative density of between 1.17 and 1.43 at 60 DEG C for later use; and the corresponding preparation is prepared according to a drug specification. The quality control method comprises the following steps of the identification of contents and the content determination of the contained compositions including the identification of the indigowoad leaves, the identification of the lonicera confusa or honeysuckle flower, the identification of the rhubarb, the identification of the incised notopterygium rhizome, the total content determination of emodin and chrysophanol in the rhubarb, and the content determination of chlorogenic acid. The methods can be applied to preparation of medicinal preparations for treating cold, influenza, parotitis and acute viral hepatitis.
Owner:RONGCHANG PHARM ZIBO CO LTD
Xiao er Anfen Huang Namin granule and preparation method thereof
ActiveCN101982179AQuality assuranceReduce dosageOrganic active ingredientsPharmaceutical product form changeChlorphenamine maleateSoft materials
The invention discloses a Xiao er Anfen Huang Namin granule, which is prepared from the following raw materials in terms of 5000g to 5600g of Xiao er Anfen Huang Namin granules through the wet-method granulation: 125 to 375g of acetaminophen, 0.5 to 1.5g of chlorphenamine maleate, 5 to 15g of artificial cow-bezoar, 4500 to 5200g of filler, 3 to 5g of adhesive, and proper amounts of essence and ethanol. The content uniformity and the stability of the granule both meet the requirement, and the product quality is stable and controllable. The invention further discloses a preparation method of the Xiao er Anfen Huang Namin granule, comprising the following steps: dissolving the chlorphenamine maleate in a proper amount of ethanol, adding the adhesive and mixing with the acetaminophen, the artificial cow-bezoar and the filler uniformly, producing the mixture into a soft material, drying, and subpackaging to prepare the Xiao er Anfen Huang Namin granule. The preparation method has simple process flow, can save the energy and the cost, and is suitable for the industrial production.
Owner:HAINAN HULUWA PHARMA GRP CO LTD
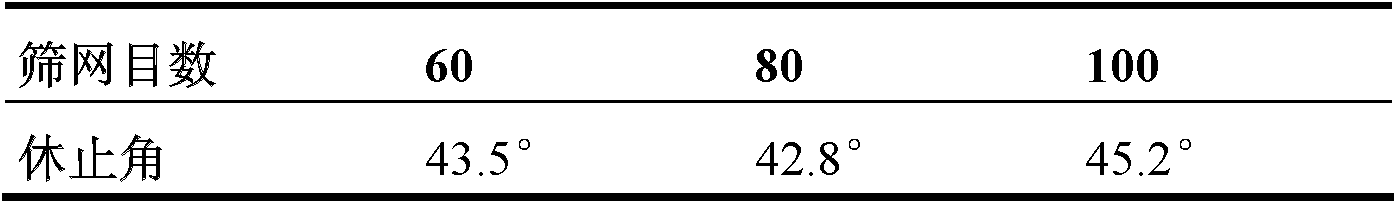
Chinese medicinal composition assistant for reducing blood fat and preparation method thereof
The invention discloses a traditional Chinese medicine composition for aiding blood fat reduction and a preparation method thereof. The method comprises: selecting 2 to 6 grams of lucid ganoderma and 1 to 4 grams of gen-seng, using 8 to 12 times of 50 to 80 percent ethanol to reflux and extract the raw materials twice, filtering the extracts respectively, combining the filtrates into filtrate I, and volatilizing the ethanol in medicine dregs for standby; decocting 1 to 3 grams of sealwort, 1 to 3 grams of polygonatum and the lucid ganoderma and gen-seng dregs by adding 6 to 10 times of water and extracting the decoction twice, filtering the extracts respectively, and combining the filtrates; decompressing and condensing the filtrate, adding ethanol into the condensed liquid, stirring the mixture evenly, keeping the mixture standing, and taking supernatant fluid II; selecting 2 to 6 grams of notoginseng, 2 to 6 grams of kudzuvine root, and 1 to 3 grams of hawthorn, using 8 to 12 times of 50 to 80 percent ethanol to reflux and extract the raw materials twice, filtering the extracts respectively, and combining the filtrates into filtrate III; and combining the filtrate I, the supernatant fluid II and the filtrate III respectively, reclaiming the ethanol by reducing pressure and condensing the combined mixture to be in shape of thick paste, drying the combined mixture in vacuum to obtain dry paste, crushing the dry paste, and sieving the dry paste by a 80-mesh sieve to obtain the composition. The traditional Chinese medicine composition has the advantages of rich raw traditional Chinese medicine, low cost, simple preparation method and process, good safety of prepared products, no any toxic and side effects, remarkable function of blood fat reduction, and stable and controllable product quality.
Owner:东方药林药业有限公司
Taxdol self assembled precusor liposome and its preparation method
ActiveCN1823734ASolve the "bottleneck" problem that is difficult for efficient industrial productionImprove product qualityOrganic active ingredientsOil/fats/waxes non-active ingredientsDispersed mediaPolyethylene glycol
A self-assembled precursor liposome of taxusol is proportionally prepared from tausol, phosphatide, polyethylene glycol and dispersing medium through mixing, dispersing, press-filtering by millipore film, and pouring it in a container full of N2.
Owner:CHINA PHARM UNIV +1
Hot stuffy processing device for steel slag
The invention relates to a hot stuffy processing device for steel slag, which comprises a tank wall, wherein the tank wall comprises a lateral surface, a bottom surface and an upper end opening; the upper part of the tank wall is provided with an exhaust pipe; the lower part of the tank wall is provided with an exhaust water pipe; a circle of water seal tank with a U-shaped cross section is arranged along the upper end opening of the tank wall, and the tank wall comprises an outer wall of heat-resisting concrete formed by pouring and tamping at one time; the lateral surface of the tank wall also comprises an inner wall formed by pouring and tamping a steel fiber reinforced fire-retarding pouring material at one time; the outer wall comprises a steel mesh M; pre-embedded parts are uniformly distributed and welded on the steel mesh M; the inner wall comprises a steel mesh N; the steel mesh N is in welding connection with exposed joints of the pre-embedded parts on the outer wall one by one; and the thickness of the outer wall is 1 to 2 times that of the inner wall. The hot stuffy processing device for the steel slag has higher hot stuffy processing efficiency, longer service life of equipment, lower maintenance cost, safer operation and simple structure.
Owner:SHAOGUAN IRON & STEEL GROUP CORP GUANGDONG PROV
High-purity ginkgolide composition
InactiveCN102293790AProduct quality is stable and controllableSimple preparation processNervous disorderGinkgophyta medical ingredientsDesorptionGinkgolide
The invention relates to a high-purity ginkgolide and its composition. It is a white powder with slightly bitter taste. The content of ginkgolide A is 10-20%, the content of ginkgolide B is 10-20%, and the content of ginkgolide C is 10-20%. 5-15%, the bilobalide content is 30-50%, and the total lactone content is 80-95%. Ginkgo biloba is used as raw material, heated and refluxed with ethanol solution with a mass fraction of 20-80% to extract, the extract is concentrated to recover ethanol, separated by a macroporous resin column, desorbed by ethanol, diluted with water, and then passed through a gel-type hydrogen bond Adsorption and desorption of the adsorption resin, collection of effluent and desorption liquid, concentration, drying, ethanol dissolution, activated carbon decolorization, concentration and drying. The preparation process is simple, reliable, low in cost and high in yield, and no toxic organic solvent is used in the process, which is an environment-friendly process. The high-purity ginkgolide is used as an active ingredient to prepare a composition that can be used as various preparations (capsules, tablets, oral liquids, granules, powders, injections, etc.) of ginkgolide medicines or health care products.
Owner:TIANJIN TAIYANG PHARMA
Compound vitamin (3) pharmaceutical composition for injection and preparation method thereof
InactiveCN103202844APlay an antioxidant roleNot easy to decomposePowder deliveryMetabolism disorderGlycineActivated carbon
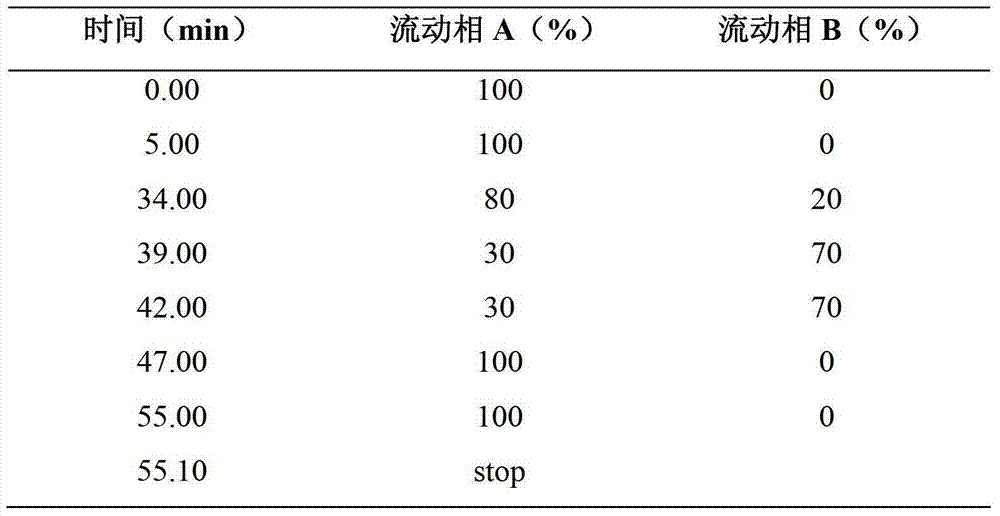
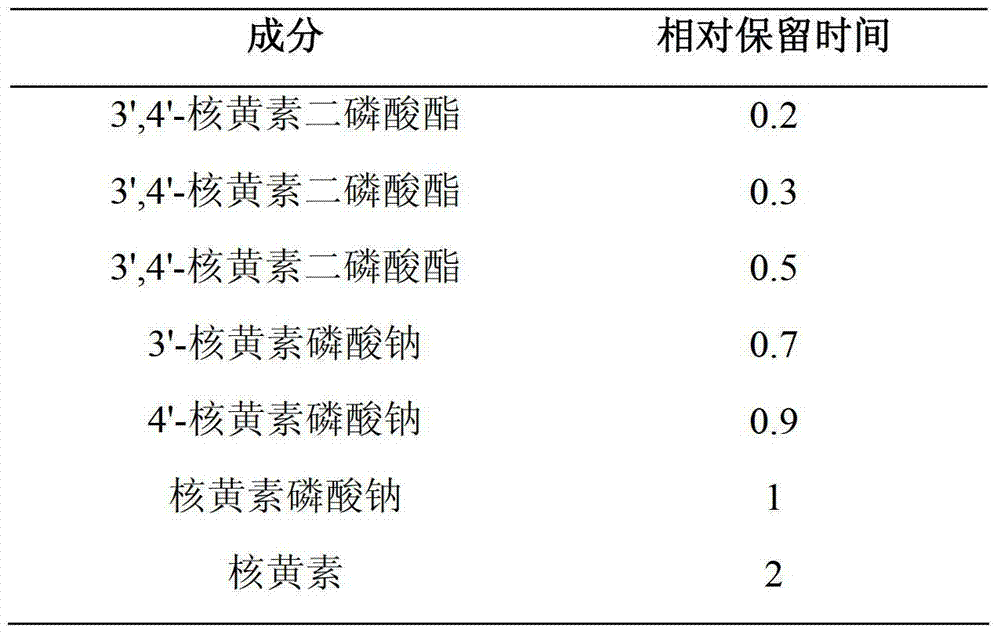
The invention provides a compound vitamin (3) pharmaceutical composition for injection and a preparation method thereof. The composition is powder injection and comprises vitamin B1, riboflavin sodium phosphate (namely vitamin B2 sodium phosphate), vitamin C, stabilizer glycine and antioxidant thiourea. The preparation method comprises the following steps: preparing liquid medicine, performing activated carbon adsorption, decarburizing, filtering and degerming, filling and performing freeze-drying. The compound vitamin (3) pharmaceutical composition for injection does not contain a pH regulator and is high in stability and high in safety.
Owner:LIAONING HAISCO PHARMACEUTICAL CO LTD
Calcium dobesilate capsule and preparation method thereof
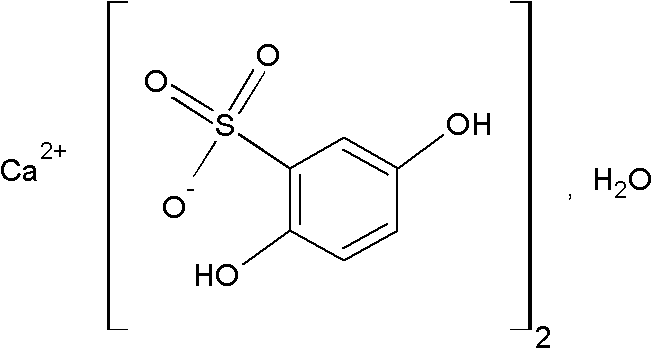
InactiveCN102091055AThe effect is fully verifiedAvoid loading differencesSenses disorderMetabolism disorderDissolutionCroscarmellose sodium
The invention discloses a calcium dobesilate capsule and a preparation method thereof. The calcium dobesilate capsule is prepared from calcium dobesilate, croscarmellose sodium, magnesium stearate and polyvinyl pyrrolidone, wherein every 1000 calcium dobesilate capsules contain 500g of calcium dobesilate, 20-50g of croscarmellose sodium and 2-6g of magnesium stearate. In addition, a traditional preparation method is improved in the invention, and the improved method comprises the following steps: on the basis of taking the croscarmellose sodium as a disintegrating agent and taking the magnesium stearate as a lubricating agent, adding a proper amount of the polyvinyl pyrrolidone to prepare a bonding agent; preparing the bonding agent and the calcium dobesilate as well as the croscarmellosesodium into granules by a multi-step granulation method; and spraying the ethanol solution of the magnesium stearate onto the surfaces of the granules to obtain calcium dobesilate capsules with stable quality, high dissolution rate and small content uniformity.
Owner:HAINAN JINRUI PHARMA CO LTD
Novel flavane derivative and its preparation method and uses
ActiveCN1765894AStrong targetingClear curative effectOrganic active ingredientsOrganic chemistryMedicinal chemistryFlavan
Owner:SHANHE PHARMA GUANGZHOU CITY
Feed additive for supplying nutrients to cows
ActiveCN102366027AImprove conception rateMake up for the negative impact of absorption and utilizationAnimal feeding stuffAccessory food factorsAnimal scienceYeast Proteins
The invention discloses a feed additive for supplying nutrients to cows, belongs to the feed additive field, and specially, relates to cow feed additive products. The feed additive for supplying nutrients to cows comprises 30 to 50 wt% of puffed soybean, 4 to 9 wt% of urea, 3 to 8 wt% of yeast protein powder, 20 to 35 wt% of gloeostereum insarnatum, 15 to 25 wt% of lucid ganoderma and 2 to 9 wt% of a sustained release agent. The feed additive for supplying nutrients to cows can provide enough nutritional reserves for cows in a perinatal period, supply enough nutrients to cows in a lactation peak period, adjust an energy negative balance of a high-yield cow in a lactation peak period, supply a body loss of a high-yield cow in a lactation peak period, improve milk daily output, lactation peak sustainability and a cow conception rate, and prolong a cow lactation peak period by 20 to 35 days.
Owner:北京东方联鸣科技发展有限公司
Elm tree mushroom feedstuff addictive, its preparation method and application
InactiveCN1806656AImprove conversion rateNo pollutionFood processingAnimal feeding stuffBiotechnologyMushroom
The invention discloses a epiphyte of ulmus feed addictive, its preparing process and application, wherein the product is prepared from epiphyte of ulmus solid zymogeneous bacteria and bacterium mass through solid fermentation culturing, disintegrating the culture and drying, finally packaging the obtained disintegrated articles.
Owner:CHINA NAT RES INST OF FOOD & FERMENTATION IND CO LTD
Liquid preparation for rhinitis/allergic rhinitis as well as preparation method and application thereof
ActiveCN103271993AImprove complianceSimple processRespiratory disorderImmunological disordersGLYCYRRHIZA EXTRACTPharmaceutical Substances
The invention relates to the technical field of liquid traditional Chinese medicine preparations and provides a liquid preparation for rhinitis / allergic rhinitis as well as a preparation method and application thereof. The liquid preparation is obtained by virtue of the following method: carrying out centrifugation after dissolving Artemisia rupetris extracts in a solubilizer solution, taking the supernatant, adding Scutellaria baicalensis extracts to the supernatant and completely dissolving the Scutellaria baicalensis extracts; then adding a thickener solution to the solution, mixing the solutions uniformly, then adding liquorice extracts to the mixture, stirring the mixture to dissolve the liquorice extracts, then adding a preservative to the solution, and fully stirring the solution to dissolve the preservative; and finally adding water for injection to the solution and mixing the substances uniformly, thus obtaining the liquid preparation for rhinitis / allergic rhinitis. The liquid preparation is simple in process, has stable and controllable quality, obvious curative effects and no toxic or side effect, directly acts on the surface of nasal mucosa, has high bioavailability, takes effect quickly, is convenient to use and has good patient compliance.
Owner:XINJIANG INST OF MATERIA MEDICA
Medicinal composition and tablet containing salicylic acid methyl ester lactoside and preparation methods thereof
ActiveCN102526080ALittle side effectsLong lasting in vivoOrganic active ingredientsAntipyreticSide effectSalicylic acid
The invention relates to a medicinal composition containing salicylic acid methyl ester lactoside. The medicinal composition comprises the following raw materials in parts by weight: 100 parts of salicylic acid methyl ester lactoside, 20-300 parts of a thinner, 1-8 parts of a disintegrating agent, 20-400 part of a bonding agent and 0.5-6 parts of a lubricating agent. The invention further discloses a salicylic acid methyl ester lactoside tablet, which consists of a tablet core and a film coating uniformly coated on the surface of the tablet core, wherein the tablet core consists of the medicinal composition containing the salicylic acid methyl ester lactoside; and the film coating comprises the following raw materials in parts by weight: 20-200 parts of a film forming material, 0-50 parts of a film forming aid and 600-2,000 parts of a solvent. The invention further discloses a preparation method of the tablet. The medicinal composition provided by the invention is convenient to take, and has low toxic and side effects; the dissolution rate of the tablet is high, and the stability is high; and the preparation methods are easy to operate, and the prepared products have stable and controllable quality.
Owner:INST OF MATERIA MEDICA CHINESE ACAD OF MEDICAL SCI
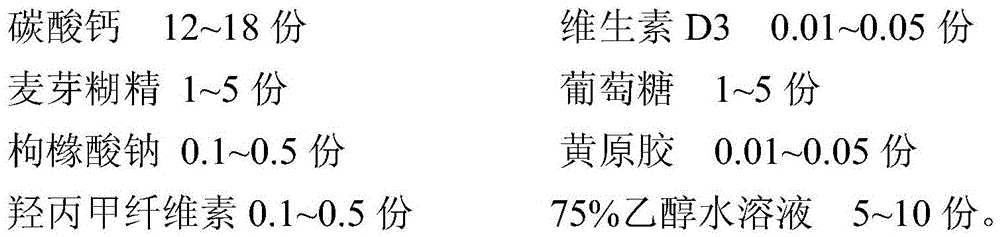
Calcium carbonate D3 granules and preparation method thereof
ActiveCN105535018AProduct quality is stable and controllableEasy to operateOrganic active ingredientsMetabolism disorderGlucose polymersHypromellose
The invention discloses calcium carbonate D3 granules and a preparation method thereof. The calcium carbonate D3 granules are prepared from the following raw materials in parts by weight: 12kg to 18kg of calcium carbonate, 0.01kg to 0.05kg of vitamin D3, 1kg to 5kg of maltodextrin, 1kg to 5kg of glucose, 0.1kg to 0.5kg of sodium citrate, 0.01kg to 0.05kg of xanthan gum, 0.1kg to 0.5kg of hydroxypropyl methylcellulose and 5kg to 10kg of 75 percent ethanol. According to the method disclosed by the invention, vitamin D3 and xanthan gum are mixed with the ethanol so as to carry out granulating, the stability of prepared granules is effectively improved, and the problems that vitamin D3 is unqualified in authentication and non-uniform in content are solved, so that the quality of products is improved; the method is simple in process flow and is energy-saving and cost-saving, thereby being applicable to industrial production.
Owner:HAINAN HULUWA PHARMA GRP CO LTD
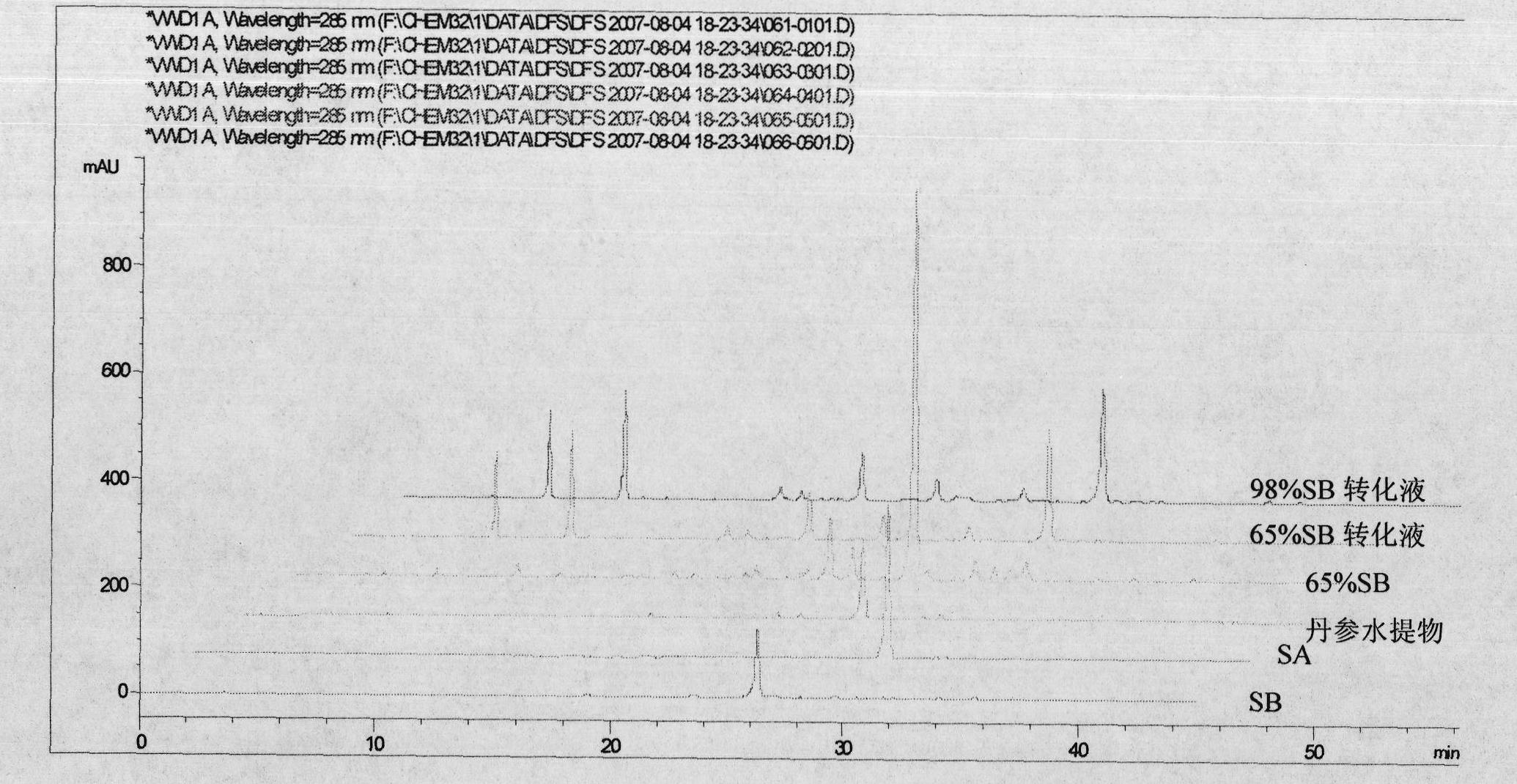
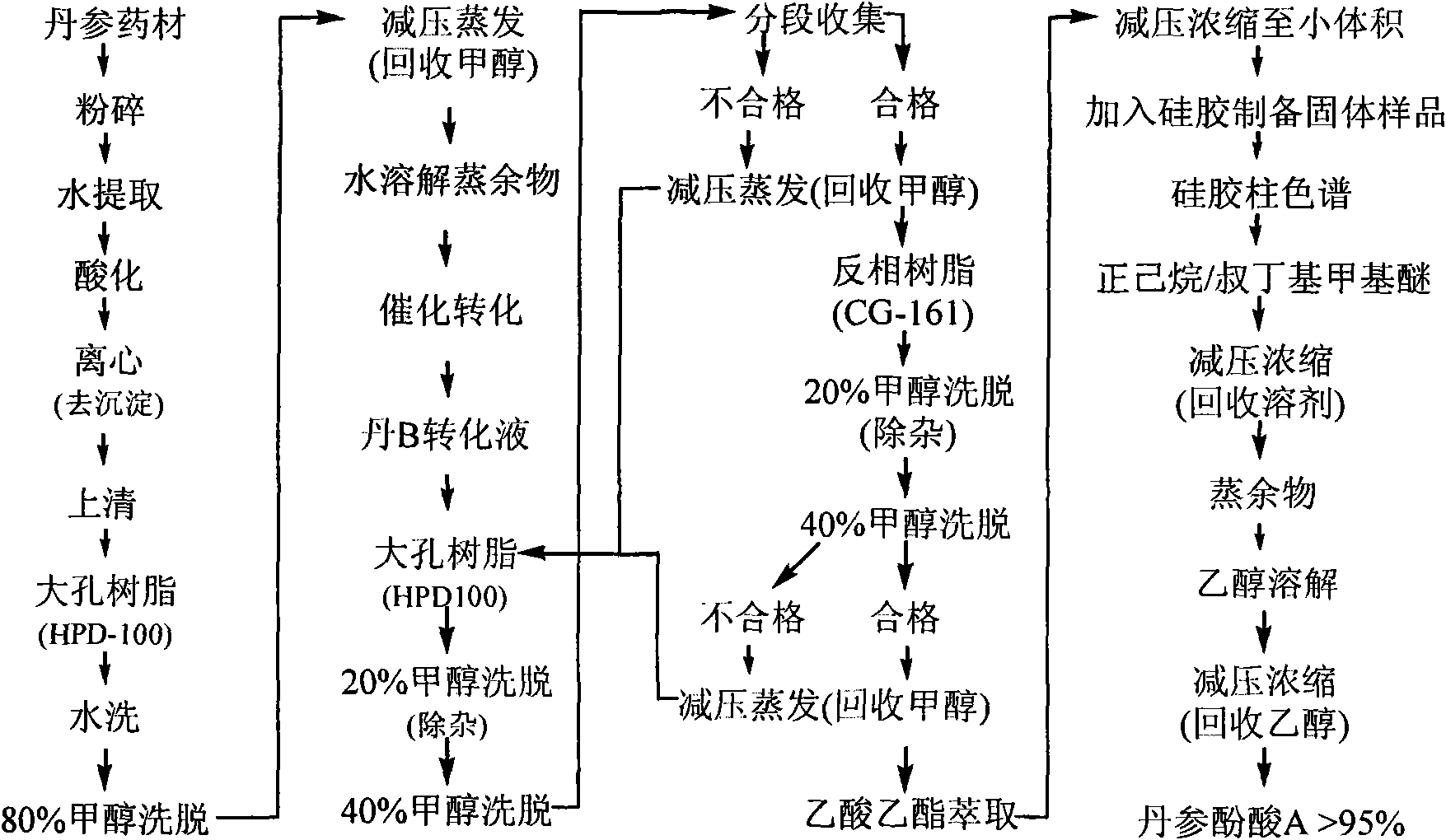
Batch preparation method of high-purity salvianolic acid A
InactiveCN102212002AEfficient separationEfficient removalOrganic compound preparationCarboxylic acid esters preparationSalvianolic acid BChemical composition
The invention relates provides a batch preparation method of high-purity salvianolic acid A based on physical and chemical properties and chromatograph behavior of a chemical component in salvia miltiorrhiza as well as adaptability of salvia miltiorrhiza to a cell separation technology. In the method, a salvianolic acid B transformation liquid containing salvianolic acid A is subjected to three-time chromatograph purification, namely hyphenated chromatography, reversed-phase chromatography and normal-phase silica gel chromatography, wherein the hyphenated chromatography refers to that macroporous resin frontal chromatography and macroporous resin displacement chromatography are jointly used. The product prepared by the method is controllable in quality, is acceptable in cost and can be used for injection medicaments. The content of salvianolic acid A prepared by the method is more than or equal to 98%, the total recovery of salvianolic acid A is more than or equal to 10% (based on salvianolic acid B), and the total yield of salvianolic acid A is more than or equal to 0.5% (based on salvianolic acid B).
Owner:YANTAI TARGET DRUG RES +1
Method for freeze-drying reducing glutathione for injection
ActiveCN102151251AIncrease productionSmall batch-to-batch variancePowder deliveryTripeptide ingredientsFreeze-dryingReduce glutathione
The invention provides a method for freeze-drying reducing glutathione for injection. In the method, after a pre-freezing process, a primary freeze-drying subliming process is performed, wherein the primary freeze-drying subliming process comprises: vacuumizing, raising temperature, raising the temperature of a partition board to -2 to 0 DEG C, keeping temperature for 10 to 20 hours, reducing the temperature of the partition board to -10 to -12 DEG C, keeping the temperature for 10 to 30 hours, and raising the temperature of the partition board to 0 DEG C within 5 to 15 hours. When the primary subliming process is adopted, the freeze-drying period is reduced obviously, and the production cost is reduced.
Owner:SHANDONG LUYE PHARMA CO LTD +1
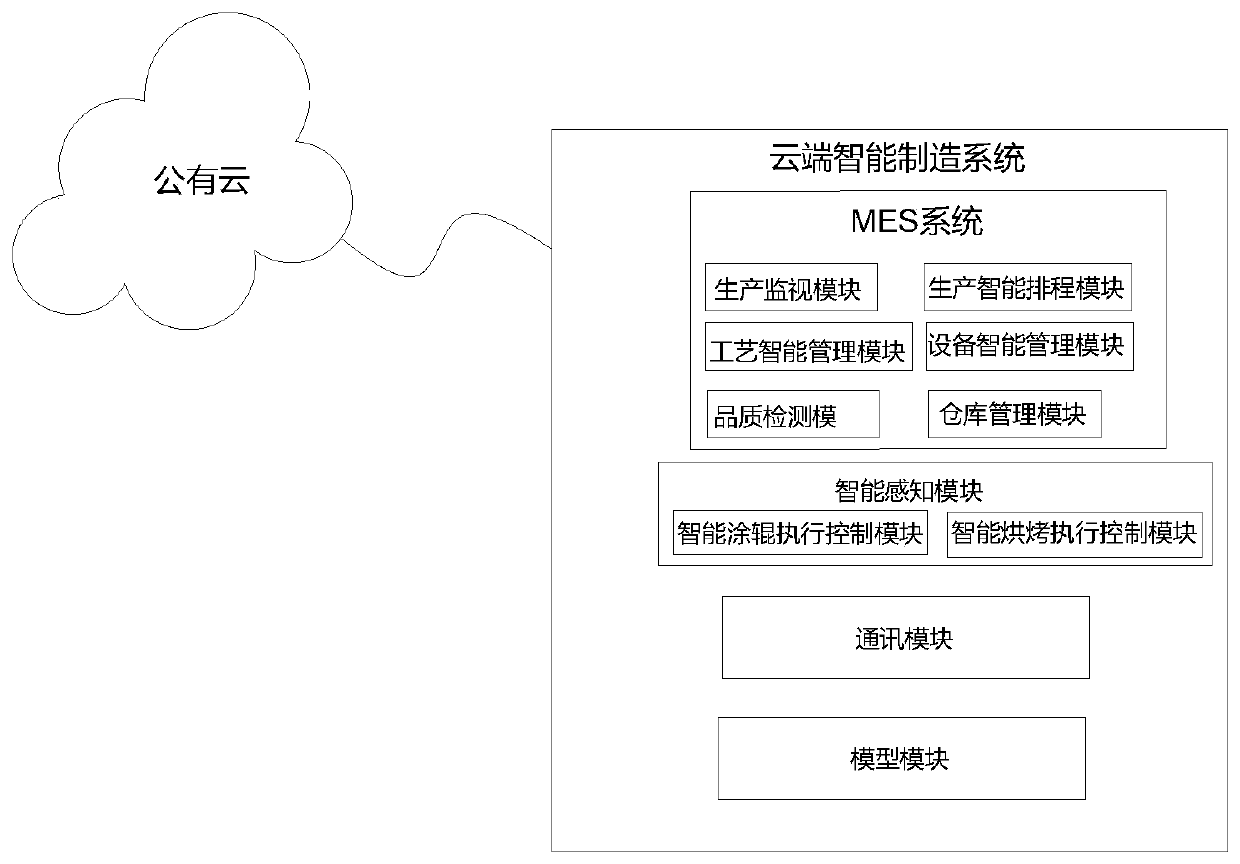
Cloud intelligent manufacturing system based on public cloud
InactiveCN110647126AAvoid job hazardsRealize data interactionTotal factory controlProgramme total factory controlThe InternetData profiling
The invention relates to the technical field of intelligent manufacturing, and specifically relates to a cloud intelligent manufacturing system based on a public cloud. The cloud intelligent manufacturing system includes a MES system that achieves interaction of cloud production data collected by a public cloud with a production system through production modeling and industrial big data analysis,and achieves intelligent control of cloud production; an intelligent perception module that exchanges data with an application module and the MES system through an ethernet communication module of a PLC, receives data instructions transmitted by an OPC for automatic production control and process adjustment while feeding back a status of each device; a communication module that is used to provideinternet services for the intelligent perception module, the application module and a model module to achieve data interaction; and a model module that is used for data interaction with the intelligent perception module, thereby achieving intelligent production.
Owner:任羲
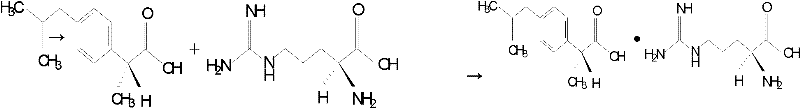
Preparation method of arginine dexibuprofen
ActiveCN102344360AHigh yieldHigh purityOrganic compound preparationMetabolism disorderArginineFiltration
The invention discloses a preparation method of an arginine dexibuprofen raw medicine. The method comprises the steps of: dissolving dexibuprofen in ethanol, heating the mixture to a temperature of 50DEG C-60DEG C, adding L-arginine in a dropwise manner slowly and continuously under stirring within 10min-20min, continuing stirring for reaction for 2h-3.5h with the temperature maintained at 50DEG C-60DEG C, leaving the mixture to stand for 25min-35min and cool to a temperature of 18DEG C-30DEG C, conducting pumping filtration so as to obtain crystals, then washing and drying the crystals, thus obtaining white crystals, i.e. arginine dexibuprofen. The preparation method of the invention has simple process, high yield, no refining step, shortened production period, and no need for any special equipment, thus being suitable for industrial production.
Owner:HAINAN HULUWA PHARMA GRP CO LTD
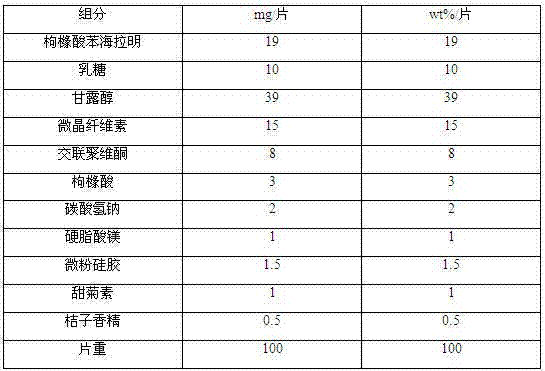
Diphenhydramine citrate orally disintegrating tablet and preparation method thereof
InactiveCN102440973AMeet quality requirementsReasonable prescription designOrganic active ingredientsNervous disorderSodium bicarbonateOrally disintegrating tablet
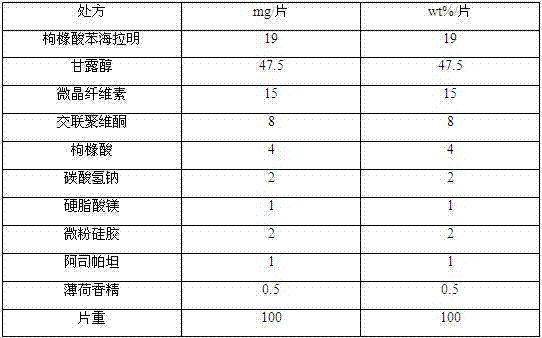
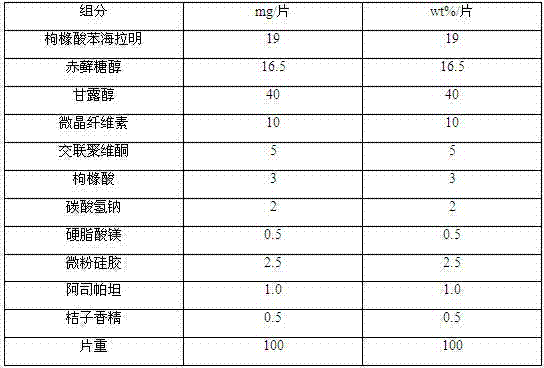
The invention discloses a diphenhydramine citrate orally disintegrating tablet, the prescription is composed by the following components in mass percent: 19% of diphenhydramine citrate, 45 swung dash 58% of filler, 15 swung dash 25% of disintegrating agent, 4 swung dash 8% of effervescent disintegrant, 0.5 swung dash 1% of lubricant, 1 swung dash 3% glidant, 1 swung dash 2% of sweetner, 0.2swung dash 0.6% aromatic and 0 swung dash 0.5% of surfactant, wherein the filler uses mannitol, or mannitol and lactose or erythritol, the disintegrating agent uses any one of polyplasdone, crosslinking sodium carboxy methyl cellulose and low substitution hydroxyl propyl cellulose together with microcrystalline cellulose, the effervescent disintegrant comprises citric acid and sodium bicarbonate which have the mass ratio of 1 swung dash 2 / 1, the lubricant is magnesium stearate, the glidant is aerosil or talcum powder, the sweetner is aspartame or stevia rebaudianum, the aromatic is the pharmaceutically acceptable essence, and the surfactant is lauryl sodium sulfate; and the invention further discloses a preparation method of the orally disintegrating tablet, which is simple to operate, the cost is low, the obtained product is in accordance with the quality requirement of the orally disintegrating tablet, and has attractive appearance, good taste and stable quality.
Owner:SOUTHWEST UNIV
Lithium hydroxide monohydrate preparation system
PendingCN107285345AHigh yieldIndustrial process stabilityLithium oxides/hydroxidesLithium carbonateIon exchange
The present invention discloses a lithium hydroxide monohydrate preparation system, which comprises: a carbonization impurity removing device provided with a crude lithium carbonate inlet, a water inlet, a carbon dioxide inlet, an EDTA inlet and a refined lithium carbonate outlet; a causticization device provided with a refined lithium carbonate inlet, a lime milk inlet and a lithium hydroxide completion liquid outlet; an ion exchange device provided with a lithium hydroxide completion liquid inlet and a refined lithium hydroxide purification liquid outlet; an evaporation crystallization device provided with a refined lithium hydroxide purification liquid inlet and a crude wet lithium hydroxide monohydrate outlet, wherein the refined lithium hydroxide purification liquid inlet is connected to the refined lithium hydroxide purification liquid outlet; and a post-treatment device provided with a crude wet lithium hydroxide monohydrate inlet and a lithium hydroxide monohydrate outlet, wherein the crude wet lithium hydroxide monohydrate inlet is connected to the crude wet lithium hydroxide monohydrate outlet. According to the present invention, with the system, the high-purity lithium hydroxide monohydrate can be prepared by using the industrial-grade or sub-industrial-grade lithium carbonate crude product, the industrial process is stable, the quality of the product is stable and controlled, and the yield is high.
Owner:CHINA ENFI ENGINEERING CORPORATION
Inorganic fullerene molybdenum disulfide/graphene composite lubricant and preparation method thereof
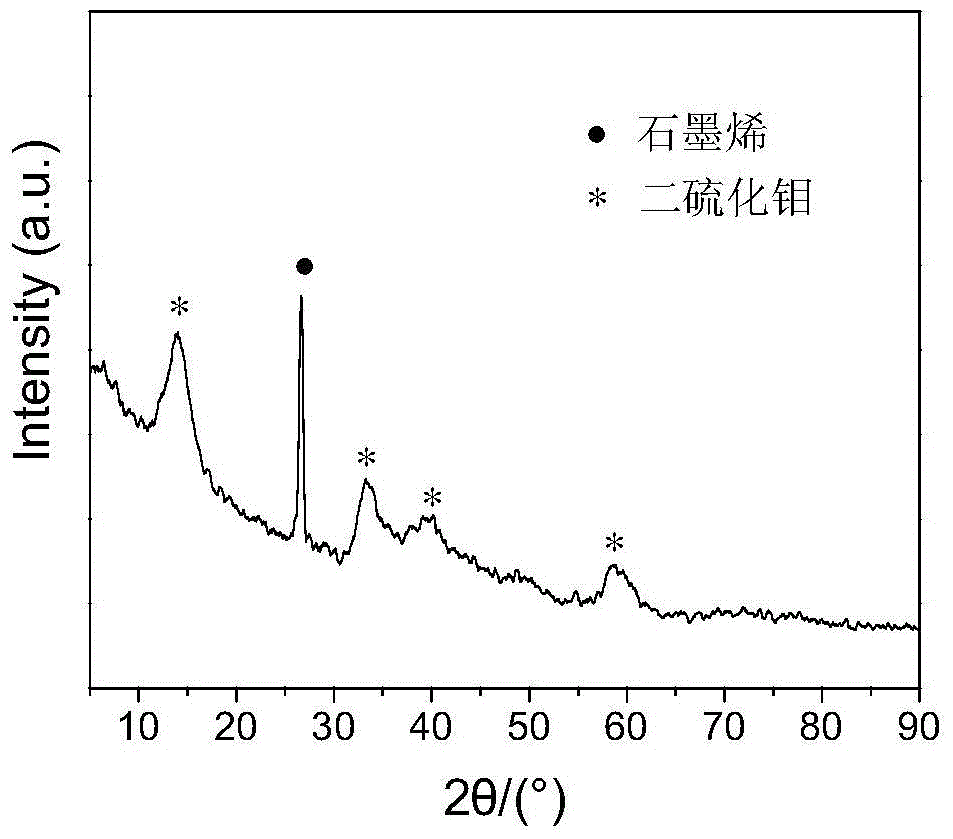
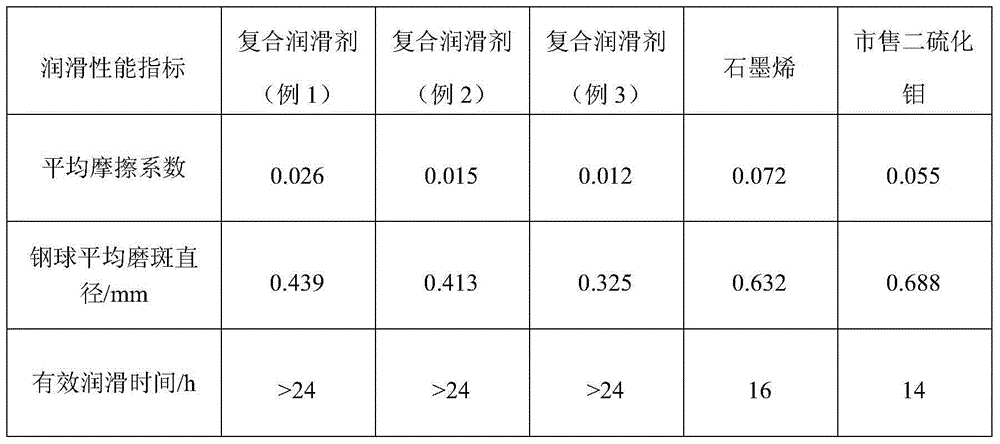
ActiveCN104962340AGuaranteed decentralizationGuarantee the firmness of the bondBase-materialsFullereneCvd graphene
The invention relates to an inorganic fullerene molybdenum disulfide / graphene composite lubricant and a preparation method thereof. The mass ratio of molybdenum disulfide to graphene in the lubricant is 1:(0.0005-0.1), the particle size of the inorganic fullerene molybdenum disulfide is 30-300nm, and the molybdenum disulfide is uniformly dispersed on the graphene surface or in the defect clearances and bonded firmly. The preparation method is convenient and controllable for operation. Under the synergistic actions of the molybdenum disulfide and graphene, the prepared composite lubricant has excellent lubricating property, and has very important application values in the fields of solid lubricating materials, precision machinery, aerospace and the like.
Owner:HEFEI UNIV OF TECH
Intelligent vertical mill control system based on distributed control system (DCS)
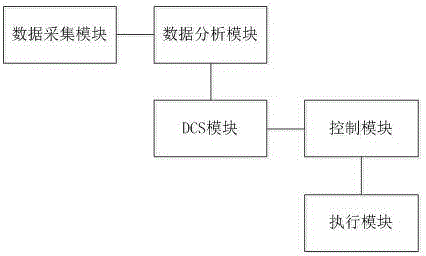
InactiveCN106269198AParameter intelligent adjustment is stable and controllableProduct quality is stable and controllableGrain treatmentsIntelligent control systemConfiguration analysis
The invention discloses an intelligent vertical mill control system based on a distributed control system (DCS). The intelligent vertical mill control system comprises a data collecting module, a data analysis module, the DCS module, a control module and an execution module, wherein the data collecting module is used for collecting the operating parameters of a vertical mill system in real time; the data analysis module is used for receiving the operating parameters, sent by the data analysis module, of the vertical mill system and carrying out configuration analysis and logic operation on the operating parameters of the vertical mill system so that a data analysis result can be obtained; the DCS module is used for receiving the data analysis result and generating a control instruction according to the data analysis result; the control module is used for receiving the control instruction from the DCS module and converting the control instruction to a control signal; and the execution module is used for receiving the control signal from the control module and carrying out corresponding operations according to the control signal. According to the intelligent vertical mill control system, the operating parameters of the vertical mill system are collected in time through a PLC and subjected to data analysis, the DCS module generates the corresponding control instruction, and therefore the vertical mill system is controlled.
Owner:四川亿欣新材料有限公司
Feed additive product with high efficiency
InactiveCN102870966ANo pollution in the processProduct quality is stable and controllableAnimal feeding stuffAnimal scienceFreeze-drying
The invention discloses a feed additive product with high efficiency. The feed additive product comprises the following constituents by weight: 10% to 15% of fructo-oligosaccharide, 10% to 20% of soybean peptide, 15% to 30% of freeze-dried bacillus subtilis powder, 20% to 40% of ganoderma solid fermentation culture, 10% to 15% of active dry yeast and 10% to 20% of freeze-dried lactobacillus rhamnosus powder, wherein the bacillus subtilis is CGMCC6012. With the adoption of the feed additive product with high efficiency provided by the invention, the application amount of the antibiotic drugs in the domesticate animals can be effectively reduced, the safety of the animal meat products is improved, the feed use efficiency of the animals and the feeding profit are improved, and the immunity of the animals is strengthened.
Owner:邵素英
Fingerprint atlas quality investigating method of ginkgo lactone material
InactiveCN1869683AAdd control indicatorsProduct quality is stable and controllableOrganic active ingredientsComponent separationPattern detectionGinkgolide
The invention discloses a fingerprint pattern detection method for gingkgo inner ester raw material, where the fingerprint pattern of ginkgo inner ester raw material has four shared peaks, and area of nonshared peaks not more than 5%, and the ginkgo inner ester raw material which accords with the fingerprint pattern has controllable quality and good stability.
Owner:JIANGSU KANION PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com