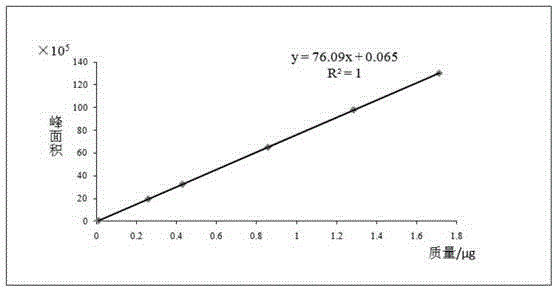
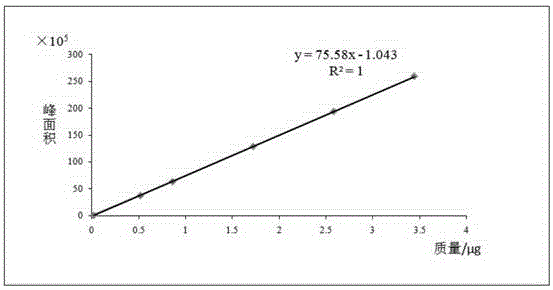
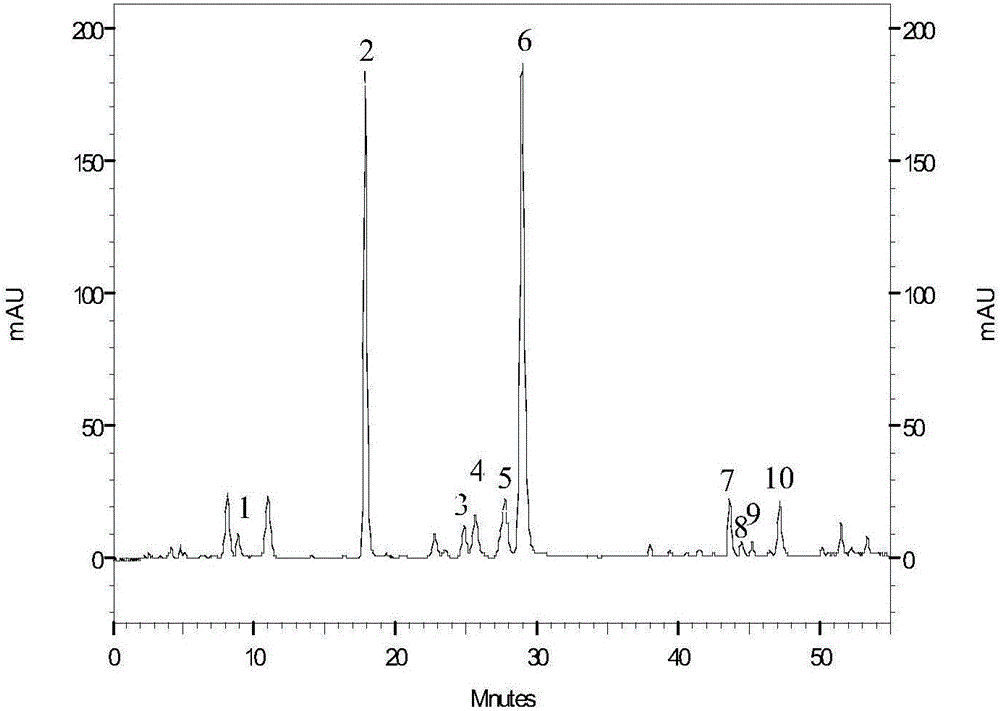
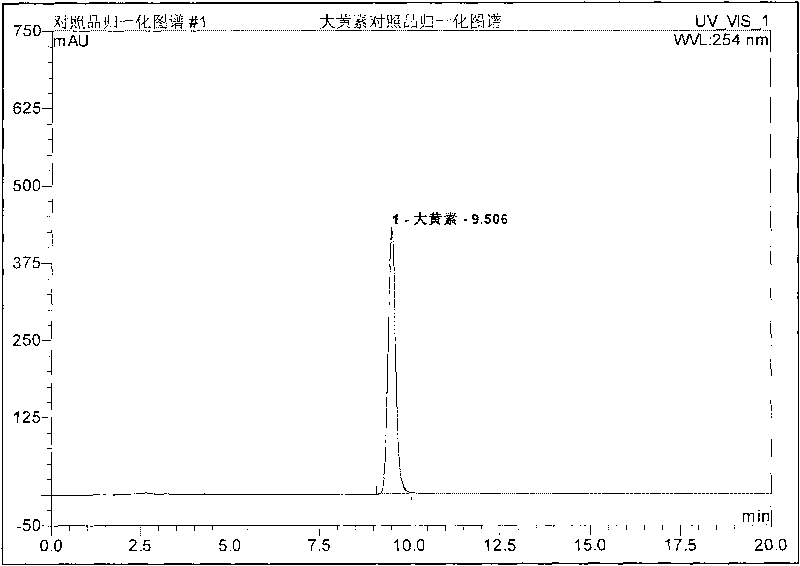
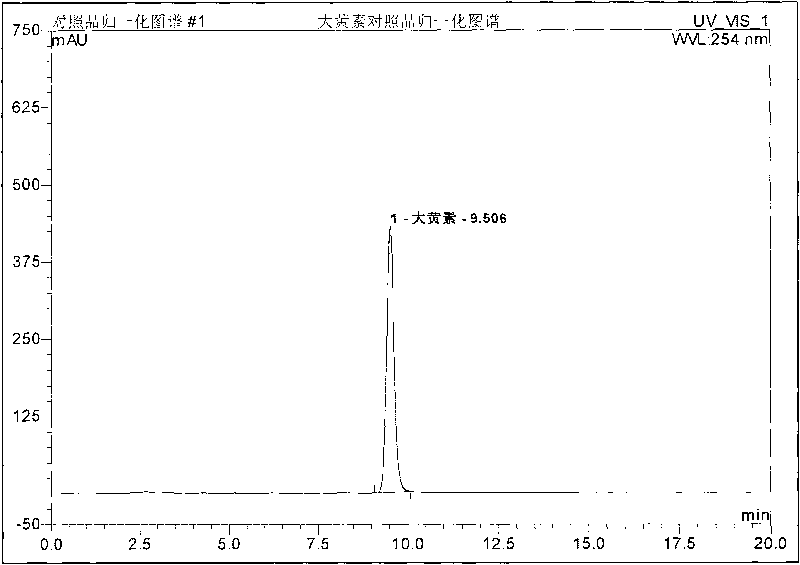
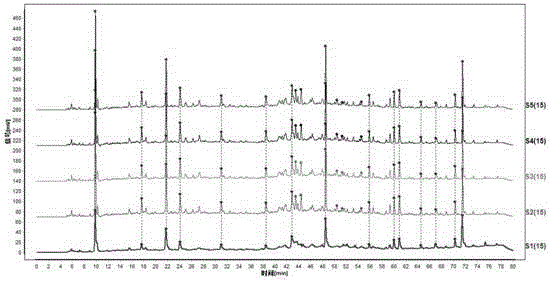
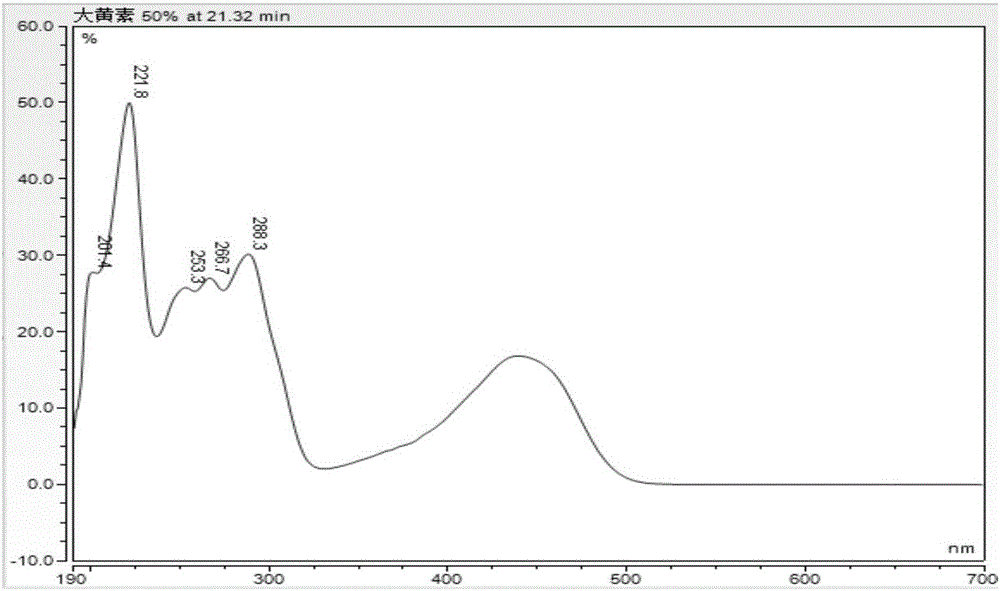
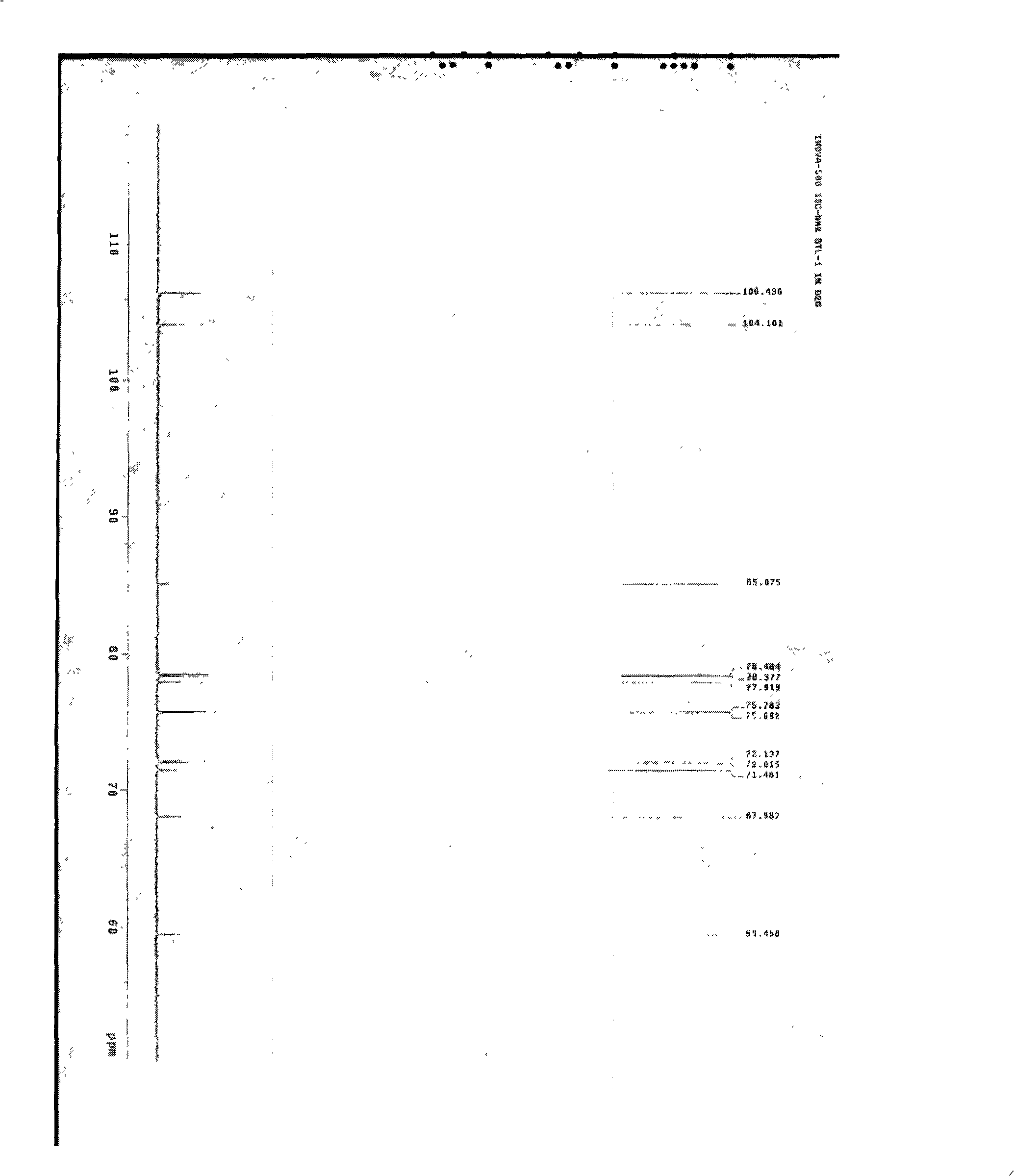
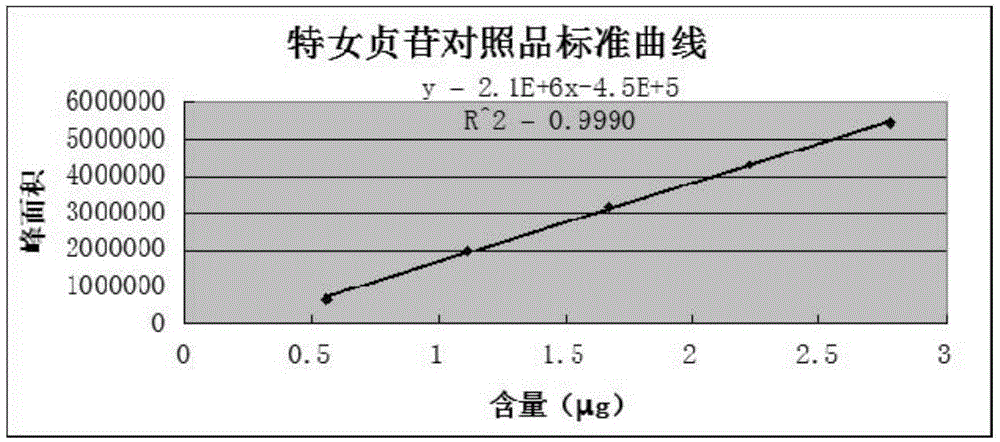
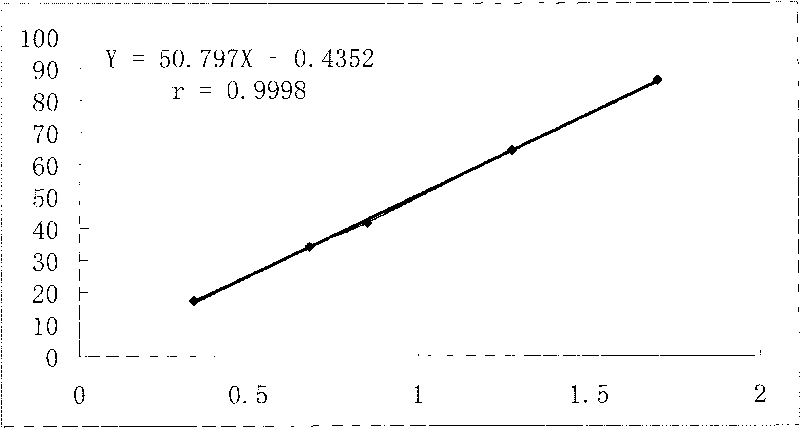Patents
Literature
56results about How to "Perfect quality control method" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for determination of organic acids and flavone components in polygonum viviparum
ActiveCN106525997APerfect quality control methodHigh sensitivityComponent separationGallic acid esterChlorogenic acid
The invention discloses a method for simultaneous determination of organic acids and flavone components in polygonum viviparum, wherein the method comprises the following steps: (1) preparing a test sample solution; (2) detecting with LC-MS / MS; and (3) carrying out component qualitative analysis. On the basis of qualitative analysis of the components of the polygonum viviparum, a reference substance solution is prepared, and the contents of chlorogenic acid and gallic acid in the polygonum viviparum are also determined. 10 kinds of substances in the two active components of the organic acids and the flavones in the polygonum viviparum are determined at the same time for the first time, and a quality control method of the polygonum viviparum medicinal material is improved. The LC-MS / MS analysis detection method is adopted to be combined with a multi-wavelength switching procedure, and the method has the advantages of being simple, stable, fast, high in efficiency, high in sensitivity, good in repeatability and low in interference, can complete the detection of 10 kinds of substances within 1 h, and obtains a chromatogram which can contain multiple pieces of wavelength information at the same time.
Owner:YICHANG SHANCHENGSHUIDU CORDYCEPS
Preparation method, quality control method and application for Chinese medicinal compound indigowoad leaf preparation
ActiveCN101708223AReduce contentMeet the requirements of healthy livingComponent separationAntiviralsAlcohol contentChlorogenic acid
The invention relates to a preparation method and a quality control method for a compound indigowoad leaf preparation. The preparation method comprises a step of preparing a basic remedy and a step of preparing a corresponding preparation. The basic remedy in the basic remedy preparation comprises the following compositions in part by weight: 360 to 440 parts of indigowoad leaves, 180 to 220 parts of lonicera confusa or honeysuckle flower, 90 to 110 parts of incised notopterygium rhizome, 90 to 110 parts of bistort rhizome, and 90 to 110 parts of rhubarb; the medicinal materials are decocted twice with conventional amount of water for 1 hour each time; the decoctions are mixed and filtered; the filtrate is concentrated to the relative density of between 1.08 and 1.32 at 60 DEG C; ethanol is added into the filtrate to ensure that alcohol content reaches 50 to 60 percent, the mixture is stood and is filtered, the filtrate is subjected to ethanol reclamation and is concentrated to an extract with the relative density of between 1.17 and 1.43 at 60 DEG C for later use; and the corresponding preparation is prepared according to a drug specification. The quality control method comprises the following steps of the identification of contents and the content determination of the contained compositions including the identification of the indigowoad leaves, the identification of the lonicera confusa or honeysuckle flower, the identification of the rhubarb, the identification of the incised notopterygium rhizome, the total content determination of emodin and chrysophanol in the rhubarb, and the content determination of chlorogenic acid. The methods can be applied to preparation of medicinal preparations for treating cold, influenza, parotitis and acute viral hepatitis.
Owner:RONGCHANG PHARM ZIBO CO LTD
Quality control method of peach kernel qi activating decoction composition
ActiveCN106483227AAvoid product qualityEasy to operateComponent separationPhosphoric acidColumn temperature
The invention discloses a quality control method of a peach kernel qi activating decoction composition and belongs to the field of traditional Chinese medicine analysis. The method includes the steps that a peach kernel qi activating decoction fingerprint spectrum is established through high performance liquid chromatography, and chromatographic conditions are characterized in that a chromatographic column adopts octadecylsilane chemically bonded silica as filler; an ultraviolet detector is adopted as a detection instrument, and the detection wavelength of the fingerprint spectrum ranges from 210 nm to 250 nm; the flow rate ranges from 0.9 ml / ml to 1.1 ml / ml, the column temperature ranges from 25 DEG C to 35 DEG C, the detection wavelength ranges from 210 nm to 250 nm, the number of theoretical plates should not be smaller than 3,000 according to calculation of catechinic acid peaks, methyl alcohol serves as a moving phase A, a 0.1% phosphoric acid solution serves as a moving phase B, and gradient elution is carried out according to the following sequence: the gradient condition of elution detection of the fingerprint spectrum is defined in the specification. The fingerprint spectrum comprehensively reflects quality information of a peach kernel qi activating decoction, and therefore the quality of the peach kernel qi activating decoction can be more comprehensively and effectively controlled.
Owner:CHINA RESOURCES SANJIU MEDICAL & PHARMA +1
Construction method of HPLC (High Performance Liquid Chromatography) fingerprint spectrum of salvia miltiorrhiza and radix puerariae depression relieving drug
The invention provides a construction method of an HPLC (High Performance Liquid Chromatography) fingerprint spectrum of a salvia miltiorrhiza and radix puerariae depression relieving drug. The method comprises the steps of preparing a sample solution, preparing a reference substance solution, preparing efficient liquid chromatogram condition, and detecting to obtain the HPLC fingerprint spectrum of the salvia miltiorrhiza and radix puerariae depression relieving drug; 17 characteristic peaks are detected from the HPLC fingerprint spectrum of the salvia miltiorrhiza and radix puerariae depression relieving drug, including 14 characteristic peaks of salvia miltiorrhiza, and 3 characteristic peaks of radix puerariae. According to the construction method, the HPLC-DAD-TOF / MS serial connecting technology is carried out to point out each chromatographic peak of the HPLC fingerprint spectrum of the salvia miltiorrhiza and radix puerariae depression relieving drug; meanwhile, the one-testing and multi-evaluation method is carried out to detect the content of main effective ingredients of the salvia miltiorrhiza and radix puerariae depression relieving drug, as well as detecting the content limit standard; therefore, the construction method of the fingerprint spectrum of the salvia miltiorrhiza and radix puerariae depression relieving drug has the advantages that the qualitative perforamnce and quantification performance are combined to construct a multi-information fingerprint spectrum with clear spectrum, clear ingredients and controllable content, thus the quality of the salvia miltiorrhiza and radix puerariae depression relieving drug can be comprehensively reflected, and the quality control method of the salvia miltiorrhiza and radix puerariae depression relieving drug can be effectively improved.
Owner:LIAONING UNIV OF TRADITIONAL CHINESE MEDICINE
Method for detecting cerebroprotein hydrolysate tablets
ActiveCN102305831ARich detection methodsPerfect quality control methodComponent separationHydrolysateAcid hydrolysis
The invention provides a method for detecting cerebroprotein hydrolysate tablets. The method comprises the following steps of identifying the micromolecule peptides in the cerebroprotein hydrolysate tablets, determining the content and the like. The total amount of amino acid in the cerebroprotein hydrolysate tablets is determined by performing complete acid hydrolysis on a sample, the content ofthe free amino acid contained in the cerebroprotein hydrolysate tablets is determined, the reasonable quality control index aiming at micromolecule peptides is set, the method for detecting the cerebroprotein hydrolysate tablets is improved, and a complete and accurate quality control method is provided from the cerebroprotein hydrolysate tablets. The content controllability of active ingredientsin a product is guaranteed, and components of a preparation reach the effective dose range, so that the curative effect is guaranteed. The method is suitable for the quality control of industrial production and has great application value.
Owner:SHANGHAI ZHONGHUA PHARMA
Discrimination method of traditional Chinese medicine Xuezhining pill
ActiveCN101653496AImprove detection abilityEasy to observe and identifyComponent separationMetabolism disorderSemenEthyl acetate
The invention relates to a discrimination method of traditional Chinese medicine Xuezhining pill; wherein discrimination of anthraquinones active ingredients in the Xuezhining pill and / or discrimination of nunciferine in Xuezhining pill all adopt thin-layer chromatography. Chrysophanol and physcion thin-layer chromatography in the discrimination method adopts acid hydrolysis method, thus greatly improving thin-layer spot detection effect, being convenient for observation and discrimination and improving discrimination efficiency; lotus leaf thin-layer chromatography in the discrimination method adopts alkalinity developing solvent cyclohexane-ethyl acetate-concentrated ammonium liquid, developing speed can be effectively improved, and discrimination speed of the whole Xuezhining pill can be improved. Repetitive operation of hawthorn thin-layer discrimination method is cancelled in the discrimination method, so that the whole quality control method is optimized, quality control time isshortened, and quality control efficiency is improved. The discrimination method of the invention is simple and easy to operate, eliminates the interference in discrimination as prepared polygonum multiflorum and semen cassiae all contain anthraquinones and is rapid and accurate for discrimination of traditional Chinese medicine Xuezhining pill.
Owner:津药达仁堂集团股份有限公司达仁堂制药厂
Detection method of compound lonicera granules
ActiveCN106290646AEfficient separationPerfect quality control methodComponent separationDrugPinoresinol
The invention belongs to analysis and detection of drugs, and particularly relates to a method for simultaneously detecting various effective components in compound lonicera granules with high performance liquid chromatography. The method is used for simultaneously detecting neochlorogenic acid, chlorogenic acid, cryptochlorogenic acid, forsythiaside A, forsythin, pinoresinol, baicalin and wogonin in the compound lonicera granules with the high performance liquid chromatography and comprises steps as follows: preparation of a reference substance solution, preparation of a solution of a testing product, determination, result calculation and the like. The method effectively solves the problems that quality evaluation of the compound lonicera granules is mainly about single-component content determination and the like in the prior art, and has the advantages of comprehensively characterizing active pharmaceutical ingredients of the compound lonicera granules and being high in repeatability and good in precision and the like.
Owner:HEBEI GOGIN PHARMA
Quality control method of jichuan decoction composition
ActiveCN108459090AAvoid product qualityEasy to operateComponent separationPhosphoric acidGradient elution
The invention discloses a quality control method of a jichuan decoction composition, wherein the method belongs to the field of traditional Chinese medicine analysis. The method comprises establishinga jichuan decoction composition fingerprint atlas according to high-efficiency liquid chromatography. Chromatogram conditions are characterized in that a chromatographic column is an octadecylsilanechemically bonded silica chromatographic column; the detecting wavelength is 320nm; the flow velocity is 0.9-1.1ml / min; the column temperature is 25-35 DEG C; a theoretical plate number is not lower than 5000 when calculated according to a ferulic acid peak; acetonitrile (A)-0.1% phosphoric acid solution (B) is used as a mobile phone; and gradient elution is performed according to a sequence whichis shown in the description. Therefore the quality control method realizes a purpose of more comprehensively and effectively controlling the quality of a jichuan decoction composition product.
Owner:CHINA RESOURCES SANJIU MEDICAL & PHARMA
Method for controlling quality of Resina Draconis drug
ActiveCN101780189AMass responseFully reflect the qualityComponent separationColor/spectral properties measurementsDrugFingerprint
The invention provides a method for controlling the quality of Resina Draconis drug. The method can be one or more of the following four methods for controlling the quality: (1) determining the content of total phenols; (2) determining the content of 7,4'-dihydroxyflavone; (3) determining the content of loureirin B; and (4) detecting high performance liquid chromatography fingerprint. The invention provides an accurate and precise method for controlling the quality with good stability, which can effectively control the quality of the Resina Draconis drug.
Owner:JIANGSU KANION PHARMA CO LTD
Quality control method of traditional Chinese medicine compound preparation for treating osteoporosis
ActiveCN104391072AAchieve visualizationEnhanced informationComponent separationGlucosidePinoresinol diglucoside
The invention discloses a quality control method of a traditional Chinese medicine compound preparation for treating osteoporosis. The quality control method comprises the following steps: (1) by taking eucommia ulmoides as a contrast medicinal material and taking pinoresinol diglucoside and geniposidic acid as comparison products, carrying out qualitative identification on eucommia ulmoides in the traditional Chinese medicine compound preparation by adopting a thin-layer chromatography; (2) by taking radix achyranthis bidentatae as a contrast medicinal material and taking beta-ecdysterone as a comparison product, carrying out qualitative identification on radix achyranthis bidentatae in the traditional Chinese medicine compound preparation by adopting the thin-layer chromatography; (3) by taking wolfberry fruits as contrast medicinal materials, carrying out qualitative identification on the wolfberry fruits in the traditional Chinese medicine compound preparation by adopting the thin-layer chromatography; and (4) determining the content of three active components (or index components) including epimedium glucoside, gentiopicroside and loganin acid in the traditional Chinese medicine compound preparation by adopting a high performance liquid chromatography. According to the quality control method, the quality of the traditional Chinese medicine compound preparation can be controlled very well, and the stability of a product production process is monitored; and the quality of the traditional Chinese medicine compound preparation is stable, uniform and controllable.
Owner:GUANGZHOU BAIYUNSHAN JINGXIUTANG PHARM CO LTD
Method for detecting liver-enhancing medicine
ActiveCN102539599AEfficient separationPerfect quality control methodComponent separationSalvianolic acid BColumn temperature
The invention provides a method for detecting a liver-enhancing medicine. The liver-enhancing medicine is composed of oriental wormwood, isatis root, angelica, white paeony root, danshen root, Radix curcumae, Astragalus mongholicus, Codonopsis pilosula, Rhizoma alismatis, sealwort, rehmannia, yam, hawthorn, large-leaved gentian, liquorice and medicated leaven. The detecting method comprises the step of detecting paeoniflorin, gentiamarin and salvianolic acid B in the liver-enhancing medicine by using a high efficiency liquid chromatography method, wherein conditions are as follows: a chromatographic column takes octadecylsilane chemically bonded silica as a filling material; mobile phases comprise a mobile phase A which is acetonitrile and a mobile phase B which is an acidic water solution, and the mobile phases are subjected to gradient elution; the flow velocity is 1.0mL / min; the column temperature is 30 DEG C; the detection wavelength is 210nm to 400nm; and the number of theoretical plates is calculated according to the paeoniflorin peak and should not be less than 6000. According to the detecting method provided by the invention, the content of active ingredients of the paeoniflorin, the gentiamarin and the salvianolic acid B in the liver-enhancing medicine can be detected at the same time, so that the active ingredients of the liver-enhancing medicine can be comprehensively represented, and the method has the characteristics of high precision, high stability and high repeatability.
Owner:SHIJIAZHUANG DONGFANG PHARMA
Fluid-increasing decoction formula granules and preparation method, application and detection method thereof
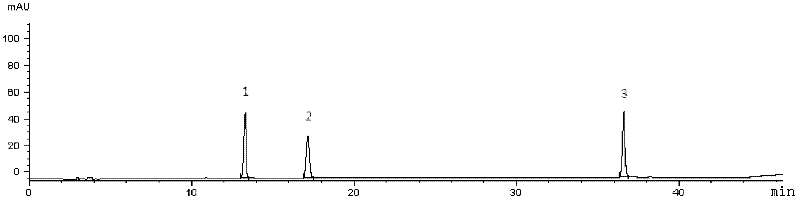
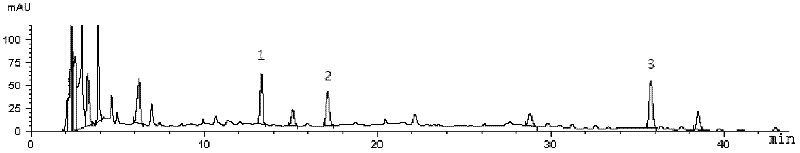
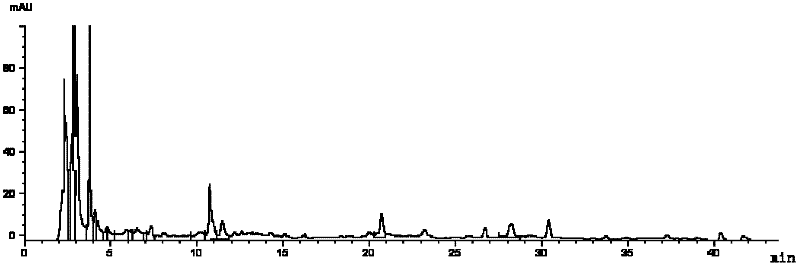
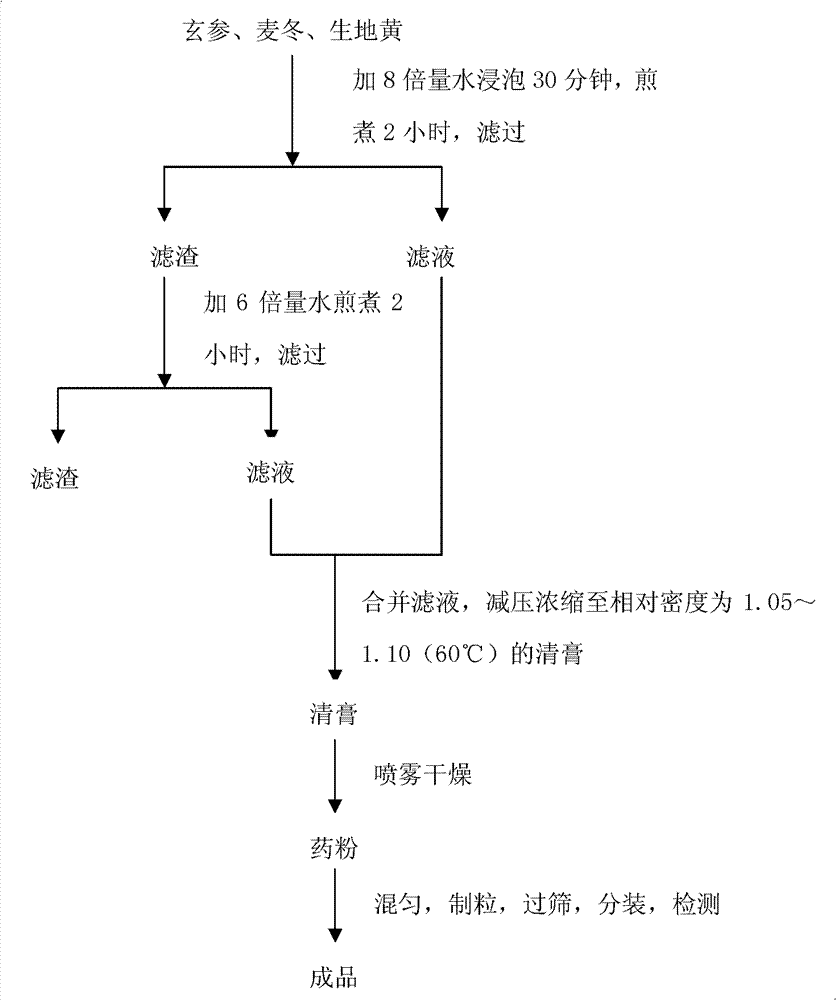
ActiveCN103169864AOvercome the disadvantage of lack of co-fryingPerfect quality control methodSenses disorderComponent separationMedicineRadix Ophiopogonis
The invention provides fluid-increasing decoction formula granules. The fluid-increasing decoction formula granules contain extracts prepared by the step of decocting radix scrophulariae, radix ophiopogonis and radix rehmanniae recen. The invention also provides a preparation method, application and a detection method of the fluid-increasing decoction formula granules. According to the fluid-increasing decoction formula granules, the defect that various herbal medicines are simply added when the conventional single-component formula granules are taken is overcome, the compatibility advantages of traditional Chinese medicine are fully exerted, the holistic thinking of traditional Chinese medicine is reflected, the aim of reducing toxicity and enhancing efficacy is ensured, the fluid-increasing decoction formula granules are quasi steady and controllable in quality and convenient to store and carry by patients, and the decoction trouble is avoided. The preparation method of the fluid-increasing decoction formula granules is simple, the herbal medicines are decocted together according to the traditional method, and the characteristics of the traditional Chinese medicine are met. According to the detection method of the fluid-increasing decoction formula granules, the perfect quality standard of the fluid-increasing decoction formula granules is established, and the quality of the formula granules can be effectively controlled.
Owner:KANGMEI PHARMA +1
Quality control method of danggui sini decoction composition
ActiveCN108459128AEffective controlEasy to operateComponent separationPhosphoric acidGradient elution
The invention discloses a quality control method of a danggui sini decoction composition, wherein the method belongs to the field of traditional Chinese medicine analysis. According to the method of the invention, a fingerprint spectrum is established by means of high-efficiency liquid chromatography; a chromatographic column is a octadecylsilane chemically bonded silica chromatographic column; the column temperature is 25-35 DEG C; the flow velocity is 0.9-1.1ml / min; the detecting wavelength is 220nm; acetonitrile (A)-0.1% phosphoric acid solution (B) is used as a mobile phase, and gradient elution is performed according to a sequence which is represented in the description. The fingerprint spectrum according to the invention comprehensively reflects quality information of the danggui sini decoction composition, thereby realizing a purpose of more comprehensively and effectively controlling the quality of the danggui sini decoction composition.
Owner:CHINA RESOURCES SANJIU MEDICAL & PHARMA +1
New compound, preparation method and application thereof
ActiveCN101293904AImprove immunityRaise countOrganic active ingredientsSugar derivativesBULK ACTIVE INGREDIENTActive ingredient
The invention discloses a novel compound and a preparation method thereof, as well as a pharmaceutical composition with the compound as an active ingredient and the effects and the uses thereof. The inventive compound and the pharmaceutical composition with the compound as the active ingredient can be used for treating tumors, relieving tumor pain, and enhancing immunity.
Owner:BEIJING ZHENDONG GUANGMING PHARMA RES INST +1
Method for simultaneously determining content of three components for traditional Chinese medicine phyllanthus emblica through whole-time three-wavelength fusion
ActiveCN102818862APerfect quality control methodInformativeComponent separationInformation processingDrug development
The invention discloses a method for simultaneously determining content of three components for traditional Chinese medicine phyllanthus emblica through whole-time three-wavelength fusion. According to the method, a modern analysis detection method and an information processing method are combined, spectrogram information of marker components of Chinese herbal medicines is effectively fused, determined components are the largest ultraviolet absorption wavelengths, the signal to noise ratio of the determined components is increased, the defect of insufficient information amount of single wavelength and index detection can be overcome, and internal quality of Chinese herbal medicines can be reflected perfectly. The quality control method of the three components is simple in operation, stable and reliable, good in reproducibility, and capable of being applied to quality evaluation and control of Chinese herbal medicines and new drug development, particularly serving as one of indexes of quality control and identification for the traditional Chinese medicine phyllanthus emblica.
Owner:LIAONING UNIV OF TRADITIONAL CHINESE MEDICINE
Quality control method of children lung clearing pills serving as Chinese medicinal preparation
ActiveCN102048864ARaise quality standardsAdd microscopic identification methodComponent separationInorganic active ingredientsInulaThin-layer chromatography
The invention relates to a quality control method of children lung clearing pills serving as a Chinese medicinal preparation. The method comprises the steps of: firstly, performing microscopical identification; secondly, identifying whether a prescription of the children lung clearing pills comprises platycodon root, pummelo peel, bitter orange, whiteflower hogfennel root and white mulberry root-bark or not by a thin layer chromatography; and lastly, detecting the baical skullcap root content of the prescription of the children lung clearing pills by taking baicalin as a comparison substance by a liquid chromatography. The quality standard of the children lung clearing pills is enhanced and perfected; based on the original standard, a microscopical identification method is provided for liquoric root, inula flower, baical skullcap root, radish seed and perilla fruit; a thin-layer identification method for whiteflower hogfennel root, white mulberry root-bark, platycodon root, bitter orange and pummelo peel in the prescription is drawn up; and a content test method for the baical skullcap root in the prescription is set up by taking baicalin as a comparison substance. By a modified quality standard, the quality control method of a product is enhanced, and counterfeit medicaments and substandard medicaments can be inspected more correctly; and the standard plays an important role in the modernization and overseas development of traditional Chinese medicines.
Owner:津药达仁堂集团股份有限公司达仁堂制药厂
Whole-time three-wavelength fusion method for simultaneously determining contents of four ingredients in Flos Carthami
ActiveCN102692461APerfect quality control methodInformativeComponent separationMedicinal herbsUv absorbance
The invention discloses a whole-time three-wavelength fusion method for simultaneously determining contents of four ingredients in Flos Carthami. The method combines modern analysis detection means and information processing means, and effectively fuses spectral information of index ingredients of Chinese medicinal materials. The detected ingredients all have maximum ultraviolet absorption wavelength, to improve signal-to-noise ratio of detected ingredients. The method can overcome defect of insufficient information amount in single-wavelength single-index detection, to perfectly reflect inherent quality of Chinese medicinal materials. The inventive quality control method of four ingredients is simple in operation, high in stability and reliability, and good in repeatability; and can be used in quality evaluation and control of Chinese medicinal materials, and research and development of new Chinese medicines; and especially can be used as one index for quality control and authentication of Flos Carthami.
Owner:LIAONING UNIV OF TRADITIONAL CHINESE MEDICINE
Quality control method for pharmaceutic preparation containing sanguis draconis extract
ActiveCN101780190AMass responseFully reflect the qualityComponent separationColor/spectral properties measurementsQuality controlDissolution
The invention provides a quality control method for pharmaceutic preparation containing sanguis draconis extract, which can adopt any one or more of the four quality control methods: (1) adopting an ultraviolet-visible spectrophotometry to measure the content of 7,4'-dyhydroxyl flavone; (2) adopting a high performance liquid chromatography to measure the content of lourerin B; (3) adopting the high performance liquid chromatography to detect finger-print; and (4) adopting the high performance liquid chromatography to detect dissolution by combining with a dissolution measurement method. The quality control method provided by the invention has good accuracy, precision and stability, and can effectively control the quality of pharmaceutic preparation containing the sanguis draconis extract.
Owner:JIANGSU KANION PHARMA CO LTD
Method for preparing ligustrum lucidum ait
InactiveCN105380997AGood inheritanceImprove developmentAntinoxious agentsPlant ingredientsEconomic benefitsHigh pressure
The invention provides a method for preparing ligustrum lucidum ait. The method comprises the following steps: weighing the raw medicinal material of ligustrum lucidum ait, and removing impurities and stems; washing, drying, and screening dust; uniformly stirring with black bean juice; braising until the ligustrum lucidum ait is soft; and steaming at high pressure, and drying. The invention further provides a ligustrum lucidum ait preparation prepared by the method. By adopting the method, the time for preparing the medicinal material can be effectively shortened, the economic benefit can be increased, and the production efficiency of enterprises can be improved; and the medicinal material prepared by the method has stable content and excellent quality.
Owner:CHENGDU UNIV OF TRADITIONAL CHINESE MEDICINE
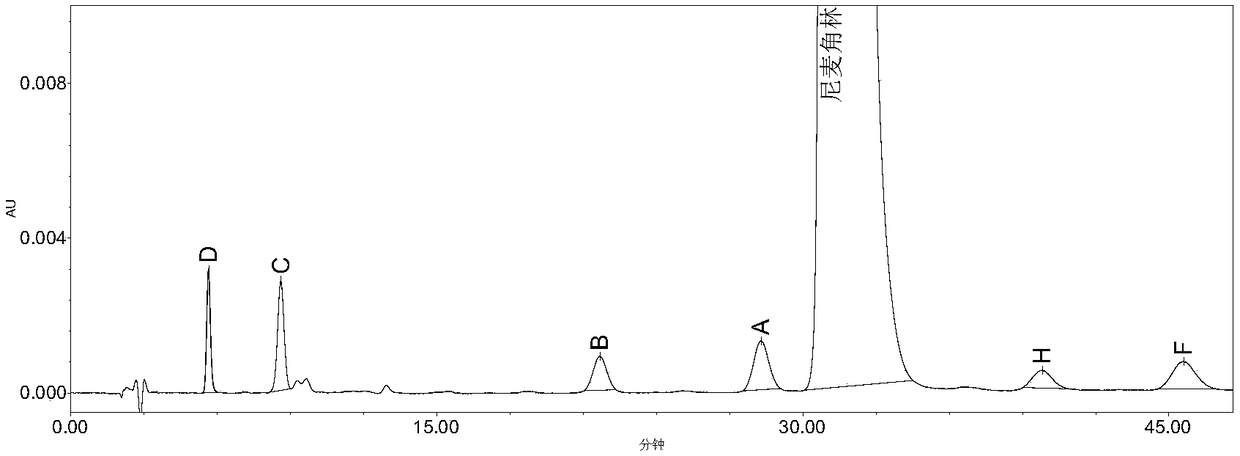
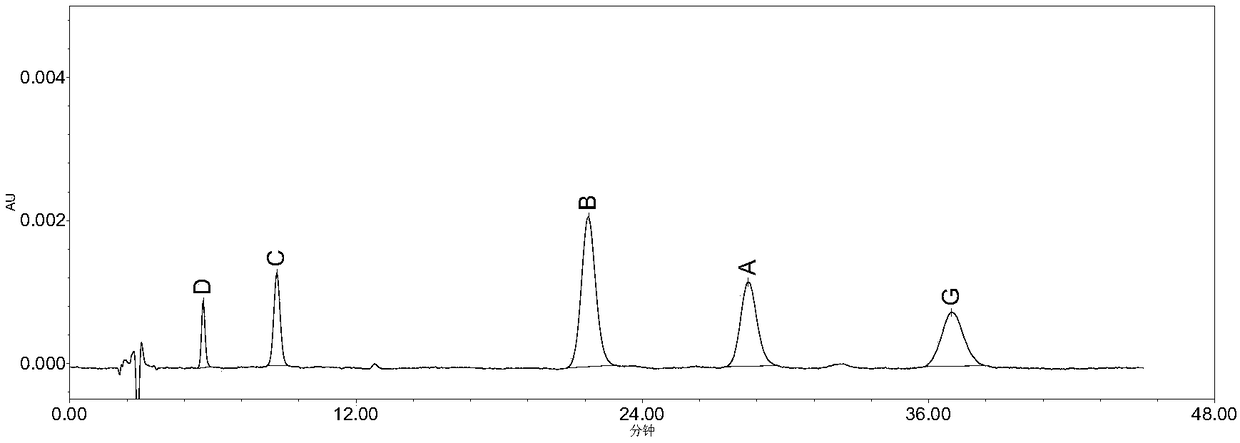
High performance liquid chromatography method for determining nicergoline related substances
ActiveCN108593818AReliable Quality Control MethodsStable quality control methodComponent separationIsocratic elutionColumn temperature
The invention belongs to the field of medicine analysis, and particularly relates to a high performance liquid chromatography method for determining nicergoline related substances. According to the high performance liquid chromatography method, a filler obtained by mixing strong cation exchange resin and a reversed phase C18 according to a volume ratio of 1 to 4 is used as a filling agent, acetonitrile is used as a mobile phase A, a phosphate buffer is used as a mobile phase B, the mobile phase A and the mobile phase B are mixed according to a ratio of 30 to 70 for isocratic elution, the detection wavelength is 288nm, and the column temperature is 30 DEG C. By the high performance liquid chromatography method, the content of an impurity D can be accurately determined and known impurities C, B, A, G, F and H and other unknown impurities in nicergoline can be simultaneously determined.
Owner:CHONGQING INST FOR FOOD & DRUG CONTROL
Quality control method of compound Chinese lobelia oral preparation and use thereof
InactiveCN101480440APerfect quality control methodQuality improvementComponent separationRespiratory disorderCoumaric acidP-Coumaric acid
The invention relates to a mass control method of a traditional Chinese medicine compound Chinese Lobelia oral formulation and the application thereof. Based on the mass control standard of compound Chinese Lobelia injecta, the content of coumaric acid in Chinese Lobelia, herba scutellariae barbatae and herba oldenlandiae is additionally measured to synthetically control the prescription. The invention improves the mass control method of compound Chinese Lobelia oral formulation, more effectively controls the quality of the compound Chinese Lobelia oral formulation, and ensures the safety of people taking the compound Chinese Lobelia oral formulation.
Owner:FUREN PHARMA GROUP
2-ethyl-1,3-hexanediol purifying process and related substance detecting method
ActiveCN105418377AReduce generationQuality assuranceOrganic compound preparationComponent separationImpurityCalcium hydride
The invention belongs to the technical field of medicine, and particularly relates to a 2-ethyl-1,3-hexanediol purifying process and a related substance detecting method thereof. The purifying process is a calcium hydride reducing process. The detecting method comprises the following steps that 1, a system suitability solution is prepared, wherein an impurity1, an impurity 2, an impurity 3 and 2-ethyl-1,3-hexanediol are taken to be prepared into the system suitability solution; 2, a test solution is prepared, wherein 2-ethyl-1,3-hexanediol is taken to be prepared into the test solution; 3, a contrast solution is prepared, wherein the test solution is taken to be prepared into the contrast solution; , the system suitability solution, the contrast solution and the test solution are injected into a gaschromatograph, and a chromatogram is obtained; 5, according to the chromatogram of the system suitability solution, the contrast solution and the test solution, the content of know impurities, unknown impurities and total impurities in the test solution is obtained through calculation.
Owner:TIANJIN CHASE SUN PHARM CO LTD
Quality control method for artemisia rupestris herb extract
ActiveCN106769964AQuality improvementControl contentColor/spectral properties measurementsMedicineRepeatability
The invention discloses a quality control method for an artemisia rupestris herb extract. The method is characterized in that the content of general flavone in the artemisia rupestris herb extract is determined by taking linarin as a reference substance, and by using ultraviolet spectrophotometry. High performance liquid chromatography is utilized to assaying the content of the linarin. The quality control method provided by the invention is good in accuracy, stability and repeatability, the quality of the artemisia rupestris herb extract can be controlled effectively, and the quality control method can be applied to massive industrial production.
Owner:XINJIANG YINDUOLAN UIGHUR MEDICINE
Method for detecting residual of solvents in idelalisib
ActiveCN107966498AAccurate detectionImprove stabilityComponent separationN dimethylformamideOrganic solvent
The invention relates to a method for detecting the residual of solvent in idelalisib, and concretely relates to a method for detecting the residual of various organic solvents in idelalisib by gas chromatography. The method comprises the following steps: 1) preparing an N,N-dimethylformamide solution of idelalisib, used as a test solution; 2) mixing all organic solvents to be detected with the N,N-dimethylformamide to form a mixed reference substance solution; 3) respectively carrying out gas chromatography detection on the test solution obtained in step 1) and the mixed reference substance solution obtained in step 2) to obtain chromatograms; and 4) calculating the content of every organic solvent to be detected in idelalisib according to peak areas through an external standard technology. The detection method can achieve easy, fast and accurate detection of the residual amounts of the organic solvents to be detected in the idelalisib. The detection method also has the advantages ofgood applicability, good durability, good precision, good sensitivity, good accuracy, good repeatability, good recovery rate, good stability of the test solution and improvement of the quality controlmethod of the above medicine.
Owner:HUBEI BIO PHARMA IND TECHCAL INST +2
Method for determining contents of seven components in lonicera and forsythia powder by adopting dual-wavelength quantitative analysis of multi-components by single marker
PendingCN113075314AImprove detection efficiencyReduce testing costsComponent separationBiotechnologyIsochlorogenic acid
The invention discloses a method for determining the contents of seven components in lonicera and forsythia powder by adopting dual-wavelength quantitative analysis of multi-components by a single marker, which comprises the following steps of: respectively taking neochlorogenic acid, chlorogenic acid, forsythiaside A, isochlorogenic acid A, isochlorogenic acid C, forsythin and arctiin as reference substances and forsythiaside A as an internal reference substance, calculating the relative correction factors of arctiin and forsythin at 237 nm, calculating the relative correction factors of neochlorogenic acid, chlorogenic acid, isochlorogenic acid A and isochlorogenic acid C at 327 nm, taking a forsythiaside A reference substance solution and a test solution, injecting the solutions into a high performance liquid chromatograph, and calculating the contents of seven components including forsythiaside A, forsythin, arctiin, neochlorogenic acid, chlorogenic acid, isochlorogenic acid A and isochlorogenic acid C in the lonicera and forsythia powder according to the relative correction factors. According to the invention, the same test solution is adopted to determine the contents of seven components under two absorption wavelengths, so that the detection efficiency is improved, and the detection cost is reduced.
Owner:SHANDONG ACAD OF CHINESE MEDICINE
Quality control method of macaque stone bovinebezoar powder
ActiveCN101168009BPerfect quality control methodQuality improvementNervous disorderHydroxy compound active ingredientsBiotechnologyCholic acid
The invention discloses a quality control method of monkey bezoar and cow-bezoar powder. The quality control standards of the former monkey bezoar and cow-bezoar powder is revised, lamella distinguish method of bilirubin, monkey bezoar, grassleaf sweelflag rhizome, alpinia zerumbet and licorice is added, lamella check method of hyodesoxycholic acid is also added, and further assay method of cinnabar and cholic acid is added. The quality control method of the monkey bezoar and cow-bezoar powder of the invention has a strong specificity, and the quality of the product is more effectively controlled, further the safety and the efficiency of medicines taking by human bodies are guaranteed.
Owner:GUANGZHOU BAIYUNSHAN QIXING PHARMA
Method for detecting liver-enhancing medicine
ActiveCN102539599BEfficient separationPerfect quality control methodComponent separationSalvianolic acid BGradient elution
The invention provides a method for detecting a liver-enhancing medicine. The liver-enhancing medicine is composed of oriental wormwood, isatis root, angelica, white paeony root, danshen root, Radix curcumae, Astragalus mongholicus, Codonopsis pilosula, Rhizoma alismatis, sealwort, rehmannia, yam, hawthorn, large-leaved gentian, liquorice and medicated leaven. The detecting method comprises the step of detecting paeoniflorin, gentiamarin and salvianolic acid B in the liver-enhancing medicine by using a high efficiency liquid chromatography method, wherein conditions are as follows: a chromatographic column takes octadecylsilane chemically bonded silica as a filling material; mobile phases comprise a mobile phase A which is acetonitrile and a mobile phase B which is an acidic water solution, and the mobile phases are subjected to gradient elution; the flow velocity is 1.0mL / min; the column temperature is 30 DEG C; the detection wavelength is 210nm to 400nm; and the number of theoretical plates is calculated according to the paeoniflorin peak and should not be less than 6000. According to the detecting method provided by the invention, the content of active ingredients of the paeoniflorin, the gentiamarin and the salvianolic acid B in the liver-enhancing medicine can be detected at the same time, so that the active ingredients of the liver-enhancing medicine can be comprehensively represented, and the method has the characteristics of high precision, high stability and high repeatability.
Owner:SHIJIAZHUANG DONGFANG PHARMA
A method for detecting solvent residues in ederaris
ActiveCN107966498BAccurate detectionImprove stabilityComponent separationOrganic solventGas liquid chromatographic
Owner:HUBEI BIO PHARMA IND TECHCAL INST +2
Quality Control Method of Taohe Chengqi Decoction Composition
ActiveCN106483227BAvoid product qualityEasy to operateComponent separationPhosphoric acidColumn temperature
The invention discloses a quality control method of a peach kernel qi activating decoction composition and belongs to the field of traditional Chinese medicine analysis. The method includes the steps that a peach kernel qi activating decoction fingerprint spectrum is established through high performance liquid chromatography, and chromatographic conditions are characterized in that a chromatographic column adopts octadecylsilane chemically bonded silica as filler; an ultraviolet detector is adopted as a detection instrument, and the detection wavelength of the fingerprint spectrum ranges from 210 nm to 250 nm; the flow rate ranges from 0.9 ml / ml to 1.1 ml / ml, the column temperature ranges from 25 DEG C to 35 DEG C, the detection wavelength ranges from 210 nm to 250 nm, the number of theoretical plates should not be smaller than 3,000 according to calculation of catechinic acid peaks, methyl alcohol serves as a moving phase A, a 0.1% phosphoric acid solution serves as a moving phase B, and gradient elution is carried out according to the following sequence: the gradient condition of elution detection of the fingerprint spectrum is defined in the specification. The fingerprint spectrum comprehensively reflects quality information of a peach kernel qi activating decoction, and therefore the quality of the peach kernel qi activating decoction can be more comprehensively and effectively controlled.
Owner:CHINA RESOURCES SANJIU MEDICAL & PHARMA +1
Method for Establishing Fingerprint of Danggui Sini Decoction Composition
ActiveCN108459091BAvoid product qualityEasy to operateComponent separationPhosphoric acidDanggui sini
The invention discloses an establishing method of a danggui sini decoction fingerprint atlas and the fingerprint atlas, wherein the method and the fingerprint atlas belong to the field of traditionalChinese medicine analysis. According to the method of the invention, the fingerprint atlas is established according to high-efficiency liquid chromatography; a chromatographic column is an octadecylsilane chemically bonded silica chromatographic column; the column temperature is 25-35 DEG C; the flow velocity is 0.9-1.1ml / min; the detecting wavelength is 220nm; acetonitrile A-0.1% phosphoric acidB is used as a mobile phase; and gradient elution is performed according to a sequence which is shown in the description. The fingerprint atlas according to the invention comprehensively reflect quality information of the danggui sini decoction, thereby realizing a purpose of more comprehensively and effectively controlling the quality of a danggui sini decoction composition product.
Owner:CHINA RESOURCES SANJIU MEDICAL & PHARMA +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com