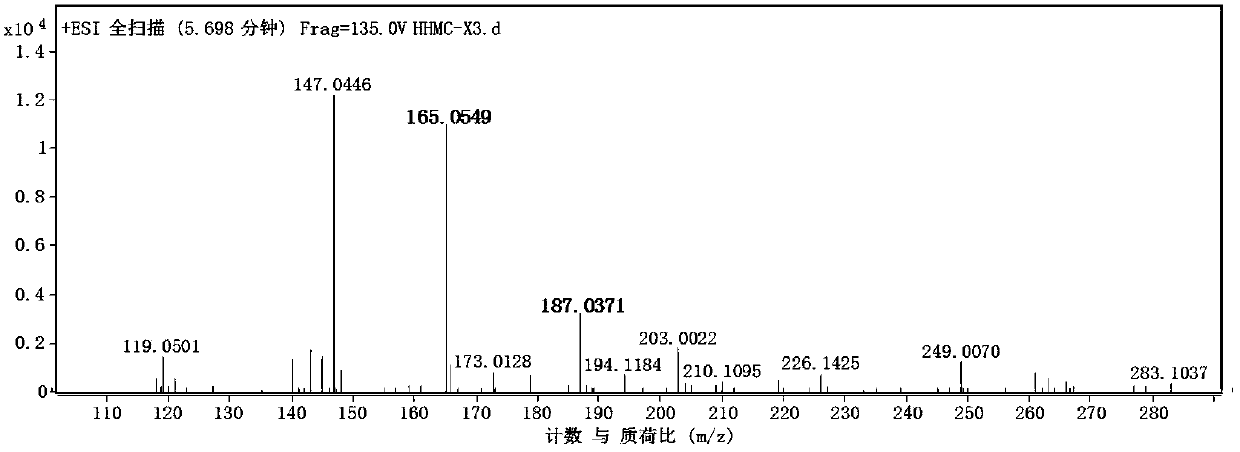
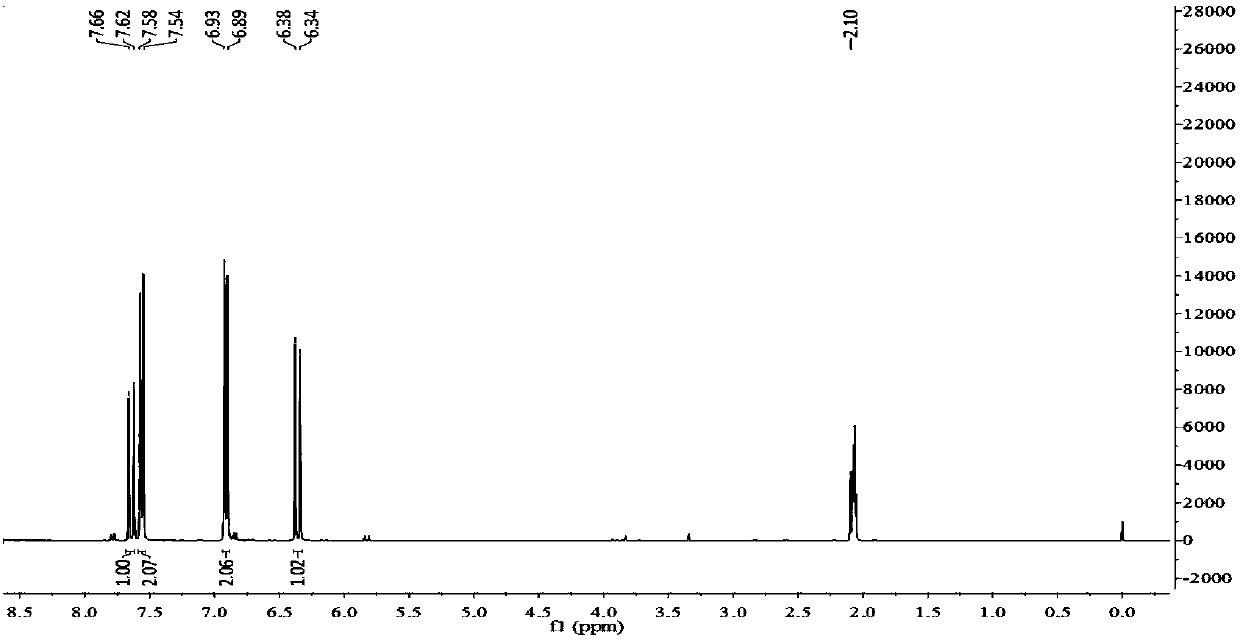
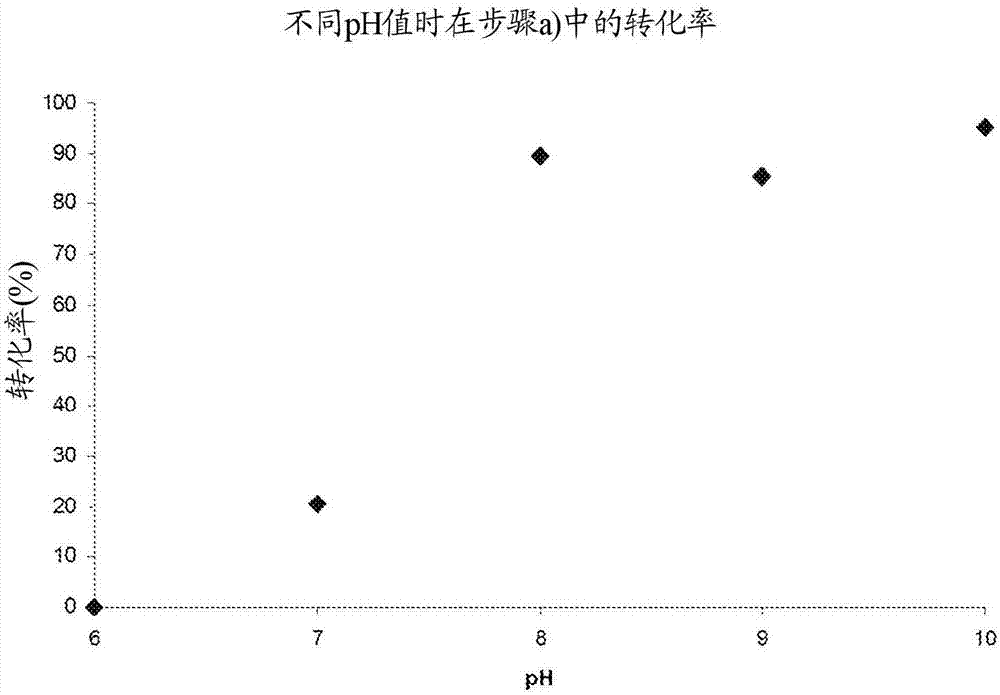
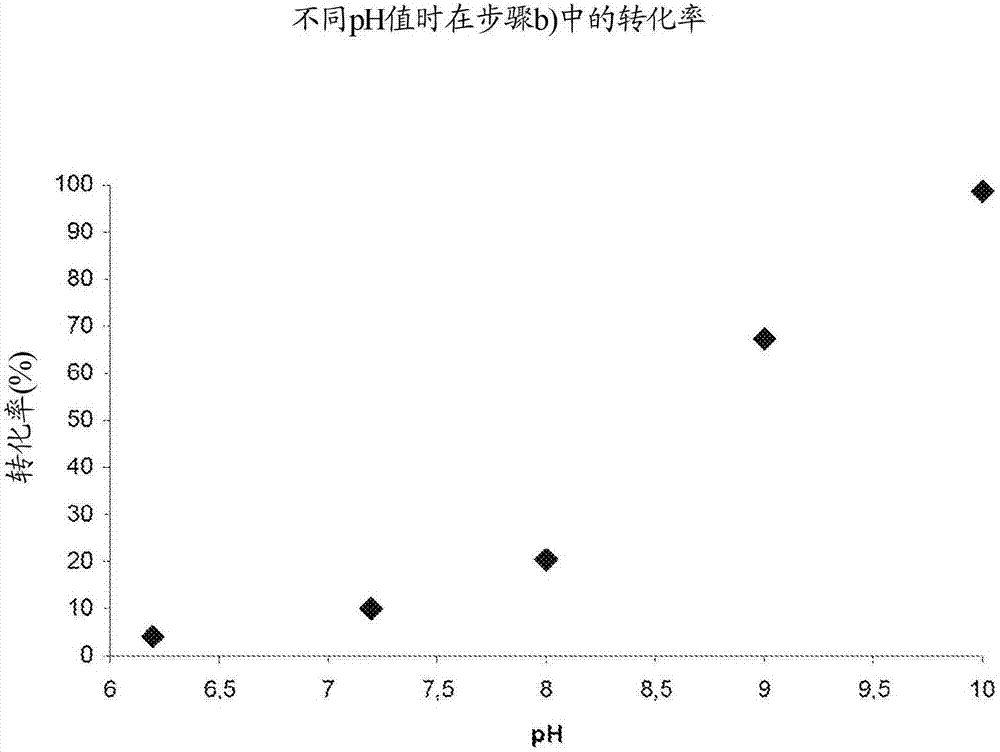
Patents
Literature
129 results about "P-Coumaric acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
P-Coumaric acid is a hydroxycinnamic acid, an organic compound that is a hydroxy derivative of cinnamic acid. There are three isomers of coumaric acid—o-coumaric acid, m-coumaric acid, and p-coumaric acid—that differ by the position of the hydroxy substitution of the phenyl group. p-Coumaric acid is the most abundant isomer of the three in nature. p-Coumaric acid exists in two forms trans-p-coumaric acid and cis-p-coumaric acid.
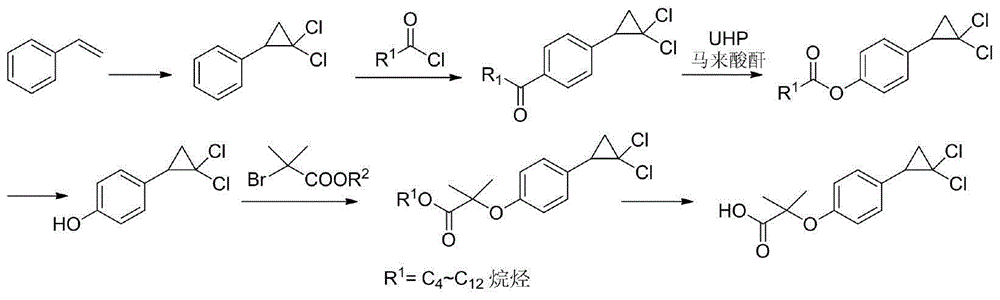
Para-coumaric acid or para-hydroxycinnamic acid derivatives and their use in cosmetic or dermatological compositions
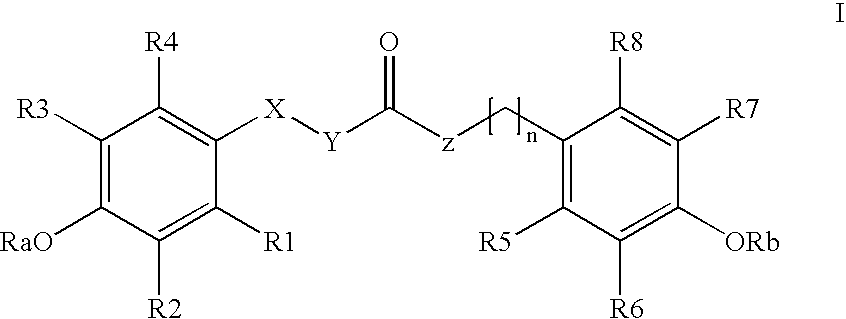
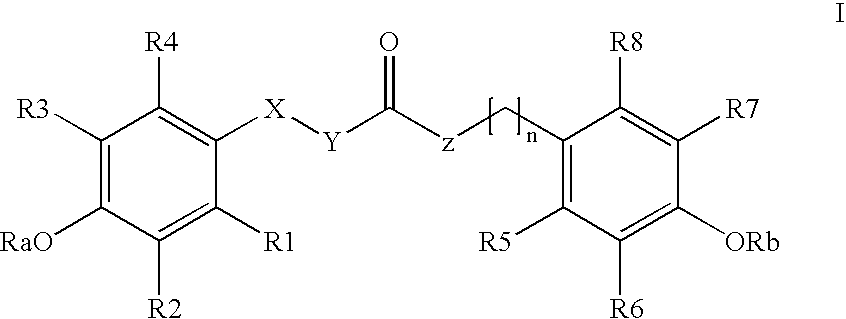
The invention relates to the use of para-coumaric acid or para-hydroxycinnamic acid derivatives in cosmetic or dermatological compositions, specifically to the use of at least one compound derived from para-coumaric acid having a general formula (I) below: in which, especially, Z represents an oxygen or an —NH— group; X and Y are identical and each represent a CH or CH2 group, as an active principle with depigmenting, free-radical-scavenging and / or antiinflammatory activity. The invention also relates to the use of the above compounds for cosmetic care or for the preparation of a pharmaceutical composition, especially for depigmenting an area of skin, having antiradical and / or antiinflammatory activity.
Owner:BASF BEAUTY CARE SOLUTIONS FRANCE SAS +1
Stabilized ascorbic acid compositions and methods therefor
ActiveUS20050154054A1Improve stabilityImprove solubilityCosmetic preparationsBiocideSolubilityCaffeic acid
The present invention relates to ascorbic acid single-phase solution compositions that provide enhanced stability, enhanced solubility and an enhanced photoprotective effect as compared to prior compositions. The compositions comprise L-ascorbic acid; a cinnamic acid derivative such as p-coumaric acid, ferulic acid, caffeic acid, sinapinic acid, a derivative thereof, and a combination thereof; a solvent comprising a glycol ether and an alkanediol; and water; the composition having a pH of no more than about 3.5. The compositions may also comprise a form of Vitamin E and are useful for treatment of radical-induced damage to a subject, particularly the skin of a subject.
Owner:LOREAL USA CREATIVE INC
Stabilized ascorbic acid compositions and methods therefor
ActiveUS7179841B2Improve stabilityImprove solubilityCosmetic preparationsBiocideCoumaric acidCaffeic acid
Owner:LOREAL USA CREATIVE INC
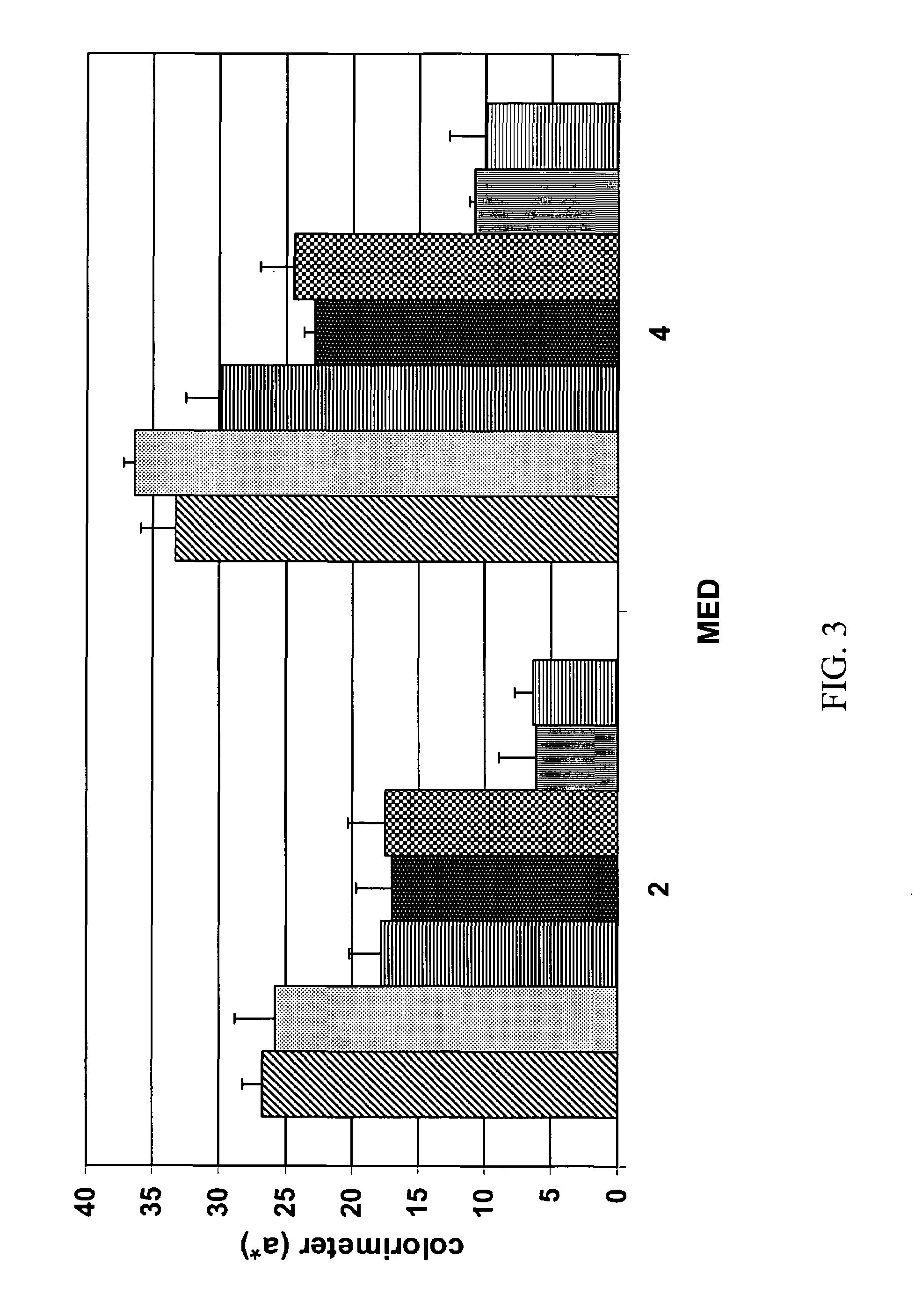
Processes for isolating bitter quinides for use in food and beverage products
InactiveUS20060286238A1Add flavorMilk preparationFood ingredient as taste affecting agentCaffeic acidBitter taste
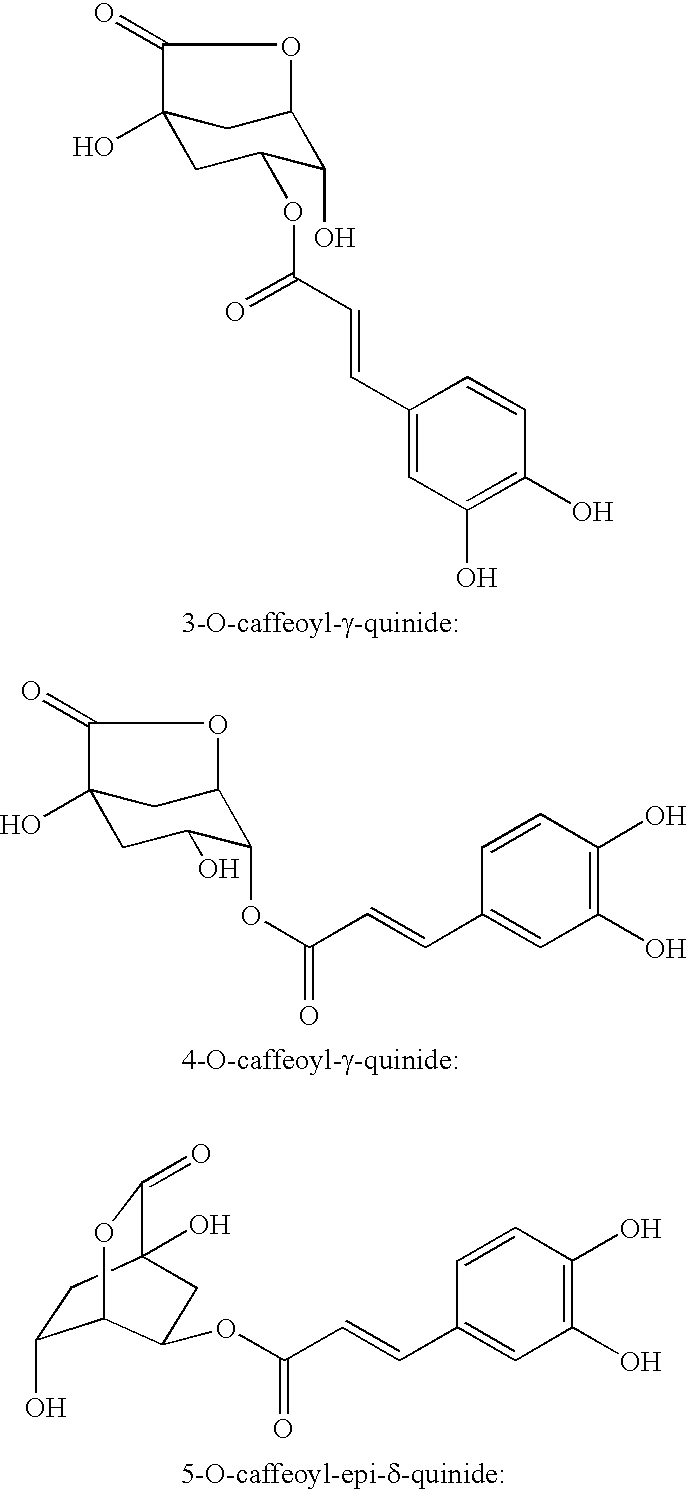
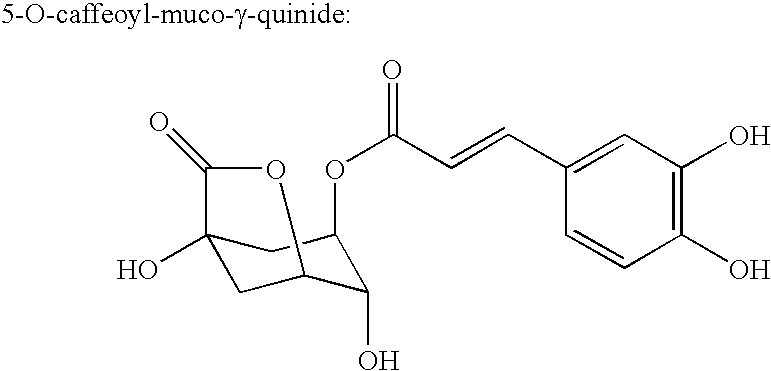
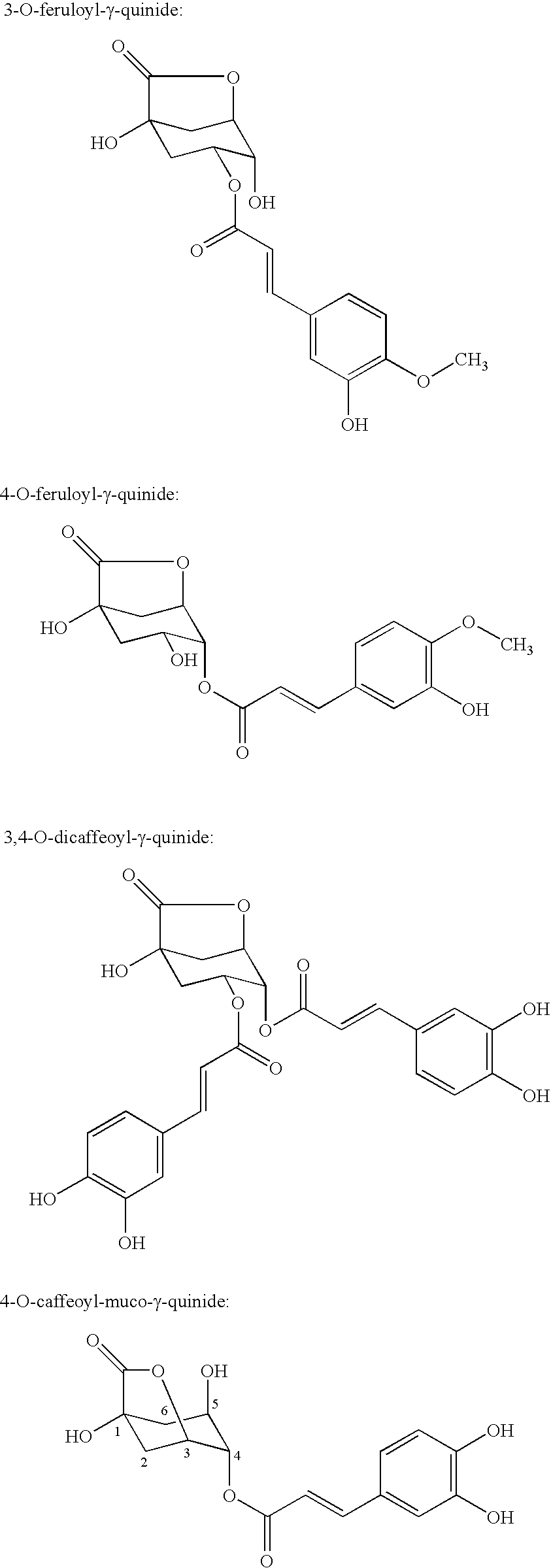
Processes for isolating bitter quinides for use in food and beverage products entailing contacting a bitter quinide solution with an adsorbent to adsorb bitter quinides from the bitter quinide solution, desorbing the bitter quinides from the adsorbent to obtain a bitter quinide isolate, and adding the bitter quinide isolate to a food or beverage product to enhance the flavor thereof. A bitter quinide isolate made up of at least one of 3-O-caffeoyl-γ-quinide, 4-O-caffeoyl-γ-quinide, 5-O-caffeoyl-epi-δ-quinide, 5-O-caffeoyl-muco-γ-quinide, 3-O-feruloyl-γ-quinide, 4-O-feruloyl-γ-quinide, 3,4-O-dicaffeoyl-γ-quinide, 4-O-caffeoyl-muco-γ-quinide, 3,5-O-dicaffeoyl-epi-δ-quinide, 4,5-O-dicaffeoyl-muco-γ-quinide, 5-O-feruloyl-muco-γ-quinide, 4-O-feruloyl-muco-γ-quinide, 5-O-feruloyl-epi-δ-quinide, quinide esterified with one or more of caffeic acid, ferulic acid, p-courmaric acid, 2,4-dimethoxycinnamic acid and mixtures thereof.
Owner:THE PROCTER & GAMBLE COMPANY +3
Method for preparing trans-ferulaic acid, p-cumaric acid and pentosan
The invention relates to a method for simultaneously preparing trans-ferulic acid, p-coumaric acid and pentosan, which comprises the following steps: processing a cellulose material with a low-concentration alkali to release coumaric acid, and hydrolyzing with a high-concentration alkali to release ferulic acid and pentosan; ultrafiltering the alkaline hydrolytic solution, precipitating the concentrated solution with ethanol to obtain pentosan; and adsorbing coumaric acid and ferulic acid in the filtrate with cation exchange resin, eluting with an ethanol-acid-water mixed eluent, removing ethanol from the eluent, crystallizing, centralizing or filtering the eluent at a low temperature or a normal temperature to obtain ferulic acid or coumaric acid crystals, and recrystallizing to obtain the high-purity product. The alkaline hydrolytic solution can be recovered and used for hydrolyzing the material, thereby obviating the production of a great amount of wastewater due to the alkaline hydrolysis. The method can produce ferulic acid and coumaric acid widely used in the industries of food, medicine and cosmetics, and pentosan used for producing xylooligosaccharide as food gum by utilizing solid wastes produced in agricultural and food processing, thereby achieving significant economic and social meanings.
Owner:JINAN UNIVERSITY
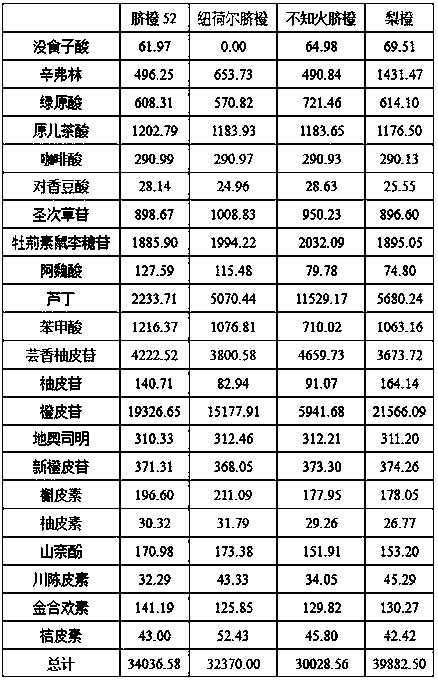
Method for simultaneously determining twenty-two flavones and phenolic acids in citrus fruits
The invention discloses a method for simultaneously determining twenty-two flavones and phenolic acids in citrus fruits by adopting high performance liquid chromatography-diode array detector-fluorescence detector (HPLC-DAD-FLD). The method is capable of simultaneously determining twenty-two phenolic compounds in citrus fruits such as gallic acid, synephrine, chlorogenic acid, protocatechuic acid,caffeic acid, p-coumaric acid, rhamnosylvitexin, eriocitrin, ferulic acid, rutin, benzoic acid, narirutin, naringin, hesperidin, diosmin, neohesperidin, quercetin, naringenin, kaempferol, nobiletin,hesperetin, acacetin and the like, derivatization is not needed, and the method is high in accuracy, high in sensitivity and excellent in repeatability.
Owner:INST OF AGRI ENG TECH FUJIAN ACAD OF AGRI SCI
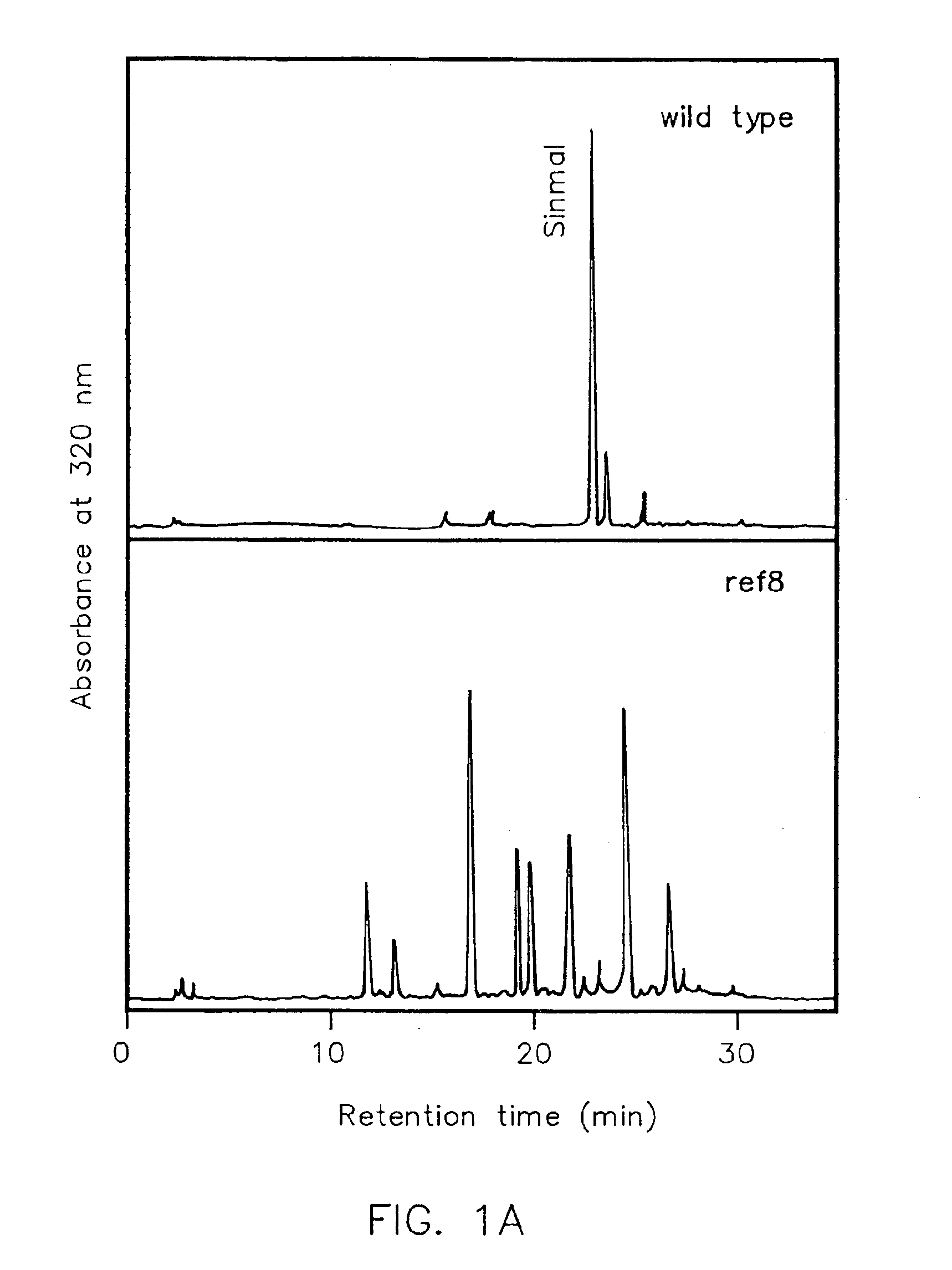
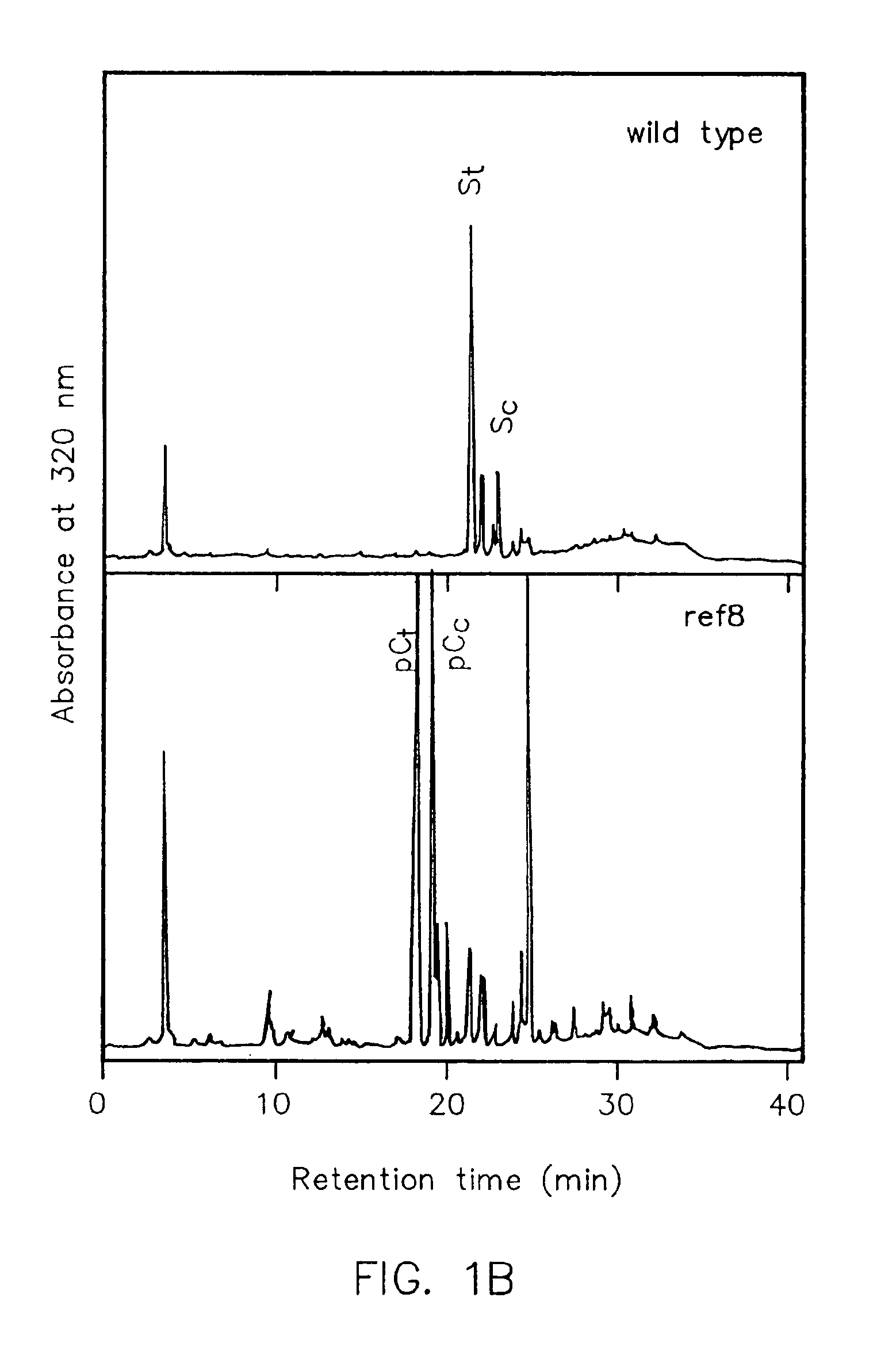
Genes encoding p-coumarate 3-hydroxylase (C3H) and methods of use
InactiveUS7071376B2Increase the content of flavonoidsIncrease contentOther foreign material introduction processesTissue cultureGMO PlantsPhenylpropanoids metabolism
The present invention is directed to a method for altering secondary metabolism in plants, specifically phenylpropanoid metabolism. The present invention is further directed to a mutant p-coumarate 3-hydroxylase gene, referred to herein as the ref8 gene, its protein product which can be used to prepare gene constructs and transgenic plants. The gene constructs and transgenic plants are further aspects of the present invention.
Owner:PURDUE RES FOUND INC
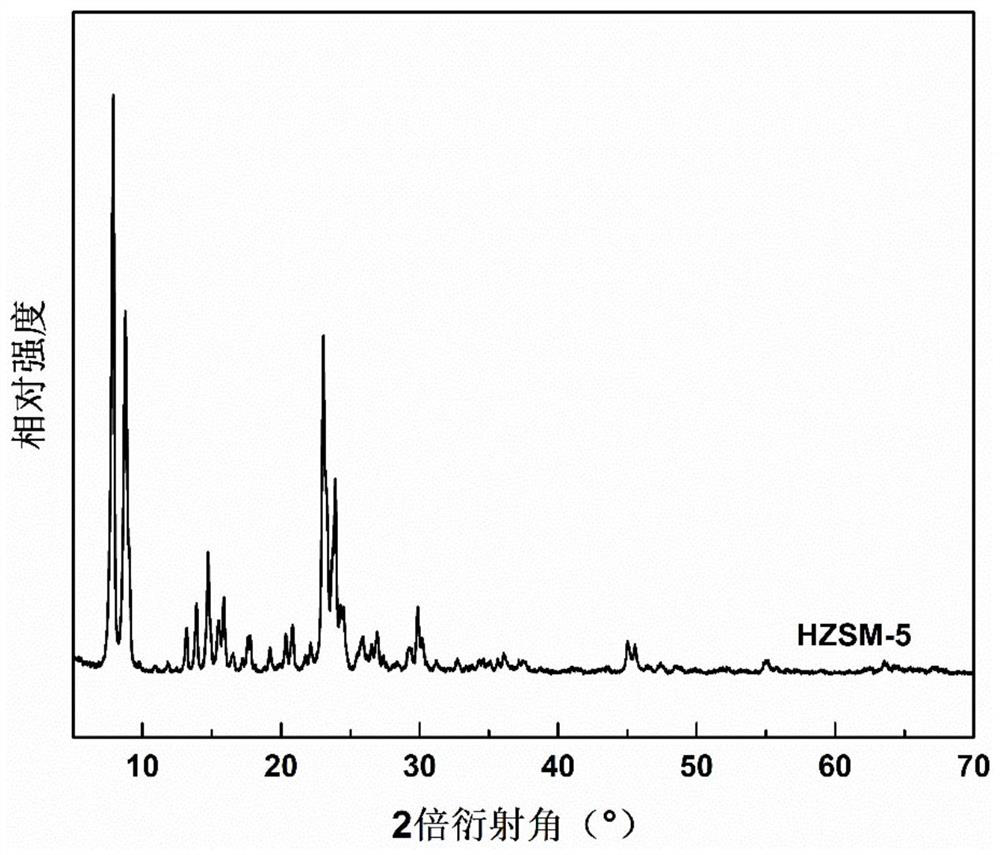
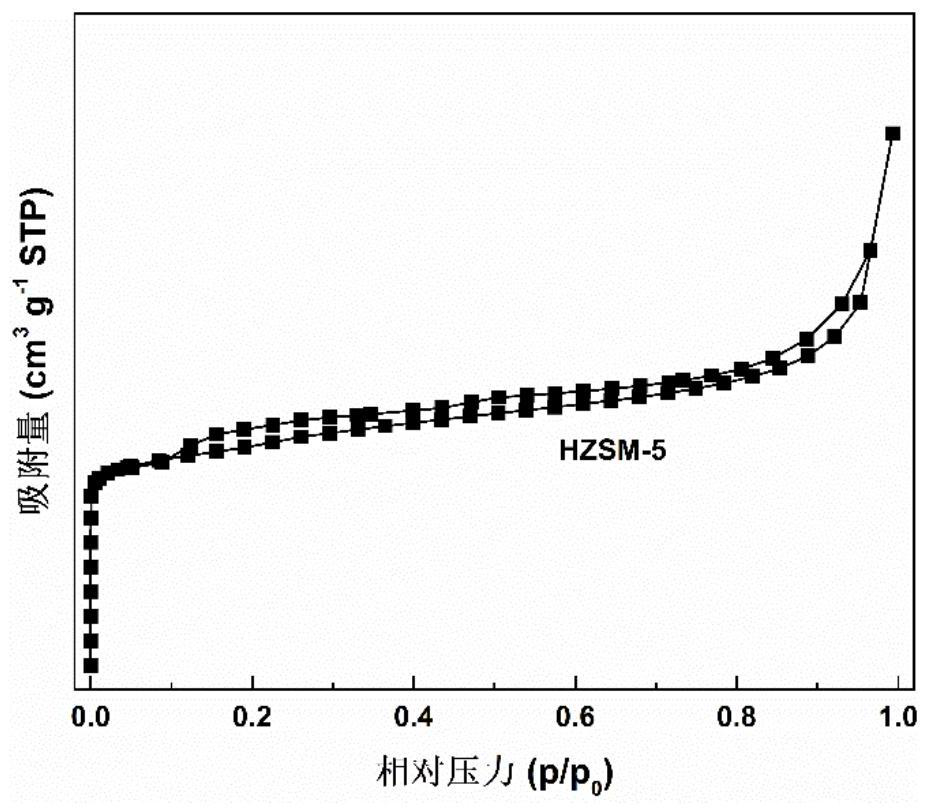
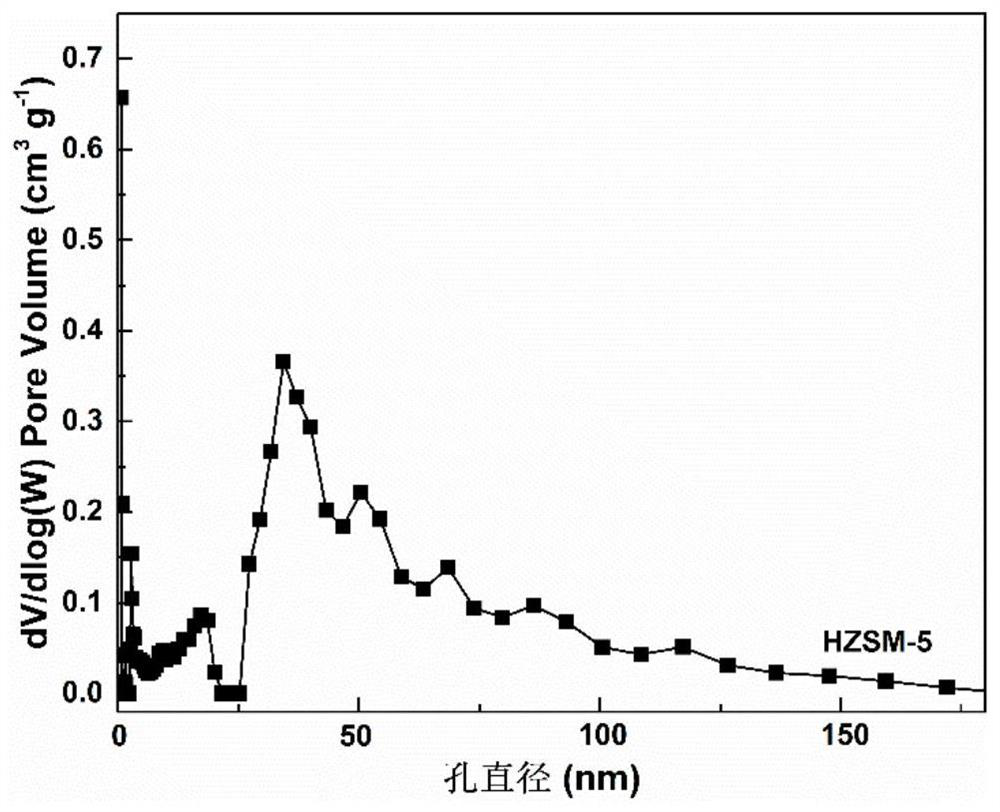
Method for preparing p-coumarate by catalyzing lignin depolymerization through hierarchical pore molecular sieve loaded molybdenum oxide
ActiveCN112495425AHigh selectivityHigh value utilizationMolecular sieve catalystsOrganic compound preparationMolecular sievePtru catalyst
The invention discloses a method for preparing p-coumarate by catalyzing lignin depolymerization through hierarchical pore molecular sieve loaded molybdenum oxide. The method comprises the following steps: by taking lignin as a raw material, adding a reaction medium and a hierarchical pore molecular sieve loaded molybdenum oxide catalyst, replacing with nitrogen, pressurizing to 0.1-1MPa, heatingto 120-160 DEG C, reacting for 2-10 hours while stirring, removing the catalyst after the reaction, and catalytically degrading the lignin into p-coumarate. By regulating and controlling the catalyststructure, the reaction medium and the reaction condition factors, high-yield and high-selectivity depolymerization of lignin is achieved, p-coumarate serving as a high-added-value chemical is obtained, and the purpose of high-valued utilization of lignin is achieved. The heterogeneous catalyst is used, the process is simple, reaction conditions are mild, lignin can be selectively converted to obtain the target product p-coumarate, and the yield and selectivity of p-coumarate can reach up to 11.78 wt.% and 85.24 wt.% respectively.
Owner:SOUTH CHINA UNIV OF TECH
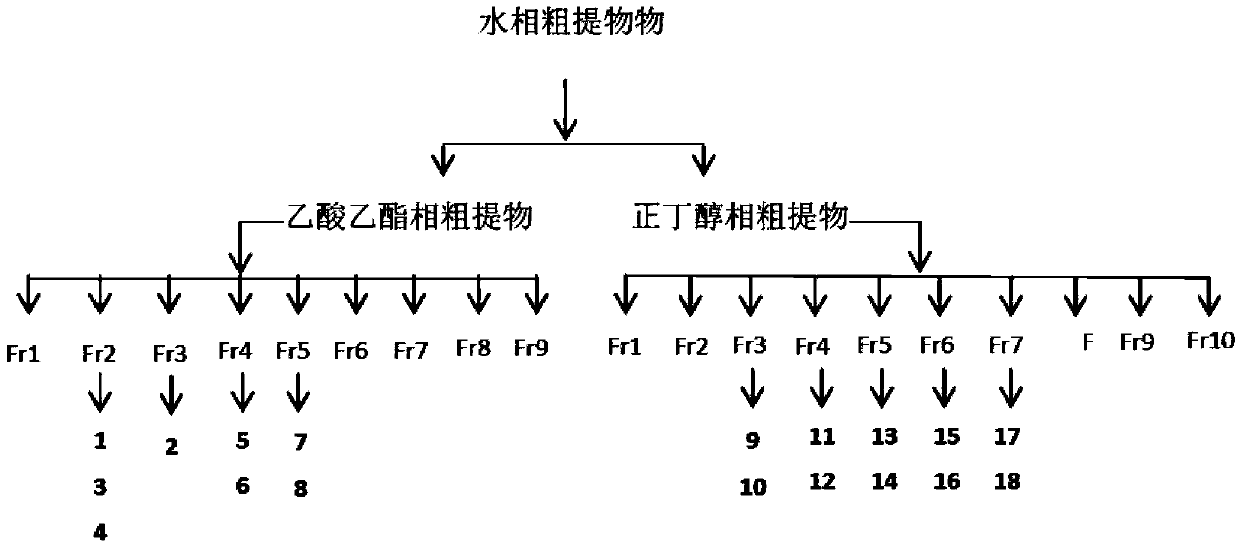
Method for preparing p-coumalic acid by using spartina alterniflora
ActiveCN109534984AReduce planting costsIncrease productionOrganic active ingredientsMetabolism disorderAcetic acidCoumalic acid
The invention discloses a method for preparing p-coumalic acid by using spartina alterniflora. Spartina alterniflora extraction liquid is subjected to ethyl acetate extraction; then, water phases aretaken; next, n-butyl alcohol is used for extraction; n-butyl alcohol phase extraction liquid is concentrated to obtain n-butyl alcohol phase crude paste; the n-butyl alcohol phase crude paste is sequentially subjected to positive phase silica gel column chromatography, ODS reversed phase chromatography and gel column chromatography; finally, separation and purification are performed through a highperformance liquid phase chromatography technology, thus obtaining the p-coumalic acid. Pharmacological research results show that the p-coumalic acid has obvious uric acid reduction and blood sugarreduction effects on mice with hyperuricemia.
Owner:南京施倍泰生物科技有限公司
Method for producing p-vinylphenols
The invention relates to a biocatalytic method for producing p-vinylphenols, comprising a three-stage one-pot reaction according to the following reaction scheme: (A) wherein a) an optionally substituted phenol (1) is bound to pyruvic acid (BTS) to form the optionally substituted tyrosine (2) by means of catalytic action of a tyrosine phenol-lyase (TPL) and in the presence of ammonium ions, b) ammonia is eliminated from the tyrosine (2) by means of catalytic action of a tyrosine-ammonia-lyase (TAL) or phenyl-ammonia-lyase (PAL), in order to produce an optionally substituted p-coumaric acid (3), and c) the p-coumaric acid (3) undergoes a decarboxylation by means of catalytic action of a phenolic acid decarboxylase (PAD), in order to produce the desired, optionally substituted p-vinyl phenol(4); d) wherein the resultant CO2 is removed from the reaction system, in order to move the chemical equilibrium of all three reaction steps in the direction of the products.
Owner:UNIVERSITY OF GRAZ
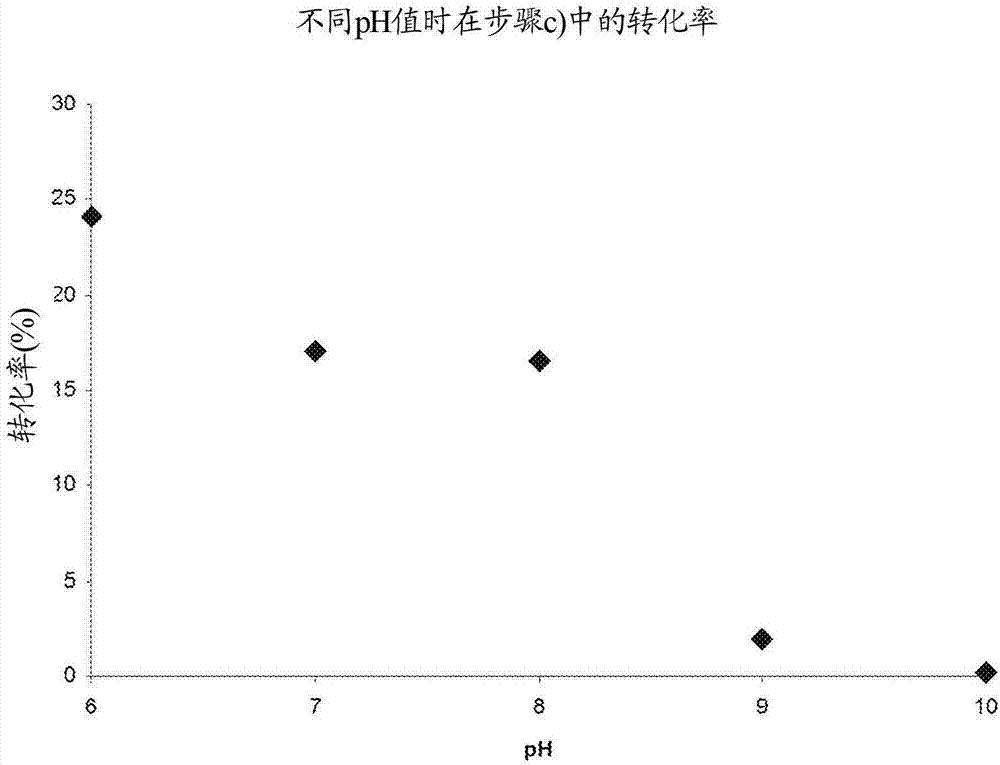
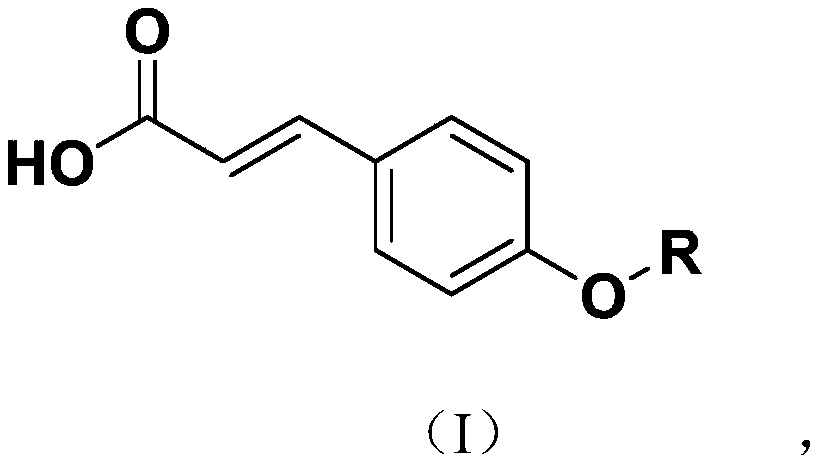
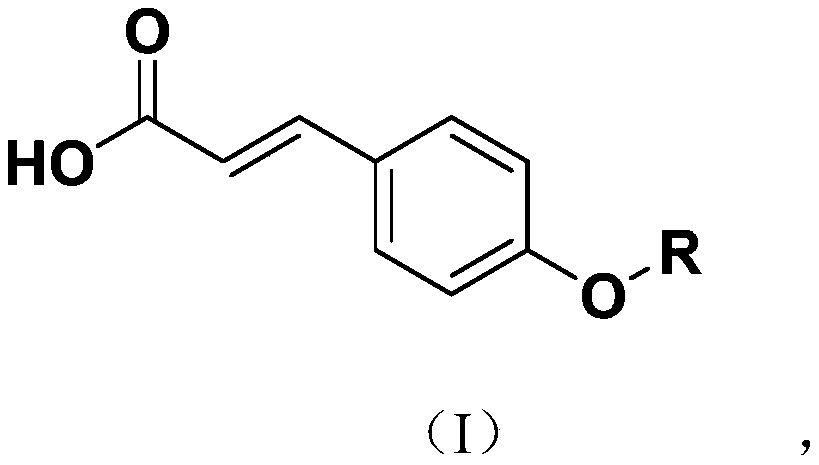
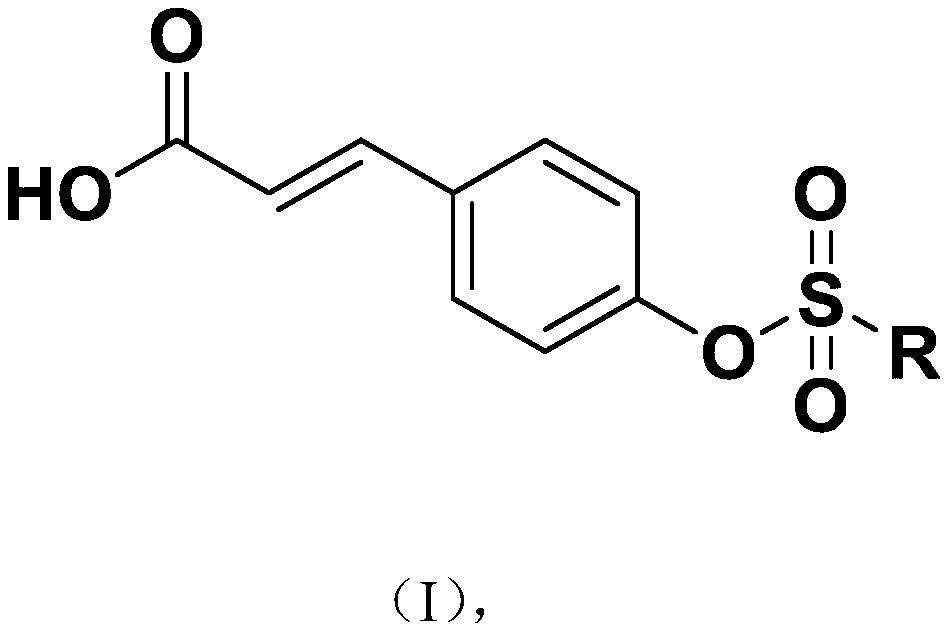
Novel p-coumaric acid sulfonate derivative, preparation method and application thereof
PendingCN110627690APharmaceutically activeRealize resource utilizationAntibacterial agentsBiocideSulfonateResource utilization
The invention discloses a novel p-coumaric acid sulfonate derivative, a preparation method and application thereof. The structural formula of the novel p-coumaric acid sulfonate derivative is shown asformula (I) in the specification, wherein R is selected from the following groups shown as the specification. The compound provided by the invention has potential pharmaceutical activity, and the preparation raw material p-coumaric acid can be obtained by natural extraction method, thus having a positive effect on resource utilization of spartina alterniflora.
Owner:南京施倍泰生物科技有限公司
Herbicide for preventing and removing semen cuscutae
ActiveCN104542609AGood prevention effectHas inhibitory effectBiocideAnimal repellantsHuman bodyWater source
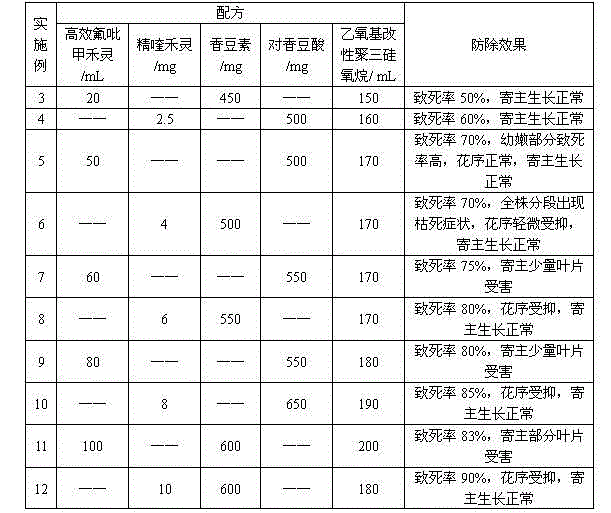
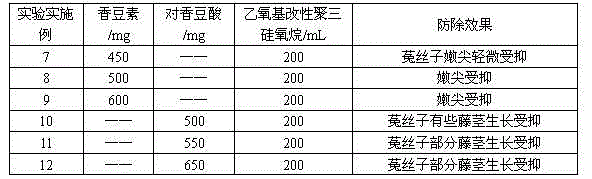
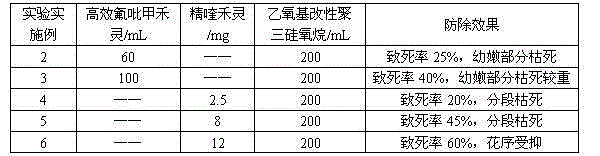
The invention relates to a herbicide for preventing and removing semen cuscutae. The herbicide comprises an effective constituent agent A, an effective constituent agent B and an additive as the following matching ratios: the agent A is one of 20-100 parts by volume of efficient haloxyfop-methyl with the mass concentration of 95% and 2.5-12 parts by weight of quizalofop-p-ethyl; the agent B is one of 450-600 parts by weight of coumarin and 500-650 parts by weight of p-coumaric acid; the additive is 150-200 parts by weight of ethoxy modified trisiloxane with the mass concentration of 100%; the parts by weight are calculated by the active compound. The herbicide has a favorable prevention effect on semen cuscutae, has the fatality rate of up to 95%, is low in toxicity, and is safe to human bodies, livestock and water sources.
Owner:广西景丽达建设工程有限公司
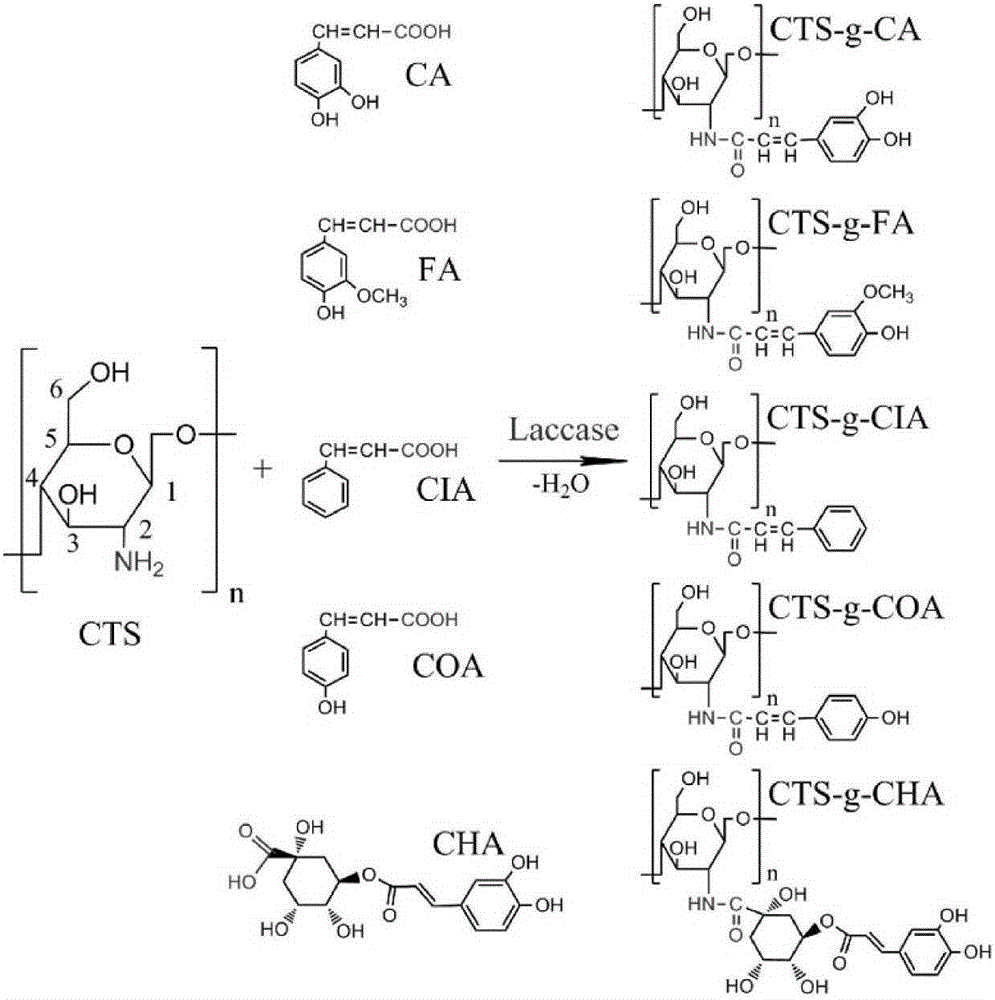
Application of chitosan derivative in bacterial wilt prevention and treatment
InactiveCN105191940AInhibition is effectiveEffective controlBiocideDisinfectantsChlorogenic acidCaffeic acid
The invention relates to application of a chitosan derivative in bacterial wilt prevention and treatment. The structure of the chitosan derivative is shown in the formula (I), wherein R represents H or caffeic acid, or ferulic acid, or cinnamic acid, or p-coumaric acid or chlorogenic acid with a decarboxylated hydroxyl group. The medial lethal concentration of the chitosan derivative used for restraining bacterial wilt pathogenic bacteria is provided, the low-concentration chitosan derivative can be used for effectively restraining bacterial wilt bacteria, an effective method for preventing and treating bacterial wilt is provided, and the environment is not polluted.
Owner:JIANGSU UNIV OF SCI & TECH
Medicinal composition for treating turtle cavity disease and preparation method thereof
InactiveCN103463387AEasy to solveGood treatment effectAntibacterial agentsAnthropod material medical ingredientsBiotechnologyCoumaric acid
The invention discloses a medicinal composition for treating a turtle cavity disease. The medicinal composition for treating the turtle cavity disease comprises the following Chinese herbs in parts by weight: 15-30 parts of hedyotis diffusa, 20-30 parts of groundsel, 5-15 parts of gallnut, 10-25 parts of golden cypress, 2-10 parts of wild chrysanthemum flower and 5-15 parts of onion. The invention further discloses a preparation of the medicinal composition. The hedyotis diffusa and the groundsel have a very strong anti-virus effect, so that the hedyotis diffusa and the groundsel as effective components can prevent and treat the turtle cavity disease better, with the cure rate as high as 99%. In the preparation method, the hedyotis diffusa is extracted with a supercritical carbon dioxide extraction method, so that effective components of the hedyotis diffusa, namely hentriacontane, stigmasterol, ursolic acid, oleanolic acid, beta-sitosterol, beta-sitosterol-D-glucoside and p-coumaric acid, have relatively high contents. The preparation method is simple and feasible and is applicable to large-scale production.
Owner:XINGHUA HENGWEI BIOTECH
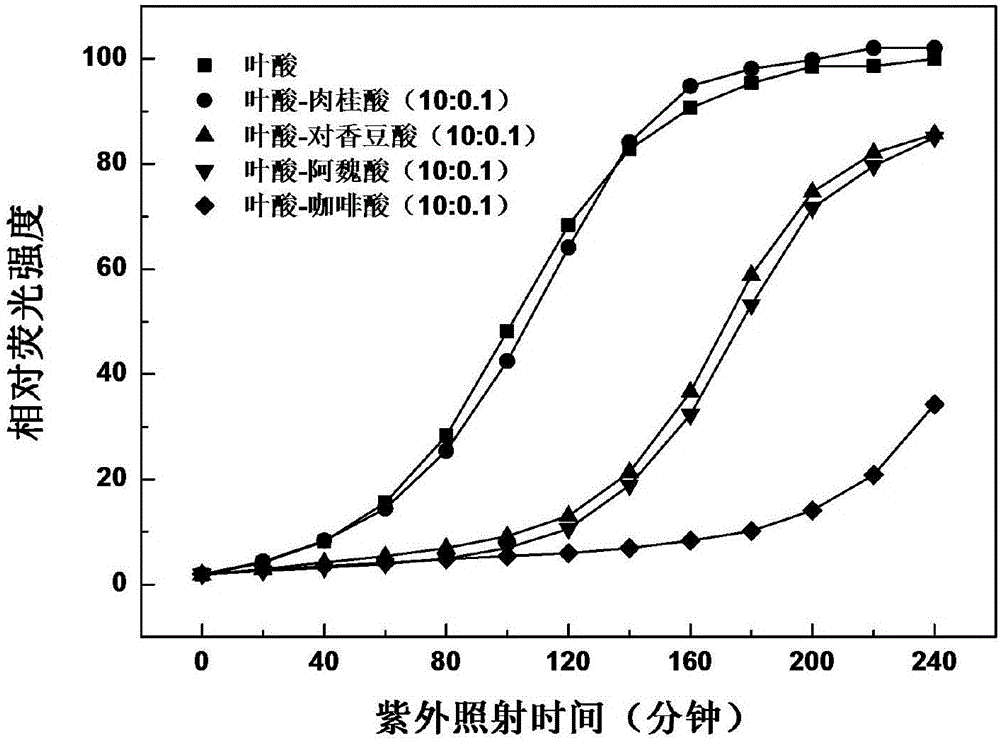
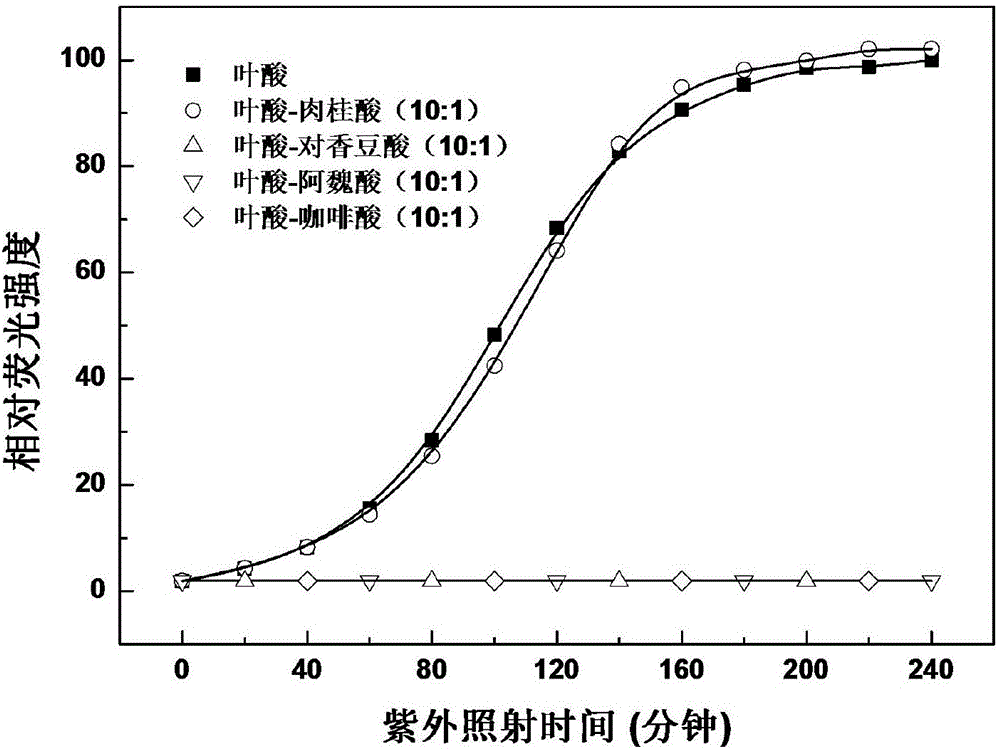
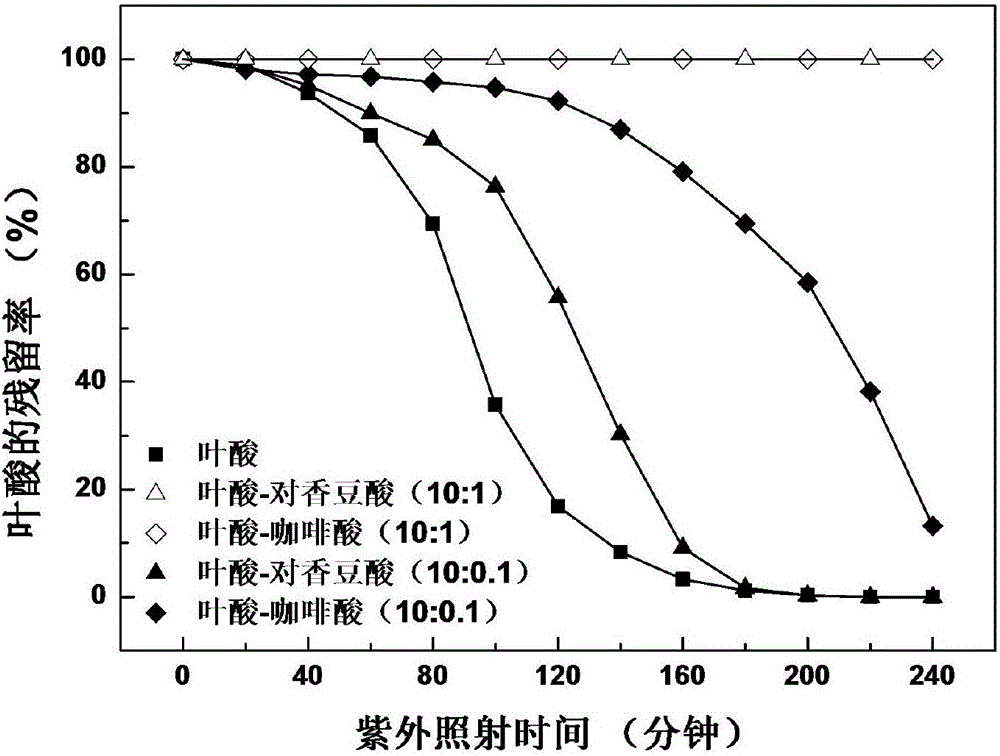
Method for enhancing photostability of folic acid
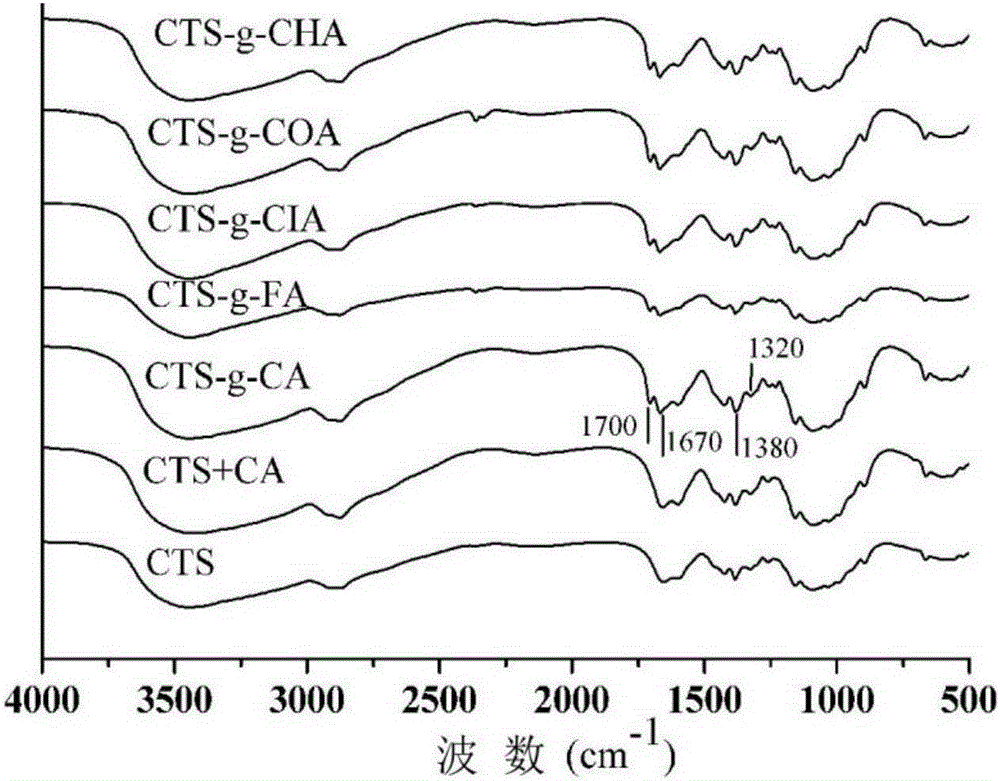
ActiveCN106420394AImprove photostabilityInhibition of photodegradationCosmetic preparationsOrganic active ingredientsCaffeic acidP-Coumaric acid
Owner:JIANGNAN UNIV
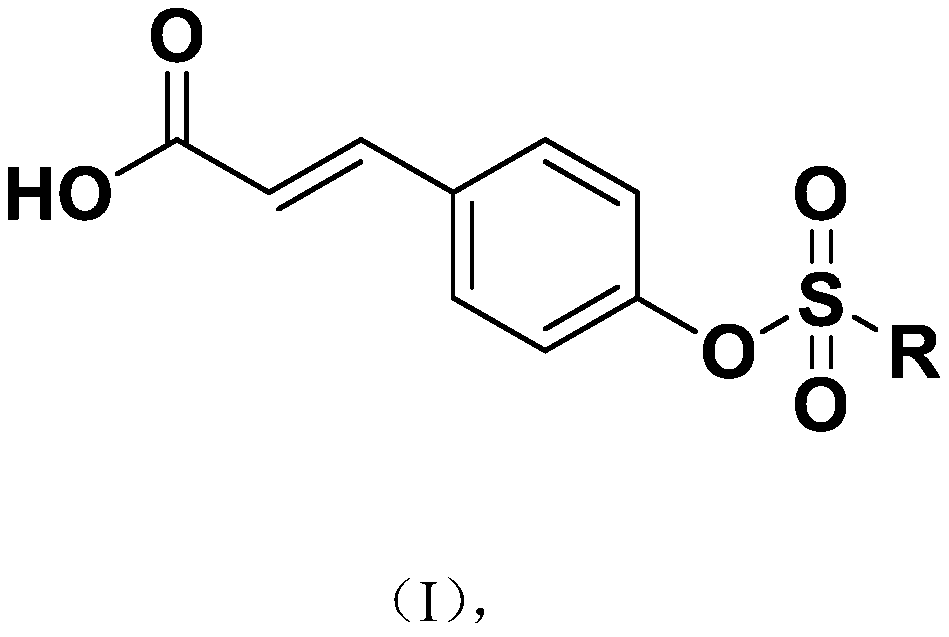
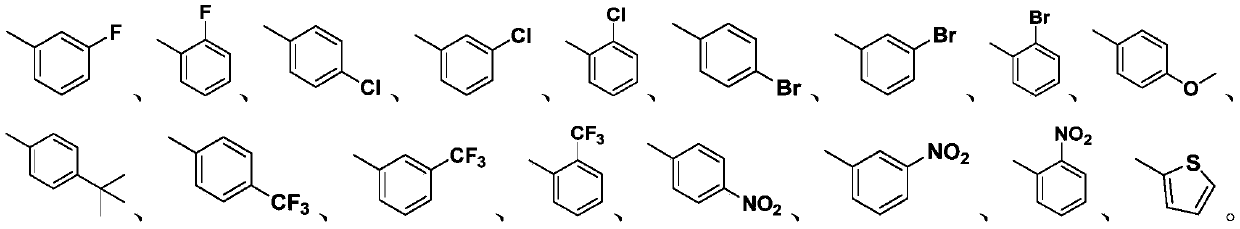
P-coumaric acid aromatic derivative and preparation method and application thereof
PendingCN110563617ARealize resource utilizationPharmaceutically activeSulfonic acid esters preparationResource utilizationP-Coumaric acid
The invention discloses a p-coumaric acid aromatic derivative and a preparation method and application thereof. The p-coumaric acid aromatic derivative of the invention has a structural formula as shown in a formula (I) which is described in the specification. In the formula (I), R is described in the specification. The p-coumaric acid aromatic derivative of the invention has potential medicinal activity; and the preparation raw material, namely p-coumaric acid, of the derivative can be obtained by using a natural extraction method, which has positive effect on resource utilization of Spartinaalterniflora Loisel.
Owner:南京施倍泰生物科技有限公司
Methods of using O-methyltransferase for biosynthetic production of pterostilbene
ActiveCN106102454ASolve the technical problem of generating pterostilbenePromote generationBryophytesNervous disorderStilbene synthaseP-Coumaric acid
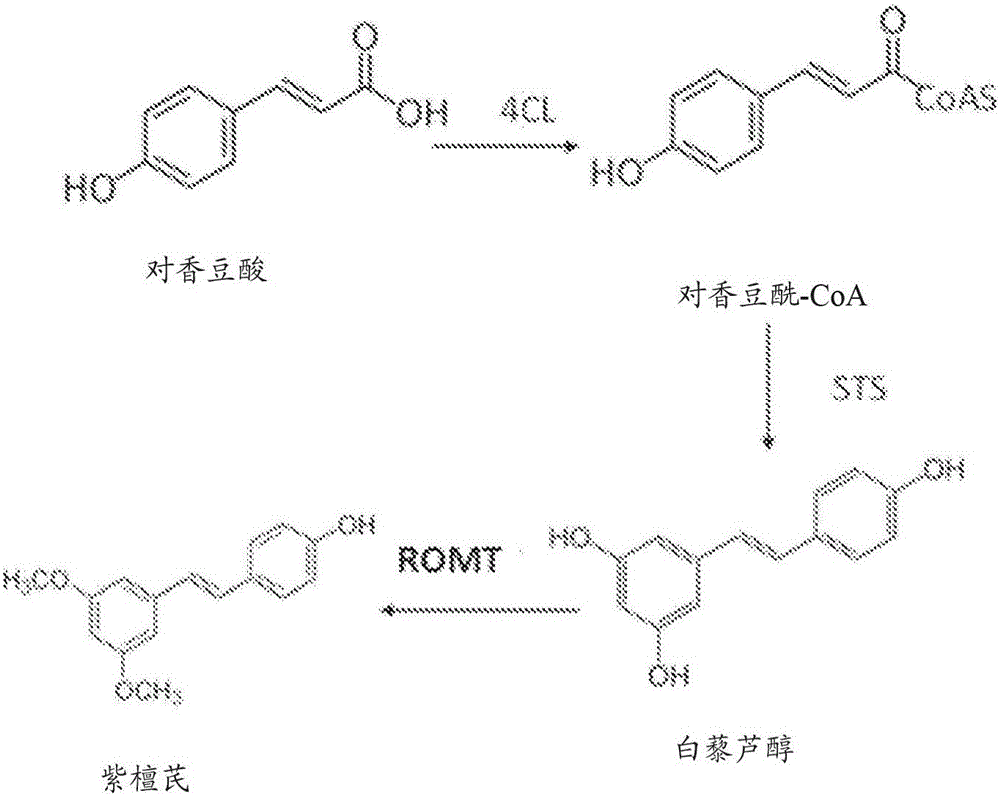
A biosynthetic method of making pterostilbene including expressing a 4- coumaratexoenzyme A ligase (4CL) in a cellular system, expressing a stilbene synthase (STS) in the cellular system, expressing a resveratrol O-methyltransferase (ROMT) in the cellular system, feeding p-coumaric acid to the cellular system, growing the cellular system in a medium, and producing pterostilbene.
Owner:CONAGEN INC
Method for detection of p-coumaric acid
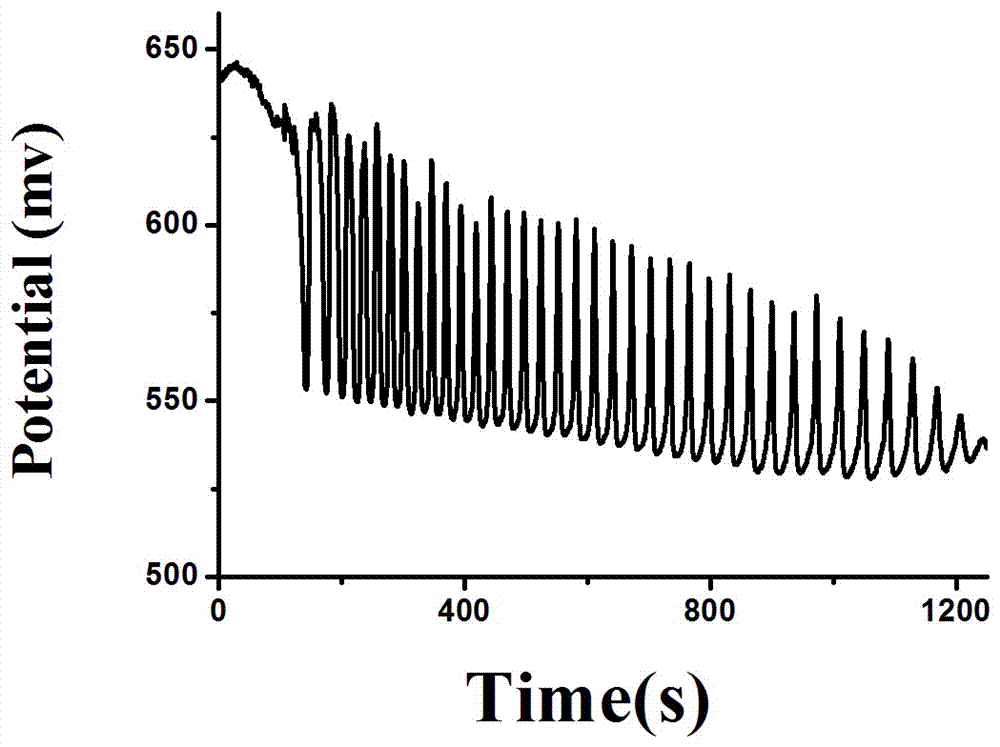
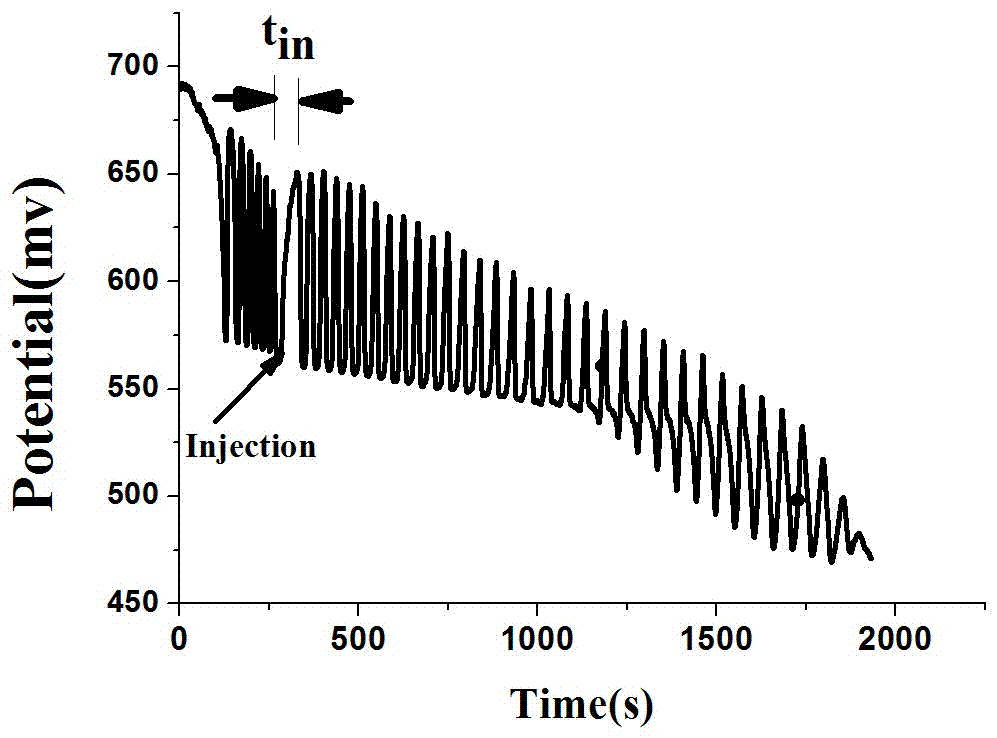
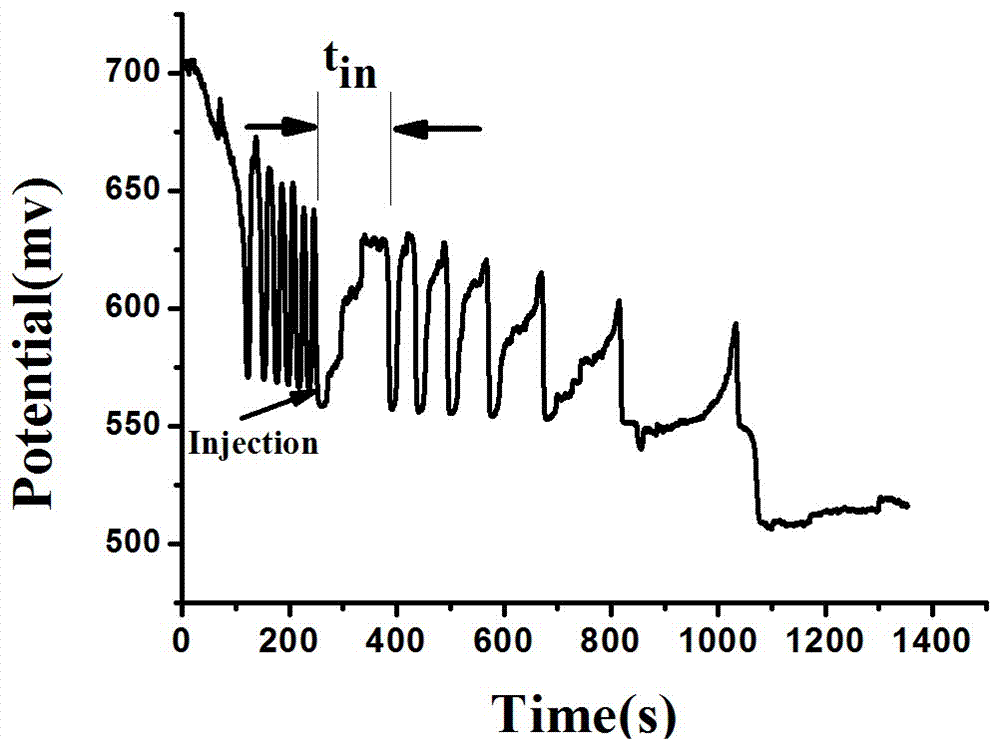
The invention relates to a method for quantitative analysis of p-coumaric acid. The method utilizes a H2SO4-KIO3-[NiL](ClO4)2-MA(malonic acid)-H2O2 non-linear chemical oscillation system as a detection solution, builds a working curve according to p-coumaric acid oscillatory response in detection and realizes quantitative analysis of p-coumaric acid. In the [NiL](ClO4)2, L represents 5, 7, 7, 12, 14, 14-hexamethyl-1, 4, 8, 11-tetraazacyclotetradecyl-4, 11-diene. The quantitative analysis method of the p-coumaric acid has the characteristics of high accuracy, operation easiness, convenience and fastness.
Owner:ANHUI UNIVERSITY
Method for preparing hypolipidemic medicine ciprofibrate with p-coumaric acid
ActiveCN105237389AShort synthetic stepsOperational securityPreparation from carboxylic acid saltsOrganic compound preparationP-Coumaric acidChloroform
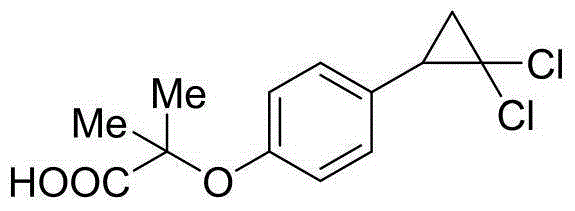
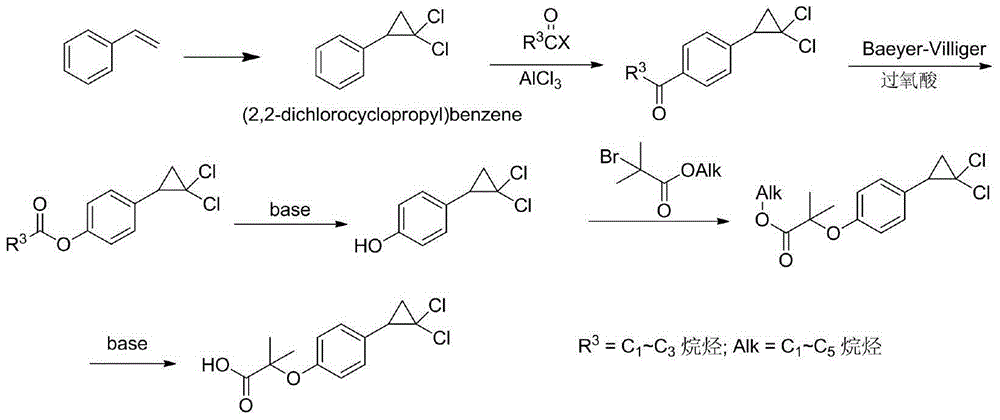
The invention discloses a method for preparing a hypolipidemic medicine ciprofibrate with p-coumaric acid. The method comprises the following specific steps: p-coumaric acid (I) is subjected to a decarboxylation reaction under the effect of an alkaline catalyst, such that p-hydroxystyrene (II) is obtained; p-hydroxystyrene (II) is subjected to a reaction with 2-haloisobutyrate under the effect of alkali, such that an etherified product (III) is obtained; under an alkaline condition, the etherified product (III) and chloroform are subjected to a cyclization reaction under the effect of a phase transfer catalyst, such that a cyclized product (IV) is obtained; the cyclized product (IV) is subjected to alcoholysis and acidification in an alkali solution; and recrystallization is carried out, such that ciprofibrate (V) is obtained. The method provided by the invention has the advantages of short synthesis process, safe operation and easy post-treatment. The method is suitable for large-scale industrialized productions, and almost has no possibility of causing accidents such as explosion. During the entire reaction process, only conventional acid, alkali and solvent are used, such that the cost is low. The solvent can be recovered and reused, such that the method is environment-friendly. With the method, the yield is improved by more than 20%.
Owner:CHENGDU LIKAI CHIRAL TECH +1
Application of p-coumaric acid ester in fruit and vegetable sterilization preservative and p-coumaric acid ester containing fruit and vegetable sterilization preservative
ActiveCN107927149AInhibition of diseaseAvoid infectionBiocideFruit and vegetables preservationAdditive ingredientFood safety
The invention belongs to the technical field of fruit and vegetable agricultural fruit storage and preservation, and particularly relates to application of p-coumaric acid ester in a fruit and vegetable sterilization preservative and the p-coumaric acid ester containing fruit and vegetable sterilization preservative. The fruit and vegetable sterilization preservative is prepared from the followingcomponents in parts by mass: 0.01-2 parts of p-coumaric acid ester, 0-50 parts of co-solvent, 0.1-10 parts of surfactant, 1-10 parts of solvent and 1000 parts of water. The fruit and vegetable sterilization preservative can be used for effectively inhibiting invasion of pathogenic bacteria, inhibiting postharvest diseases of fruits and vegetables and reducing rot loss, has high food safety sincethe main ingredient which is p-coumaric acid ester is healthy and harmless for human bodies and cannot cause environmental pollution, and can be used as a substitute product of traditional synthesizedbactericides and chemical preservatives.
Owner:CHINA AGRI UNIV
Method for biosynthesizing high-added-value compound by utilizing lignocellulose derivatives
ActiveCN112481336ASynthesis fastEfficient use ofBacteriaMicroorganism based processesEscherichia coliHydroxytyrosol
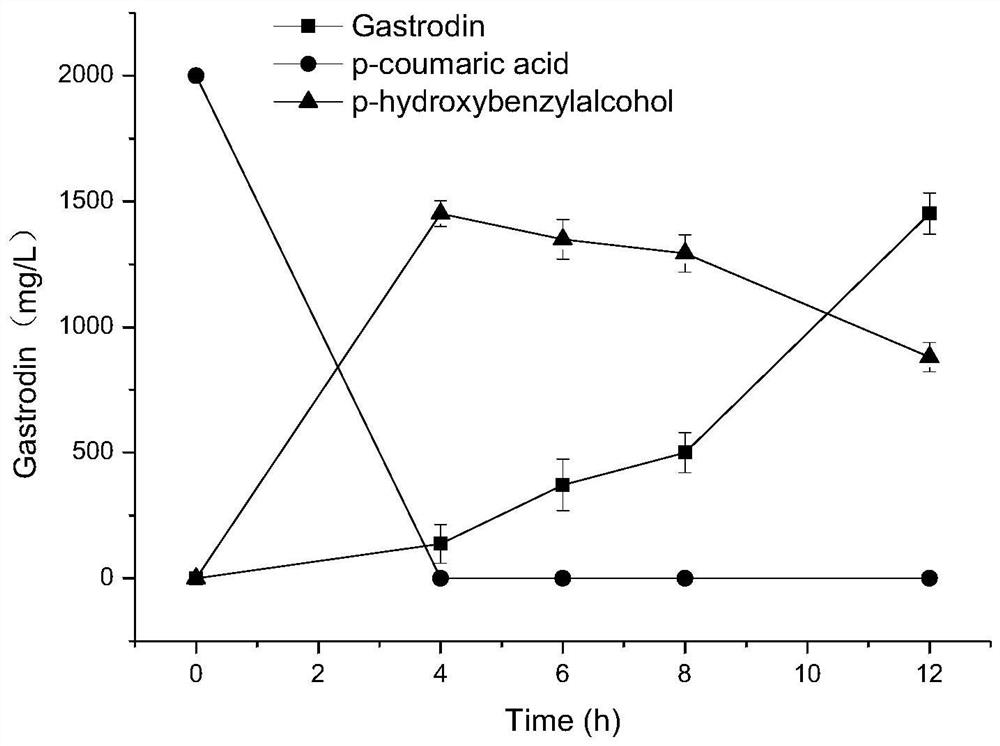
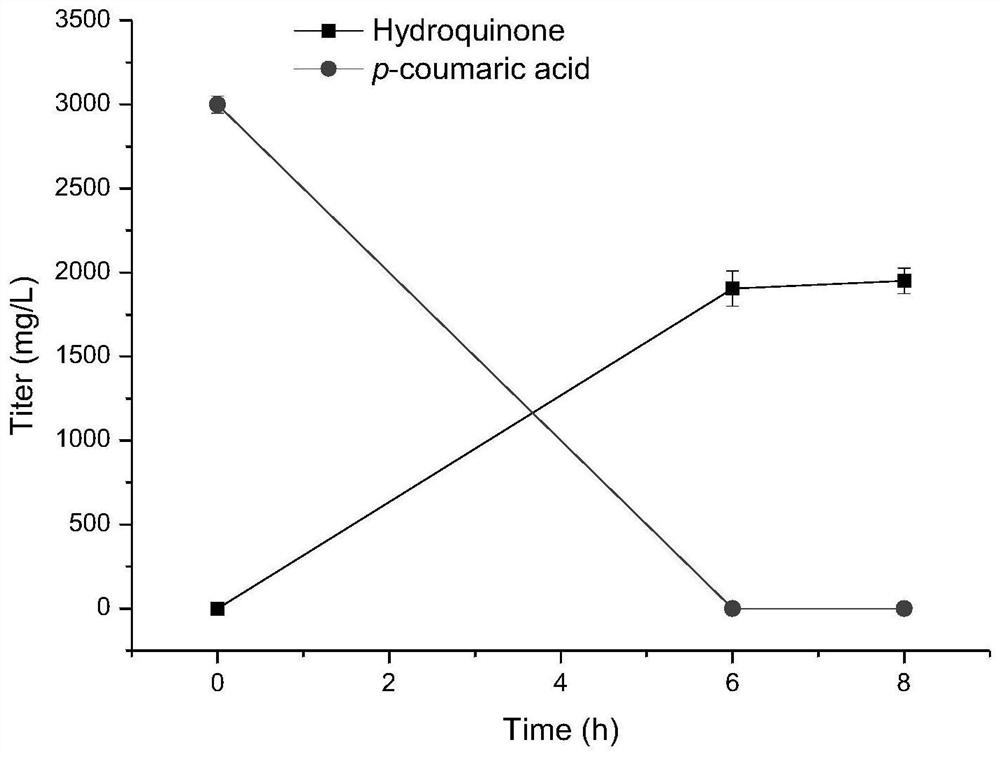
The invention discloses a method for biosynthesizing a high-added-value compound by using lignocellulose derivatives, which comprises the following steps: A, modifying Escherichia coli to obtain a biocatalyst; B, synthesizing a high-added-value compound by taking a lignocellulose derivative as an initial raw material through a biocatalyst, wherein the lignocellulose derivative comprises at least one of p-coumaric acid and ferulic acid, and the high-added-value compound comprises at least one of gastrodin, arbutin, salidroside and derivatives thereof such as hydroquinone, tyrosol, hydroxytyrosol and styracitol. According to the method, escherichia coli is modified through a genetic engineering means, three new enzymatic reaction ways are constructed, and various high-added-value compounds including gastrodin, arbutin, salidroside and the like are efficiently synthesized by utilizing lignocellulose derived aromatic compounds p-coumaric acid and ferulic acid, wherein the product yield reaches gram-level or above.
Owner:SHANGHAI JIAO TONG UNIV
A genetically engineered bacterium for synthesizing resveratrol and a constructing method thereof
InactiveCN106032525AIncrease productionAchieve truly industrial outputBacteriaMicroorganism based processesBiotechnologyTyrosine
A genetically engineered bacterium for synthesizing resveratrol is disclosed. The genetically engineered bacterium is obtained by knocking tyrR and trpED which are genes limiting tyrosine synthesis from glucose out of E.coli BW25113, and the chromosome is recombined with a tyrosine deaminase gene tal of rhodotorula glutinis, a petroselinum crispum p-coumaric acid: coenzyme A ligase gene 4cl and a stilbene synthase gene sts of grapes. The genetically engineered bacterium adopts glucose as a substrate to synthesize the resveratrol.
Owner:SHANGHAI INST OF PHARMA IND +1
Cytochrome P450 reductase 1 of lycoris aurea as amaryllidaceae plant as well as coding gene and application thereof
The invention relates to cytochrome P450 reductase as well as coding gene and application thereof. The invention discloses cytochrome P450 reductase 1 of lycoris aurea as an amaryllidaceae plant for the first time; the cytochrome P450 reductase 1 has favorable coenzyme specificity and can be used for assisting a cytochrome P450 enzyme in exerting catalytic activity to modify a synthetic product of a substrate of the cytochrome P450 enzyme in an oxidative manner. The invention also discloses a polynucleotide for coding the cytochrome P450 reductase, a carrier and a host cell for expressing the cytochrome P450 reductase and a method for producing p-coumaric acid.
Owner:INST OF BOTANY JIANGSU PROVINCE & CHINESE ACADEMY OF SCI
P-coumaric acid-3-hydroxylase as well as coding gene and application thereof
ActiveCN106906189AGood substrate specificityHigh regional selectivityOxidoreductasesGenetic engineeringRegioselectivityCaffeic acid
The invention relates to p-coumaric acid-3-hydroxylase as well as a coding gene and an application thereof. The p-coumaric acid-3-hydroxylase of lycoris plant lycoris aurea is disclosed for the first time, has good substrate specificity and region selectivity, and can catalyze hydroxylation of 3-position C of p-coumaric acid to produce coffeic acid. The invention further discloses polunecleotide coding the p-coumaric acid-3-hydroxylase, a vector and a host cell expressing the p-coumaric acid-3-hydroxylase as well as a coffeic acid production method.
Owner:INST OF BOTANY JIANGSU PROVINCE & CHINESE ACADEMY OF SCI
Composition comprising polyphenol
InactiveUS20090011103A1Promote loweringAcceptable colourOrganic active ingredientsMilk preparationCow milkingP-Coumaric acid
The invention relates to a food product comprising p-coumaric acid, wherein the level of p-coumaric acid is from 0.05 to 1.0 wt %, wherein said product is a fat based spread comprising from 10-85 wt % of fat and 10-90 wt % of water, or a drink, especially a dairy based drink, wherein the drink comprises from 10 to 95 wt % of a dairy base such as cow milk, soy milk or yoghurt, especially preferable cow milk or yoghurt. The food product has vaso-relaxating properties but still has an acceptable taste and color.
Owner:CONOPCO INC D B A UNILEVER
Method for extracting ferulic acid, p-coumaric acid and pentosan from corn husks
InactiveCN102381960AReduce consumptionIncrease productivitySugar derivativesOligosaccharidesChemical industryCoumaric acid
The invention discloses a method for extracting ferulic acid, p-coumaric acid and pentosan from corn husks, which belongs to the technical field of chemical industry. The method includes firstly, soaking corn husks into alkali solution, filtering and extracting p-coumaric acid after filtrate is neutralized to meet PH (potential of hydrogen) = 3-4 and concentrated, secondly, soaking the corn husks filtered from the first step into alkali solution, filtering and extracting ferulic acid by ethyl acetate after the filtrate is neutralized to meet PH (potential of hydrogen) = 3-4 and vacuum concentration is completed, thirdly, merging aqueous phase after the extraction of ethyl acetate in the step one and the step two, fourthly, desalinizing and condensing by means of nanofiltration, and fifthly, collecting pentosan by means of centrifugal settling after precipitated pentosan is dissolved out. The method for extracting ferulic acid, p-coumaric acid and pentosan from corn husks is short in production cycle and lower in solvent consumption, and thereby production efficiency can be further improved for enterprises.
Owner:刘启民
Pharmaceutical composition for post-operative nursing for cholangitis and preparation method of pharmaceutical composition
InactiveCN105582382AAvoid infectionAvoid siltingHeavy metal active ingredientsOrganic active ingredientsIntestinal structureNaringin
The invention discloses pharmaceutical composition for post-operative nursing for cholangitis and a preparation method of the pharmaceutical composition. The pharmaceutical composition for post-operative nursing for the cholangitis comprises components in parts by weight as follows: 12-30 parts of p-coumaric acid, 15-32 parts of naringin, 10-28 parts of cordacin, 10-25 parts of a water shield extract, 15-26 parts of mangiferin, 8-18 parts of red halloysite nano-powder, 12-24 parts of limonene, 16-28 parts of eucalyptol, 14-20 parts of chlorogenic acid, 6-12 parts of levamisole hydrochloride, 40-80 parts of a magnesium sulfate solution, 50-100 parts of corn steep liquor, 8-16 parts of potassium sorbate and 13-25 parts of mixed amino acid. Effective components of Chinese herbal medicines are added on the basis of Western medicines, the pharmaceutical composition has effects of resisting bacteria, diminishing inflammations, inhibiting intestinal infection and preventing cholestasis, effectively regulates functions of the liver, the gall bladder and intestines of a patient and improves various anomaly indexes of the body, so that relapse of the cholangitis is prevented, and post-operative body recovery of the patient can be accelerated. The pharmaceutical composition is simple in preparation process and convenient to use and has the great clinical significance.
Owner:李萍
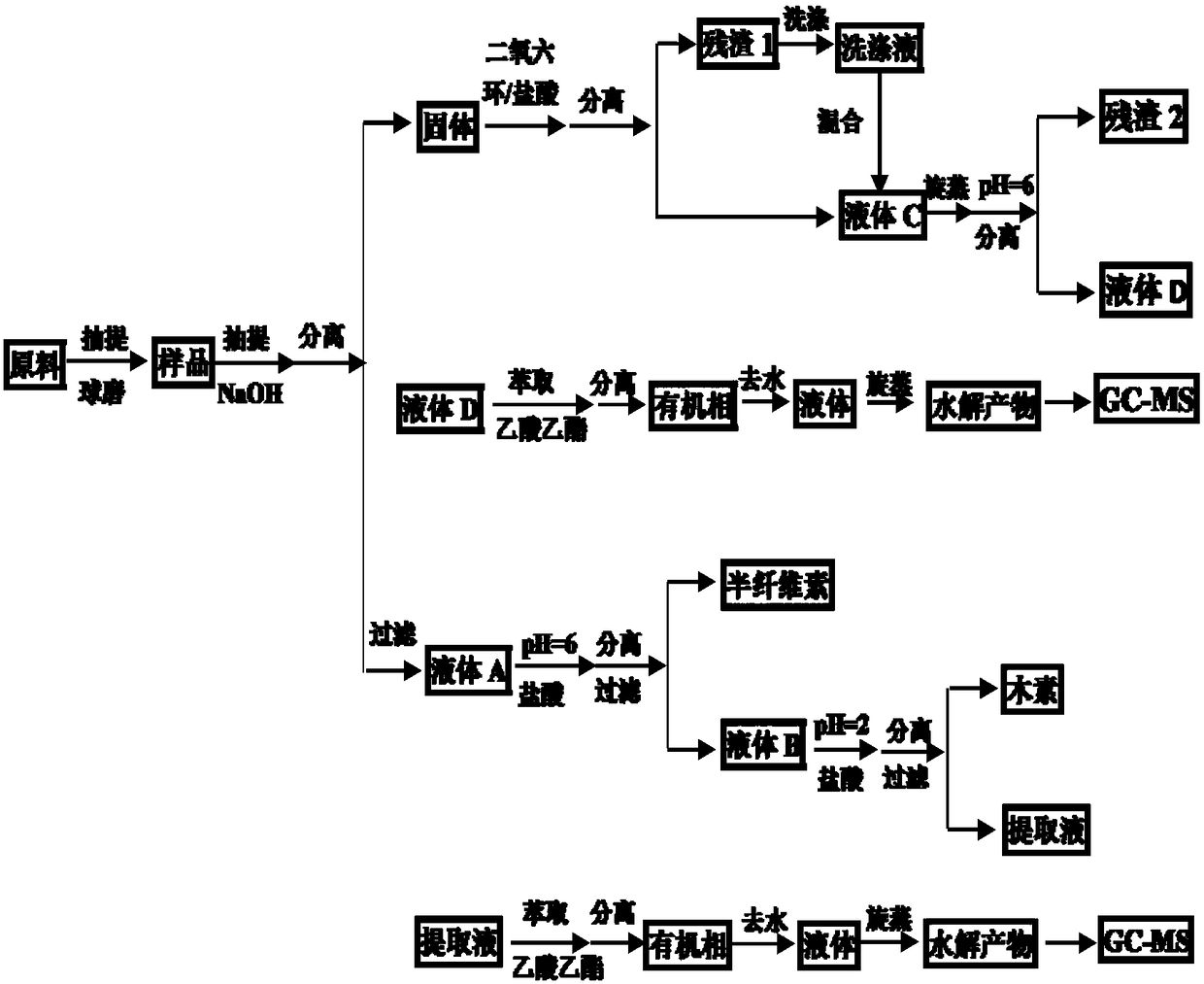
Method for improving efficiency of ferulic acid and p-coumaric acid separation
InactiveCN108168987AReduce the impact of extractionImprove extraction efficiencyComponent separationPreparing sample for investigationAcetic acidAcid hydrolysis
The invention discloses a method for improving the efficiency of ferulic acid and p-coumaric acid separation. According to the method, basic hydrolysis and acid hydrolysis are adopted in sequence to separate ferulic acid and p-coumaric acid from bagasse cell walls, and GC-MS quantitative analysis is carried out on the content of ferulic acid and p-coumaric acid. According to the method, a comprehensive method including basic hydrolysis and acid hydrolysis in sequence is adopted to separate ferulic acid and p-coumaric acid from bagasse cell walls, thereby eliminating interference of hemicellulose and lignin; ethyl acetate is taken as an extraction solvent, thereby obviously improving the yield of ferulic acid and p-coumaric acid; and GC-MS fast efficient quantitative analysis is carried outon ferulic acid and p-coumaric acid. The method establishes the foundation for efficient separation and industrial application of ferulic acid and p-coumaric acid.
Owner:GUANGXI UNIV
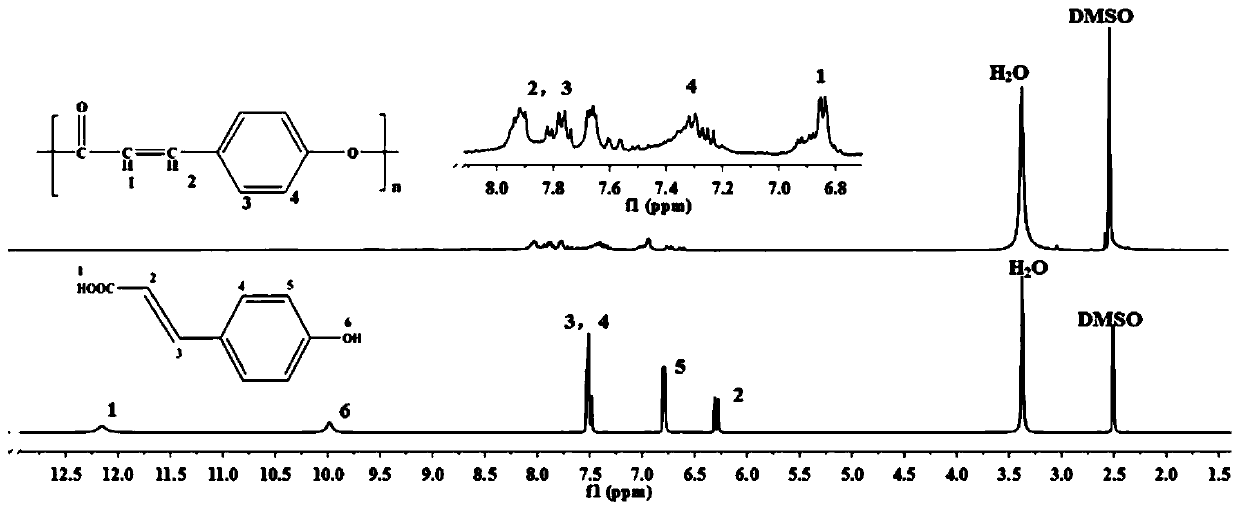
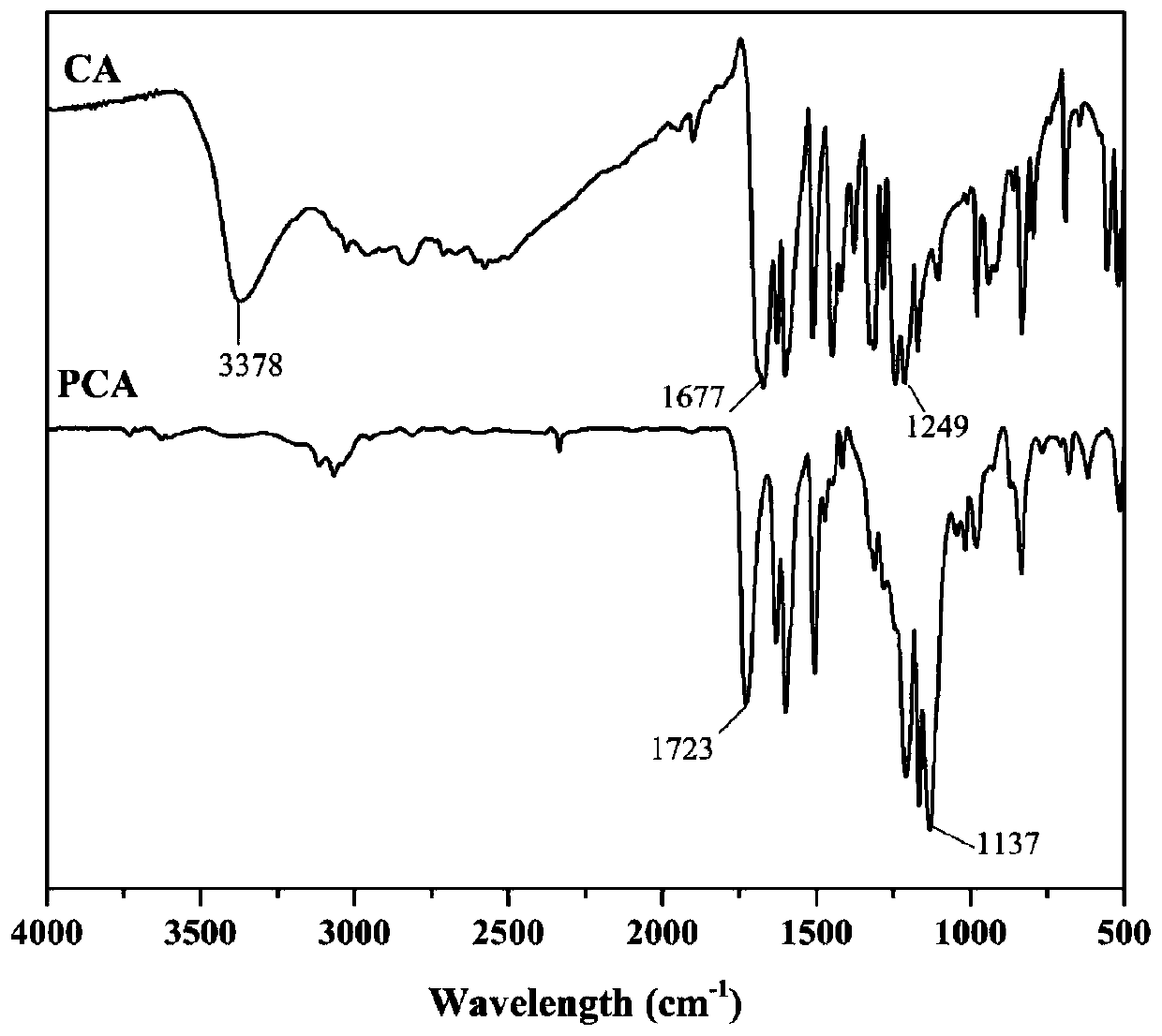
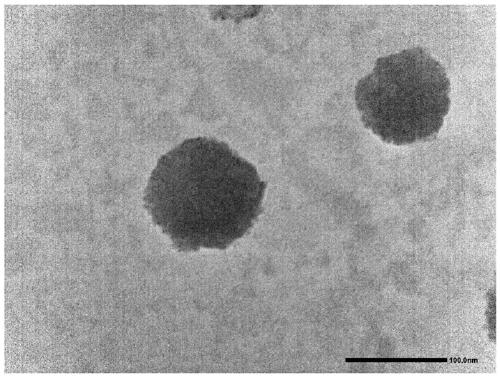
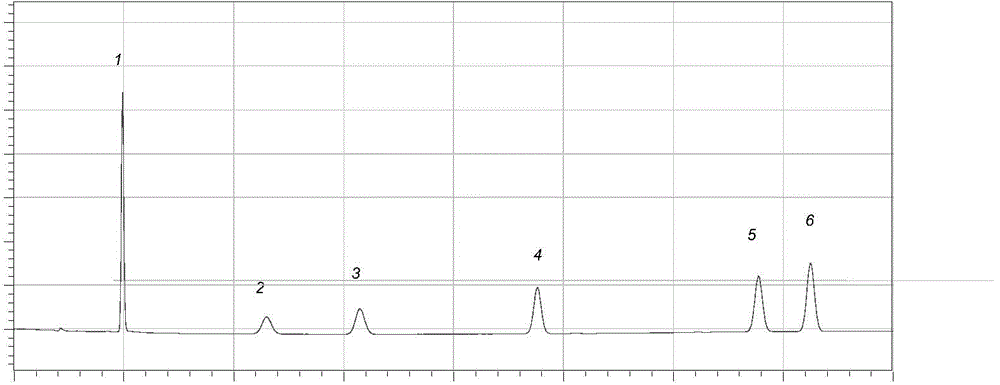
Nano drug delivery system based on p-coumaric acid polymer and preparation method and application thereof
PendingCN110302174APromote degradationGood biocompatibilityOrganic active ingredientsPharmaceutical non-active ingredientsTreatment effectSide effect
The invention discloses a nano drug delivery system based on a p-coumaric acid polymer and a preparation method and an application thereof. The method synthesizes a biocompatible and biodegradable polymer PCA by a solution polycondensation method by using natural phenolic acid and p-coumaric acid as monomers; and based on the polymer PCA, the PCA nano drug delivery system is prepared by a nanoprecipitation method. The system is uniform in size and stable in nature, and can effectively load hydrophobic anticancer drugs and improve their bioavailability, significantly enhances the anti-tumor treatment effect in vitro and in vivo, and reduces the toxic side effects of the drug. In addition, a polymer carrier itself also exhibits certain anti-tumor activity, which can help enhance the efficacyof anti-cancer drugs, and opens up a new way for the application of p-coumaric acid in anti-tumor. The preparation method of the polymer and the nano-drug delivery system thereof have the advantagesof easy reaction operation, less reaction steps, short reaction period and high repeatability.
Owner:SUN YAT SEN UNIV
HPLC separate detection for polyphenols and rhodiola crenulata quality detection method
InactiveCN104569186AImprove practicalityImprove applicabilityComponent separationSalidrosideHplc method
The invention discloses an HPLC separate detection for polyphenols. Aiming at the defects of limited quantity of components, which can be simultaneously separated and detected, in a rhodiola crenulata medicinal material in the prior art, and poor comprehensive quality control effect on the rhodiola crenulata medicinal material, the invention provides a high performance liquid chromatography method for six polyphenol components comprising gallate, rhodioloside and the like, and application of the high performance liquid chromatography method in quality detection of the rhodiola crenulata medicinal material. A test article and / or a reference substance for the detection method disclosed by the invention comprise at least one of gallate, rhodioloside, butyl alcohol, catechinic acid, ethyl gallate and p-coumaric acid; the chromatographic conditions are that a C18 chromatographic column is adopted, and the column temperature is 25 DEG C; the mobile phase is a mixed solution of acetonitrile and 0.04% phosphoric acid solution; the volume percent of the acetonitrile is 7%-20%; and gradient elution is carried out. The invention further provides a rhodiola crenulata quality detection method. The HPLC method in the detection method disclosed by the invention has conventional chromatographic conditions; special treatment on samples is not required; and the practicability and the applicability are high.
Owner:西藏藏医药大学
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com