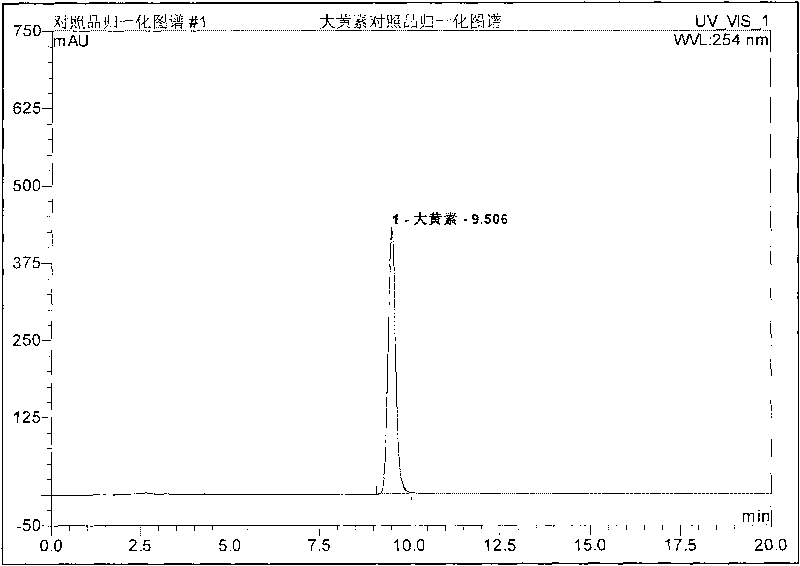
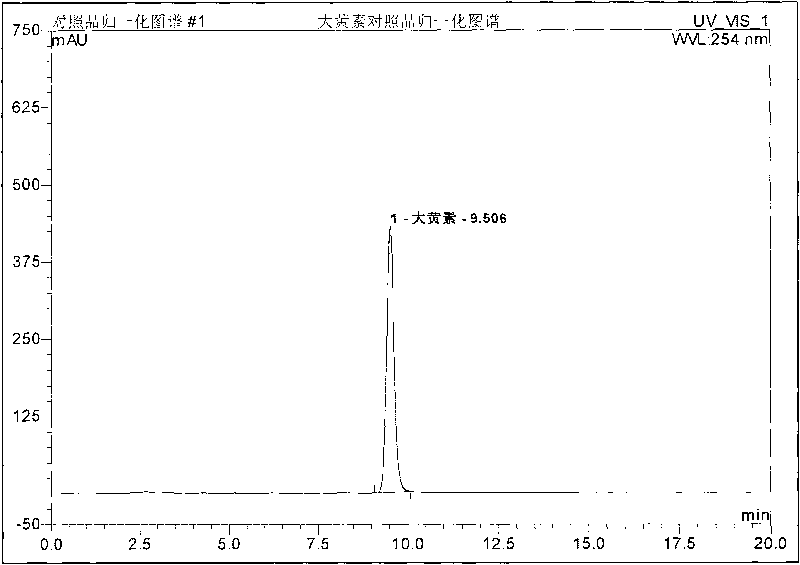
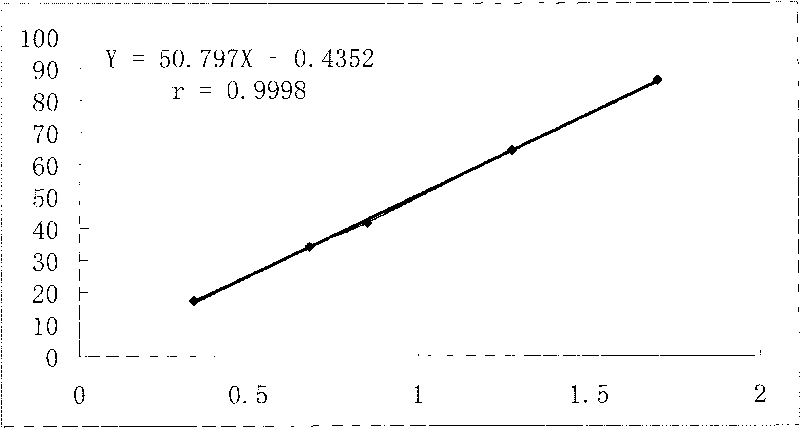Preparation method, quality control method and application for Chinese medicinal compound indigowoad leaf preparation
A quality control method, the technology of Folium Folium, applied in the direction of pharmaceutical formulations, antiviral agents, measuring devices, etc., can solve the problems of ineffective control of the quality of compound Folium Folium preparations, no content determination, and few quality control indicators
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0106] (1) Main drug preparation
[0107] Proportion of main ingredients: Daqingye 360g, Sanyinhua 180g, notopterygium 100g, ginseng 90g, rhubarb 100g.
[0108] The above medicinal materials are decocted twice with a conventional amount of water, 1 hour each time; the decoction liquid is combined, filtered, and the relative density of the filtrate is 1.08-1.32 when the filtrate is concentrated to 60°C; ethanol is added to make the alcohol content reach 50-60%. Stand still, filter, recover ethanol from the filtrate and concentrate to a clear paste with a relative density of 1.17-1.43 at 60°C, and set aside;
[0109] (2) Preparation of oral liquid
[0110] Add 2 to 3 times water to the upward step clear cream, stir well, refrigerate for ≥12 hours, filter, heat and boil the filtrate for 0.5 hours, when the temperature drops to 60°C, add preservatives, refrigerate for ≥12 hours, filter; add flavoring agent and syrup, stir well to obtain the drug stock solution, and set aside;
...
Embodiment 2
[0311] Embodiment 2 prepares granules
[0312] Proportion of main ingredients:
[0313] Daqingye 360g, honeysuckle 180g, notopterygium 90g, boxing ginseng 90g, rhubarb 90g.
[0314] The above medicinal materials are decocted twice with a conventional amount of water, 1 hour each time; the decoction liquid is combined, filtered, and the relative density of the filtrate is 1.08-1.32 when the filtrate is concentrated to 60°C; ethanol is added to make the alcohol content reach 50-60%. Stand still, filter, recover ethanol from the filtrate and concentrate to a clear paste with a relative density of 1.25-1.30 at 60°C, and set aside;
[0315] (2) Preparation of granules
[0316] Shangbuqing Paste is added with appropriate pharmaceutical excipients according to pharmaceutical routines, mixed evenly and made into granules, and dried below 60°C. The above-mentioned dosage can make 1000 parts of granules, which are packed according to the pharmaceutical specifications and sealed.
[0...
Embodiment 3
[0363] Embodiment 3 prepares capsule
[0364] Proportion of main ingredients:
[0365] 300g Daqingye, 200g Shanyinhua, 100g Qianghuo, 100g Quanshen, and 100g Rhubarb.
[0366] The above medicinal materials are decocted twice with a conventional amount of water, 1 hour each time; the decoction liquid is combined, filtered, and the relative density of the filtrate is 1.08-1.32 when the filtrate is concentrated to 60°C; ethanol is added to make the alcohol content reach 50-60%. Stand still, filter, recover ethanol from the filtrate and concentrate to a clear paste with a relative density of 1.25-1.30 at 60°C, and set aside;
[0367] Preparation of capsules
[0368] Shangbu clear paste is vacuum-dried or microwave-dried below 80°C, crushed into fine powder, mixed with suitable pharmaceutical excipients according to pharmaceutical routines, mixed and made into granules, dried, filled in empty capsules, and the above-mentioned dosage can be made into capsules 1000 capsules, packe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Column length | aaaaa | aaaaa |
| The inside diameter of | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com