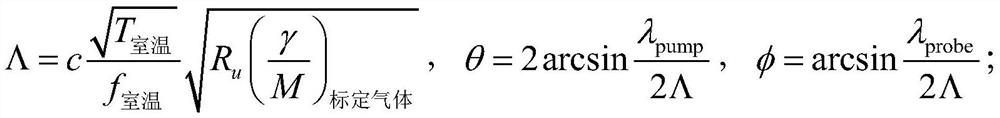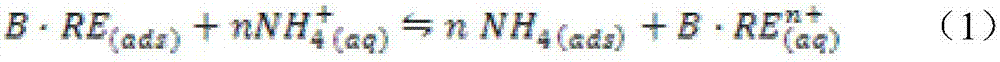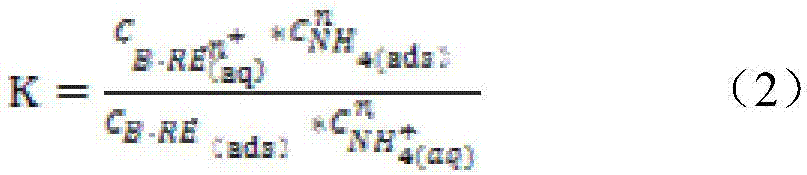Patents
Literature
133 results about "Chemical equilibrium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In a chemical reaction, chemical equilibrium is the state in which both reactants and products are present in concentrations which have no further tendency to change with time, so that there is no observable change in the properties of the system. Usually, this state results when the forward reaction proceeds at the same rate as the reverse reaction. The reaction rates of the forward and backward reactions are generally not zero, but equal. Thus, there are no net changes in the concentrations of the reactant(s) and product(s). Such a state is known as dynamic equilibrium.
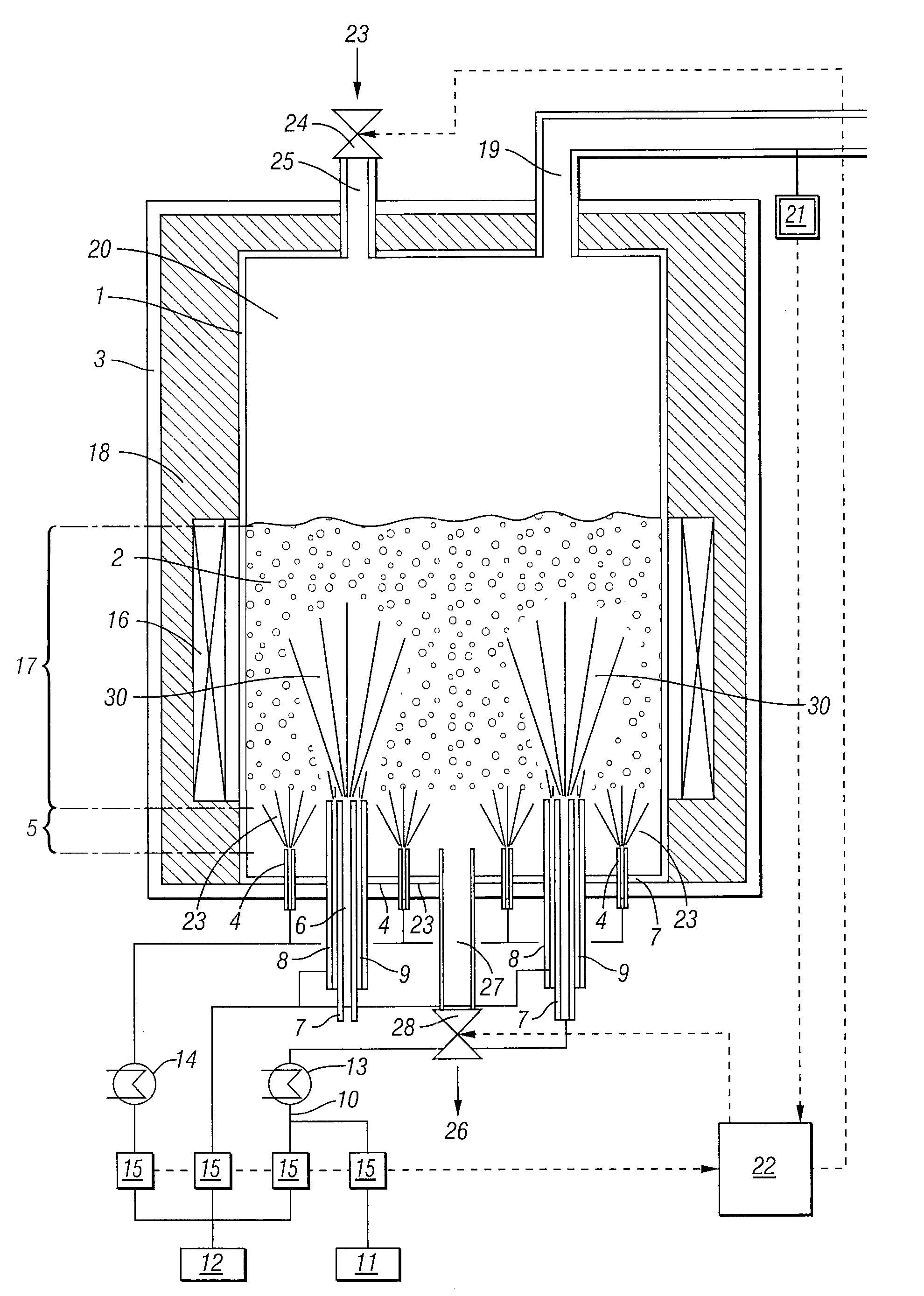
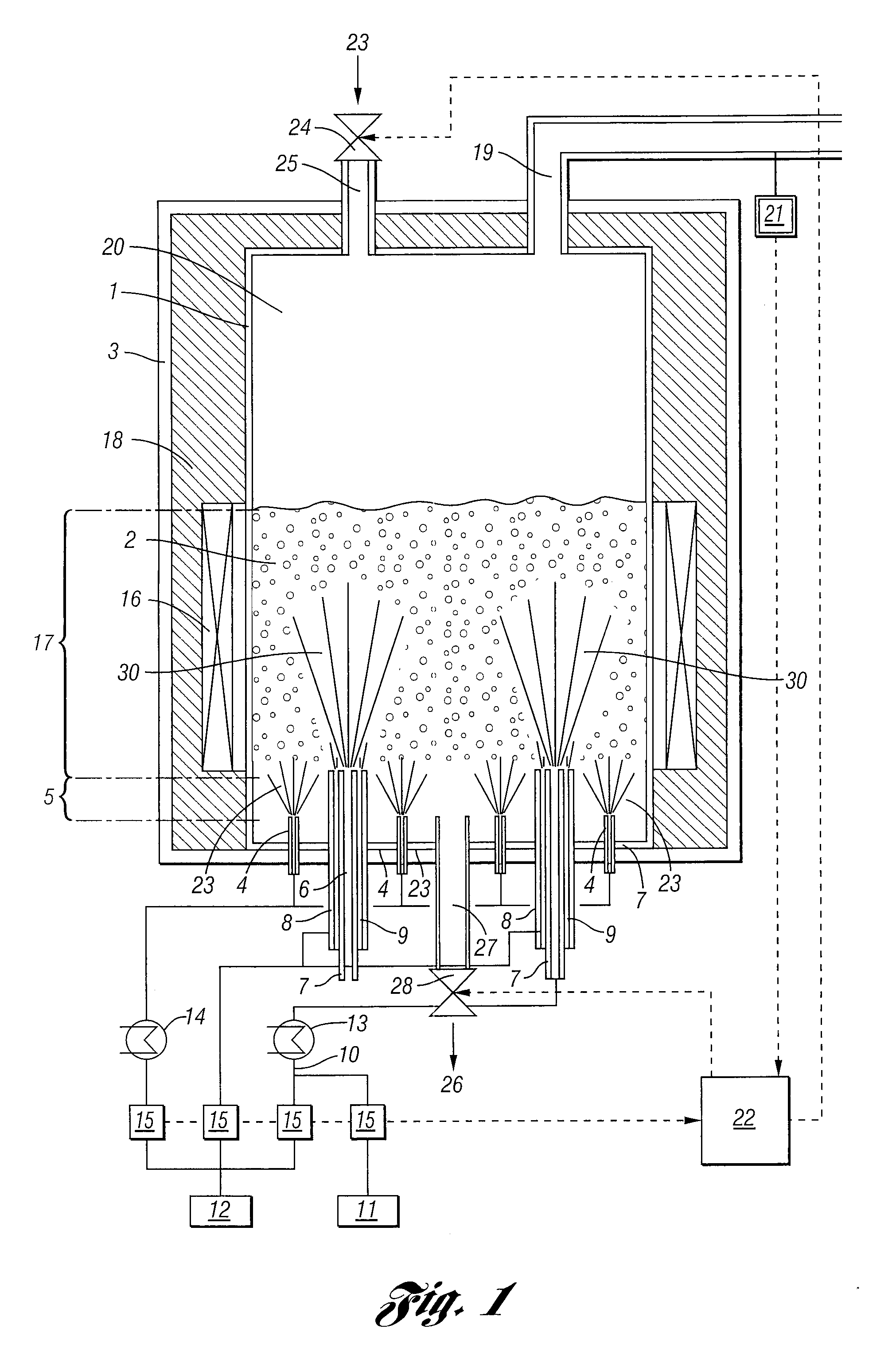
Process For The Continuous Production Of Polycrystalline High-Purity Silicon Granules
ActiveUS20080299291A1Efficient heatingLong operating campaignPolycrystalline material growthLiquid surface applicatorsFluidized bedReaction zone
High-purity polysilicon granules are prepared by depositing reaction gas on silicon granules in a fluidized bed reactor having:a reactor space comprising at least two zones lying one above the other, the lower zone weakly fluidized by introduction of a silicon-free gas into silicon granules in the lower zone by a plurality of individual dilution gas nozzles, and a second, reaction zone directly abutting the lower zone,the reaction zone heated via its outwardly bounding wall,introducing silicon-containing reaction gas as a vertical high speed gas jet into the reaction zone by reaction gas nozzle(s), forming local reaction gas jets surrounded by bubble-forming fluidized bed, gas decomposing leading to particle growth,wherein the reaction gas has fully or almost fully reacted to chemical equilibrium conversion before reaching the wall or bed surface.
Owner:WACKER CHEM GMBH
Method of manufacturing semiconductor device
A catalyst element for accelerating crystallization is added to an amorphous silicon film containing an impurity element for threshold voltage control, and a heat treatment is then performed to obtain a crystalline silicon film. Thereafter, the catalyst element is gettered by performing a heat treatment in an atmosphere containing a halogen element. In this step, a chemical equilibrium state is established for the impurity element for threshold voltage control by mixing a compound gas containing the impurity element into the atmosphere, thereby preventing the impurity element from escaping into the vapor phase.
Owner:SEMICON ENERGY LAB CO LTD
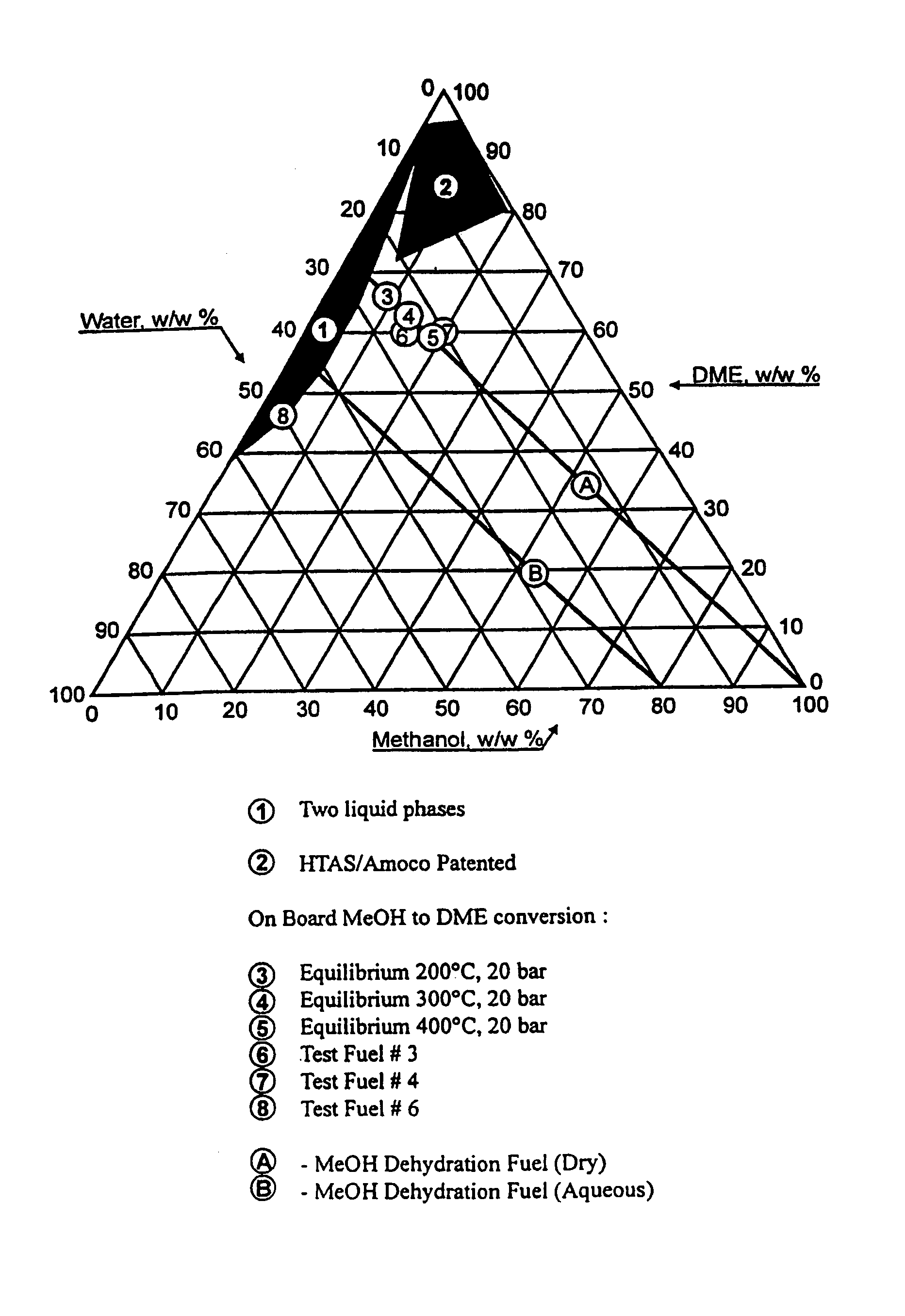
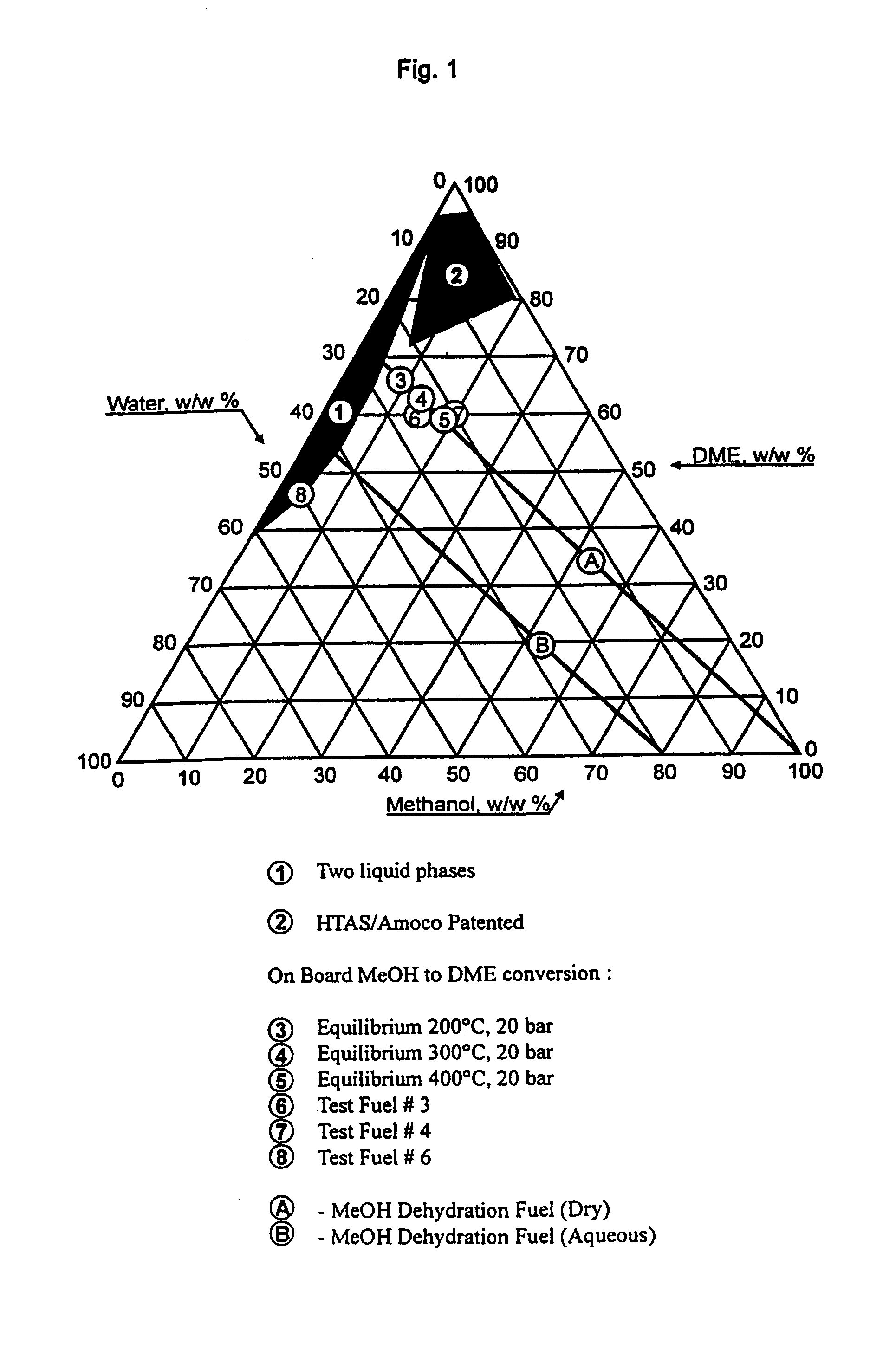
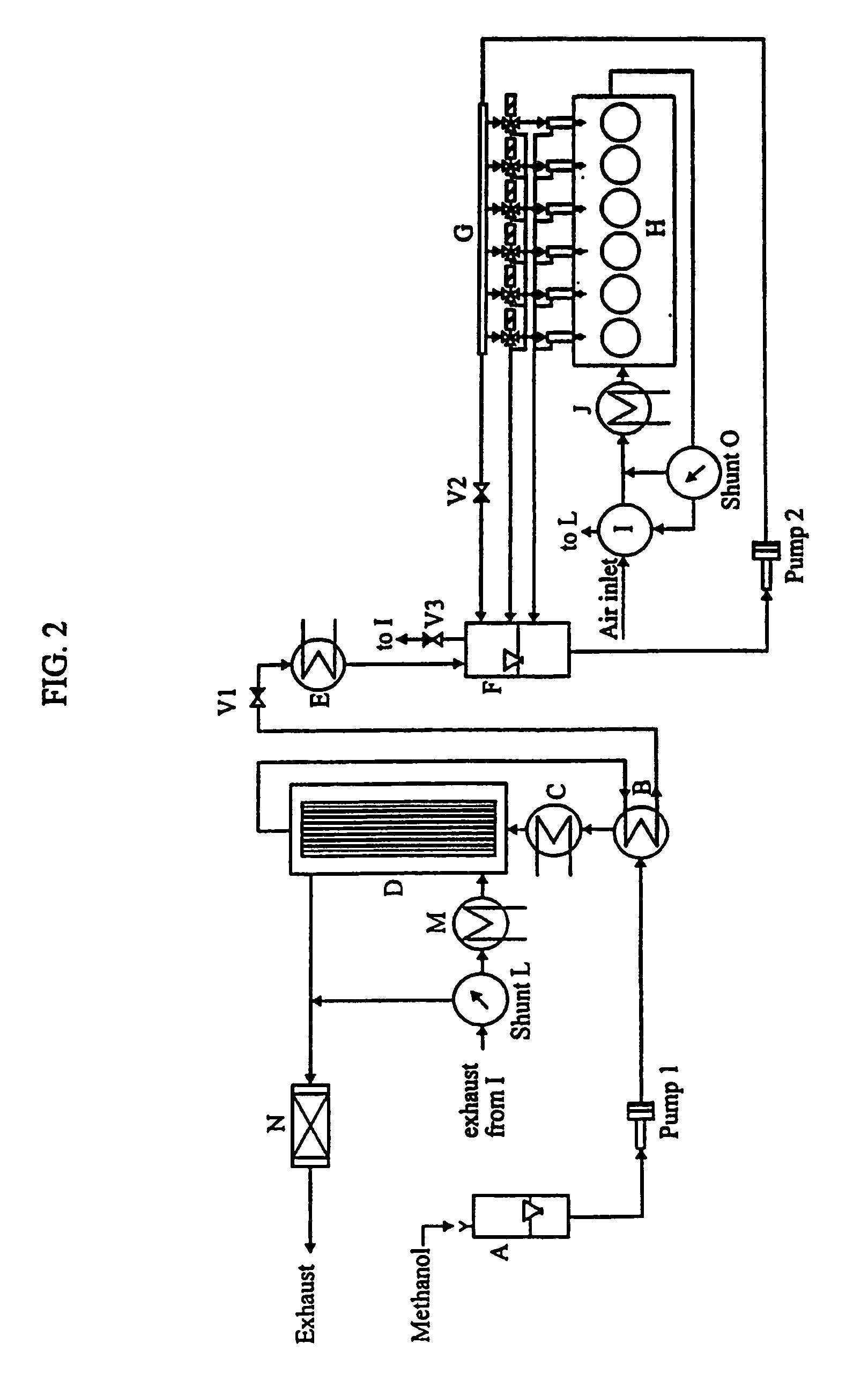
Continuous dehydration of alcohol to ether and water used as fuel for diesel engines
InactiveUS7449034B1Improve fuel efficiencyEmission reductionDomestic stoves or rangesExhaust apparatusWater useCombustion
The present invention relates to an oxygenated fuel composition suitable for use in compression ignition internal combustion engines, equipped with inlet air heater and catalytic alcohol dehydration equipment suitable for chemical equilibrium conversion of methanol and higher alcohol to their associated ether plus water.
Owner:HALDOR TOPSOE AS
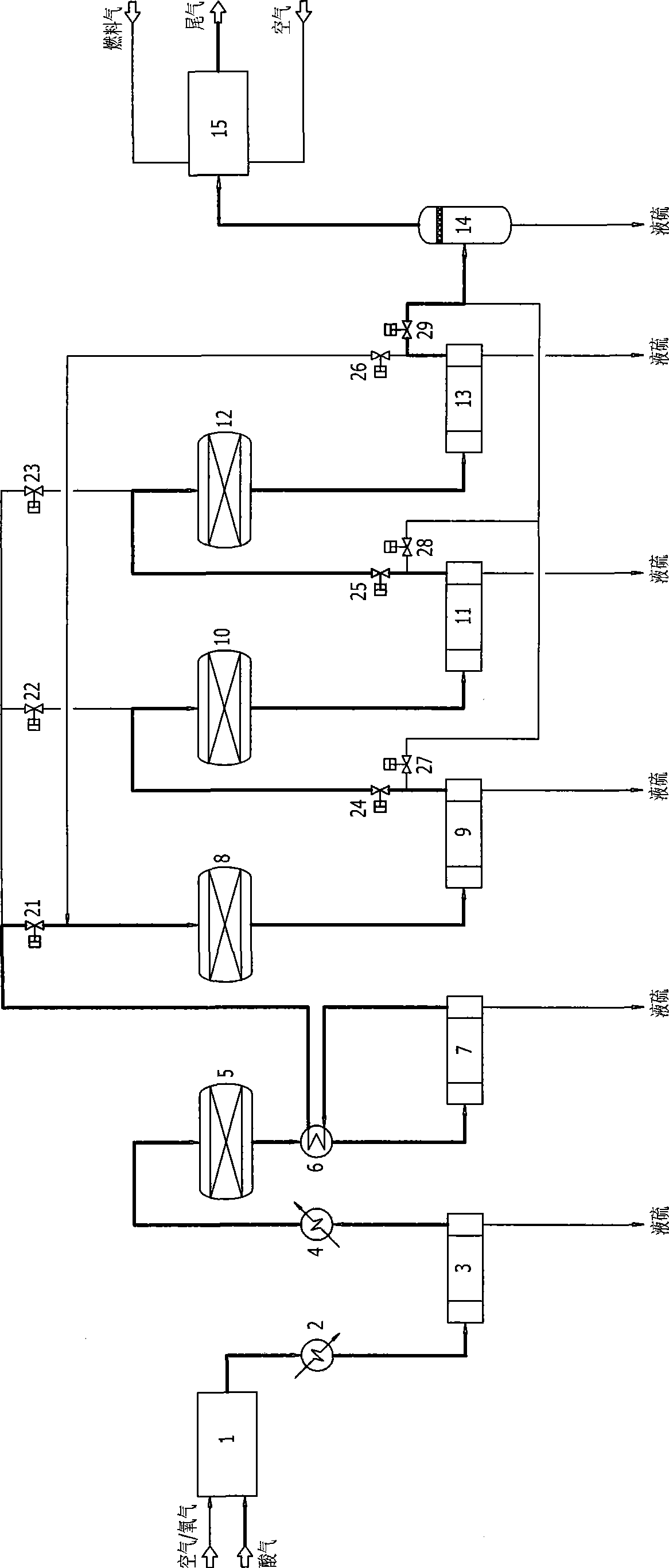
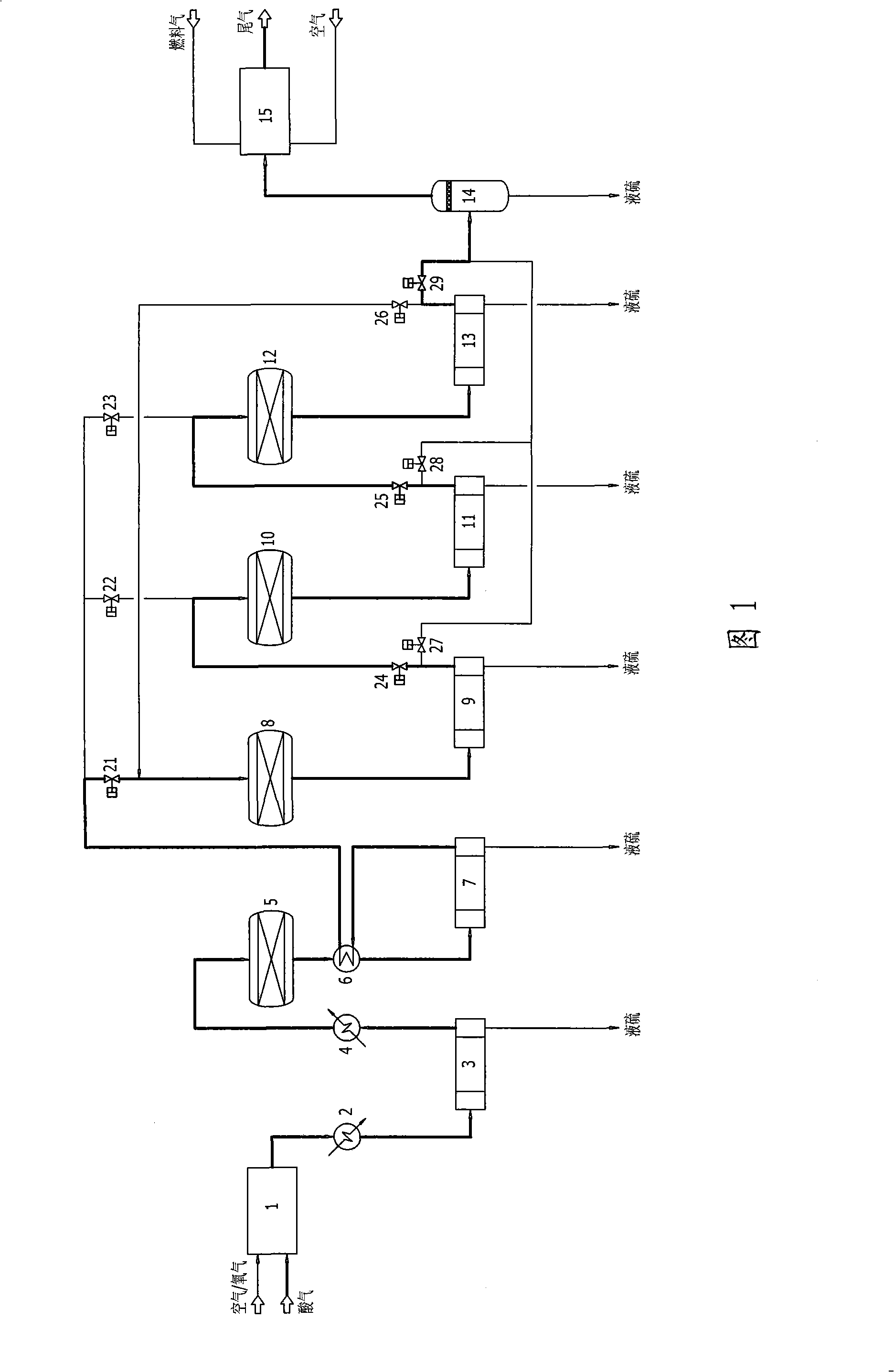
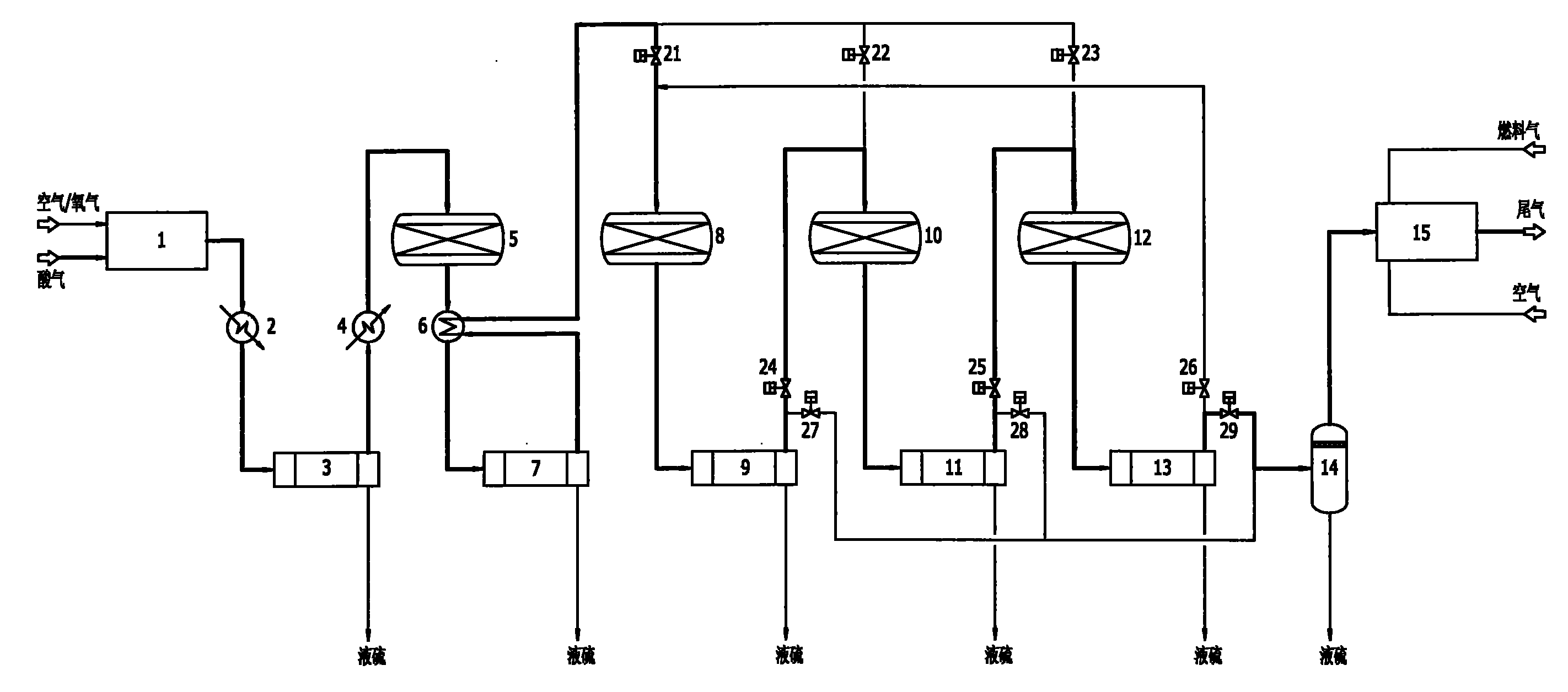
Low temperature Claus sulfur recovery process and device therefor
InactiveCN101519192AAchieve regenerationImprove conversion rateEnergy inputSulfur preparation/purificationReaction temperatureOxygen
Owner:CHENGDU SEPMEM SCI & TECH
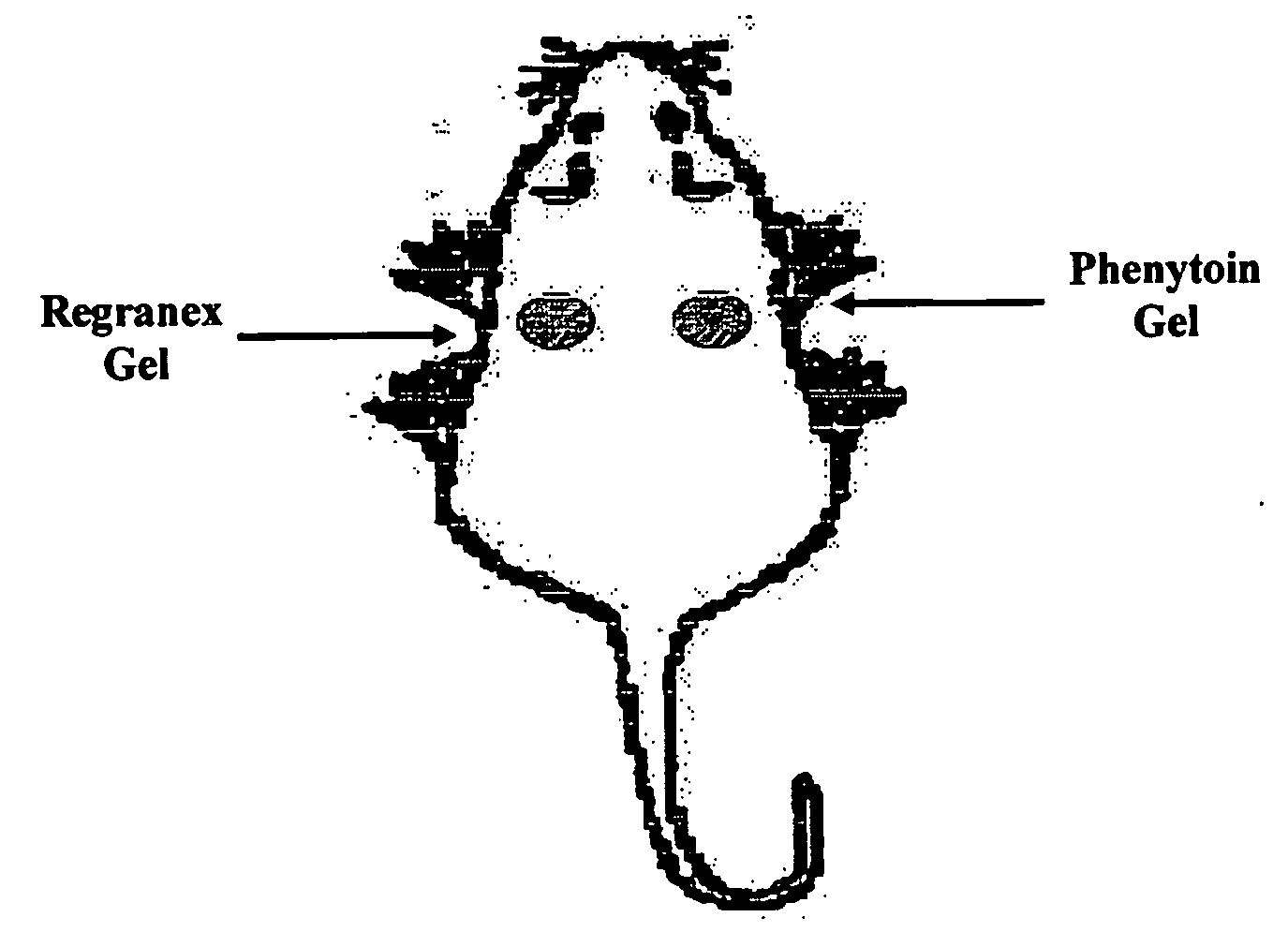
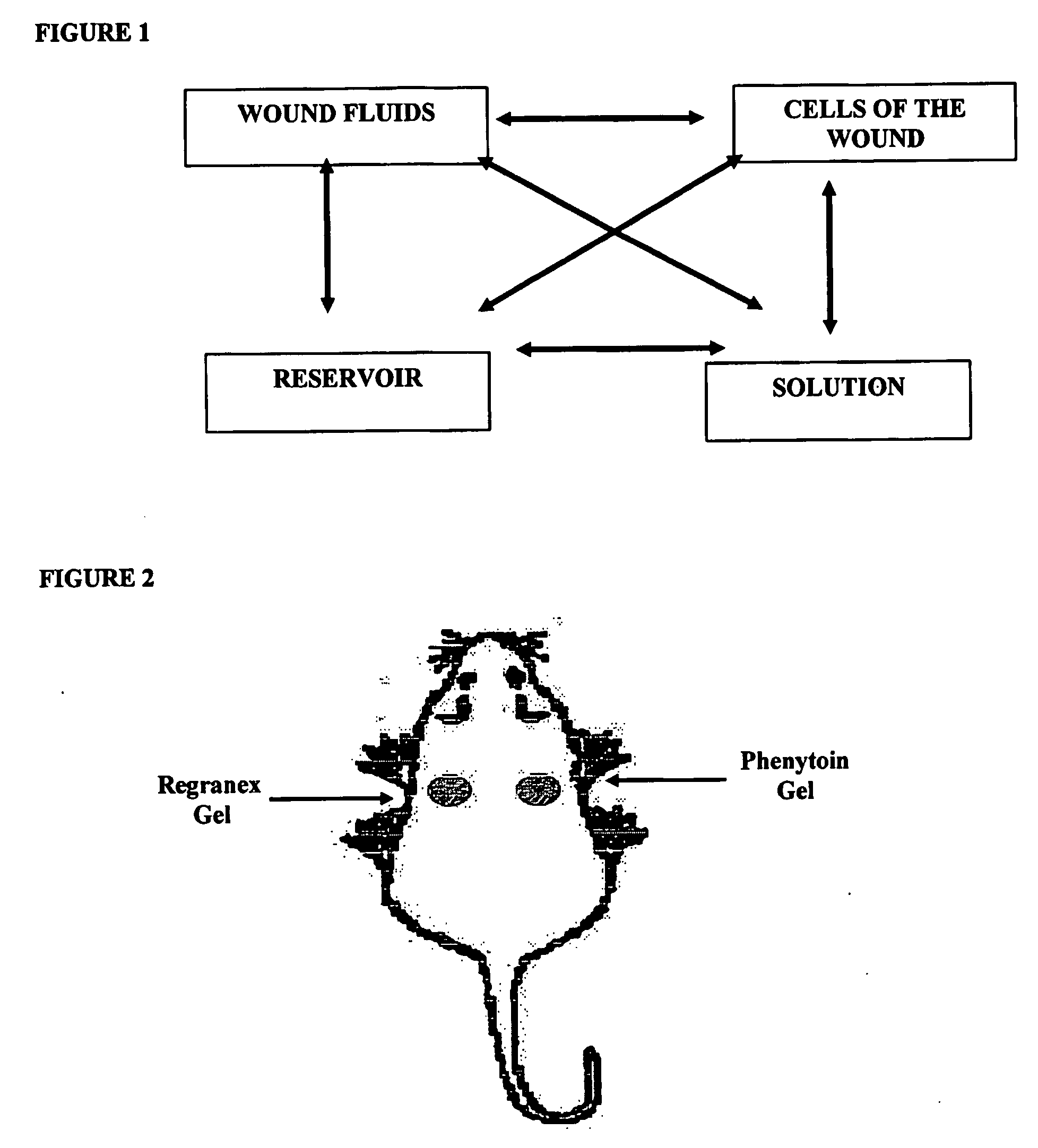
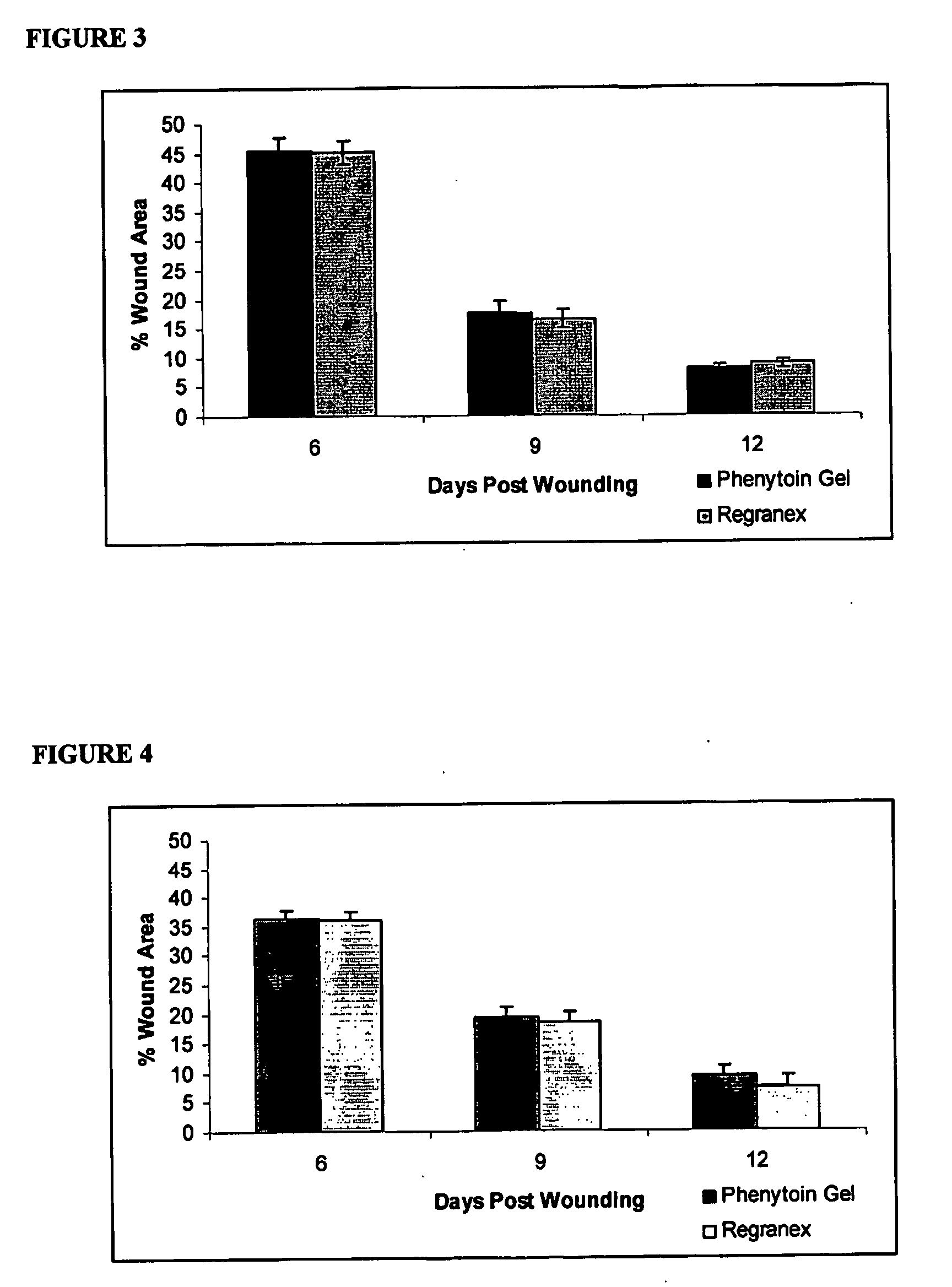
Phenytoin Formulations, and Uses Thereof in Wound Healing
InactiveUS20090022779A1Promote wound healingCut woundOrganic active ingredientsPowder deliveryWound healingPhenylbutyrate
A formulation of phenyloin suitable for topical application to a wound comprises a reservoir of phenyloin entrapped within a stabilising matrix, and an amount of dissolved phenyloin, wherein the dissolved phenyloin is in chemical equilibrium with the phenyloin entrapped within the stabilised matrix. The stabilised matrix may comprise a gel matrix, especially a gel matrix in which the polymer of the gel forms ion-pairs with the phenyloin. Also described are methods of treating a wound in diabetic and non-diabetic patients using a formulation according to the invention.
Owner:ROYAL COLLEGE OF SURGEONS & IRELAND
Device and method for recovering ammonia during wastewater treatment
InactiveCN102167454AAchieve purificationUp to L)Multistage water/sewage treatmentWater/sewage treatment by neutralisationBoiling pointGas phase
The invention belongs to the field of wastewater treatment technologies and resource recycling and relates to a device and method for recovering ammonia during wastewater treatment. In the invention, on the basis of chemical equilibrium and the difference of boiling points of NH3 and H2O, a principle that ammonia volatilizes and water does not volatilize when the temperature is controlled at 40-60 DEG C and the vacuum degree is controlled at 0.04-0.06MPa is adopted, and processes of vacuum and bubbling reinforced stirring and falling film heating are utilized under a lower temperature by utilizing a vacuum falling film bubbling and deaminizing device to enlarge the contact surface between a gas phase and a liquid phase and enhance the removal effect of ammonia nitrogen, and the volatilized ammonia is recovered by utilizing the negative pressure to avoid air pollution. By means of the method and the device disclosed by the invention, the advantages of high recovery rate, low energy consumption, convenience for operation and capability of continuous and intermittent production are achieved.
Owner:DALIAN UNIV OF TECH
Low temperature Claus sulfur recovery process and device therefor
InactiveCN101519192BImprove conversion rateReduce emission concentrationEnergy inputSulfur preparation/purificationReaction temperatureOxygen
The invention discloses a low temperature Claus sulfur recovery process. The process mainly comprises a thermal reaction section, a catalytic reaction section and a tail gas incineration section, wherThe invention discloses a low temperature Claus sulfur recovery process. The process mainly comprises a thermal reaction section, a catalytic reaction section and a tail gas incineration section, whertion temperature, contributes to performance of chemical equilibrium towards the direction of sulfur generation, so that conversion rate and recovery rate of sulfur are improved. Moreover, the processtion temperature, contributes to performance of chemical equilibrium towards the direction of sulfur generation, so that conversion rate and recovery rate of sulfur are improved. Moreover, the process has the advantages of simple process flow, relative small equipment and investment, low operation cost and more contribution to environmental protection. The invention also discloses a device for thehas the advantages of simple process flow, relative small equipment and investment, low operation cost and more contribution to environmental protection. The invention also discloses a device for the low temperature Claus sulfur recovery process.low temperature Claus sulfur recovery process.ein in a combustion furnace of the thermal reaction section, partial hydrogen sulfide reacts with oxygen to be converted into sulfur dioxide, the hydrogen sulfide and the sulfur dioxide undergo a Clauein in a combustion furnace of the thermal reaction section, partial hydrogen sulfide reacts with oxygen to be converted into sulfur dioxide, the hydrogen sulfide and the sulfur dioxide undergo a Claus reaction to generate sulfur, and process gas after sulfur separation enters the catalytic reaction section; in the reactor of each stage of the catalytic reaction section, the hydrogen sulfide and ts reaction to generate sulfur, and process gas after sulfur separation enters the catalytic reaction section; in the reactor of each stage of the catalytic reaction section, the hydrogen sulfide and the sulfur dioxide under conventional Claus reaction, catalyst reactivation, sub-dewpoint and sub-solid point low temperature Claus reaction in sequence; after the catalyst reaction section, the tail ghe sulfur dioxide under conventional Claus reaction, catalyst reactivation, sub-dewpoint and sub-solid point low temperature Claus reaction in sequence; after the catalyst reaction section, the tail gas which is subjected to sulfur separation enters the tail gas incineration section, and the tail gas is incinerated and exhausted in the tail gas incineration furnace. The process adopts a lower reacas which is subjected to sulfur separation enters the tail gas incineration section, and the tail gas is incinerated and exhausted in the tail gas incineration furnace. The process adopts a lower reac
Owner:CHENGDU SEPMEM SCI & TECH
Preparation method of plasticizer diethylene glycol dibenzoate
InactiveCN103086891AImprove conversion rateGood choiceOrganic compound preparationCarboxylic acid esters preparationBenzoic acidReflux
The invention relates to a preparation method of a plasticizer, particularly a preparation method of a plasticizer diethylene glycol dibenzoate, which comprises the following steps: heating benzoic acid and diethylene glycol until the benzoic acid is dissolved, adding a catalyst to carry out reflux reaction, starting a vacuum pump, heating to 200-220 DEG C, keeping the temperature to react under reduced pressure, and stopping the reaction when no water is distilled out, thereby obtaining a diethylene glycol dibenzoate crude product; and cooling the obtained diethylene glycol dibenzoate crude product, neutralizing with an Na2CO3 water solution to remove excessive benzoic acid, washing, separating out the ester layer, and dehydrating by vacuum distillation to obtain a colorless transparent oil liquid which is the plasticizer diethylene glycol dibenzoate product. The technique is simple, does not use any water-carrying agent, continuously distils out water in the reaction under negative pressure, breaks the chemical equilibrium of the esterification reaction, shortens the reaction time, greatly enhances the benzoic acid conversion rate, has the advantages of high esterification rate (up to higher than 98%), favorable catalyst selectivity, fewer side reactions, light product color and no need of activated carbon for decolorization, and is suitable for industrial production and environment-friendly.
Owner:XIAMEN UNIV
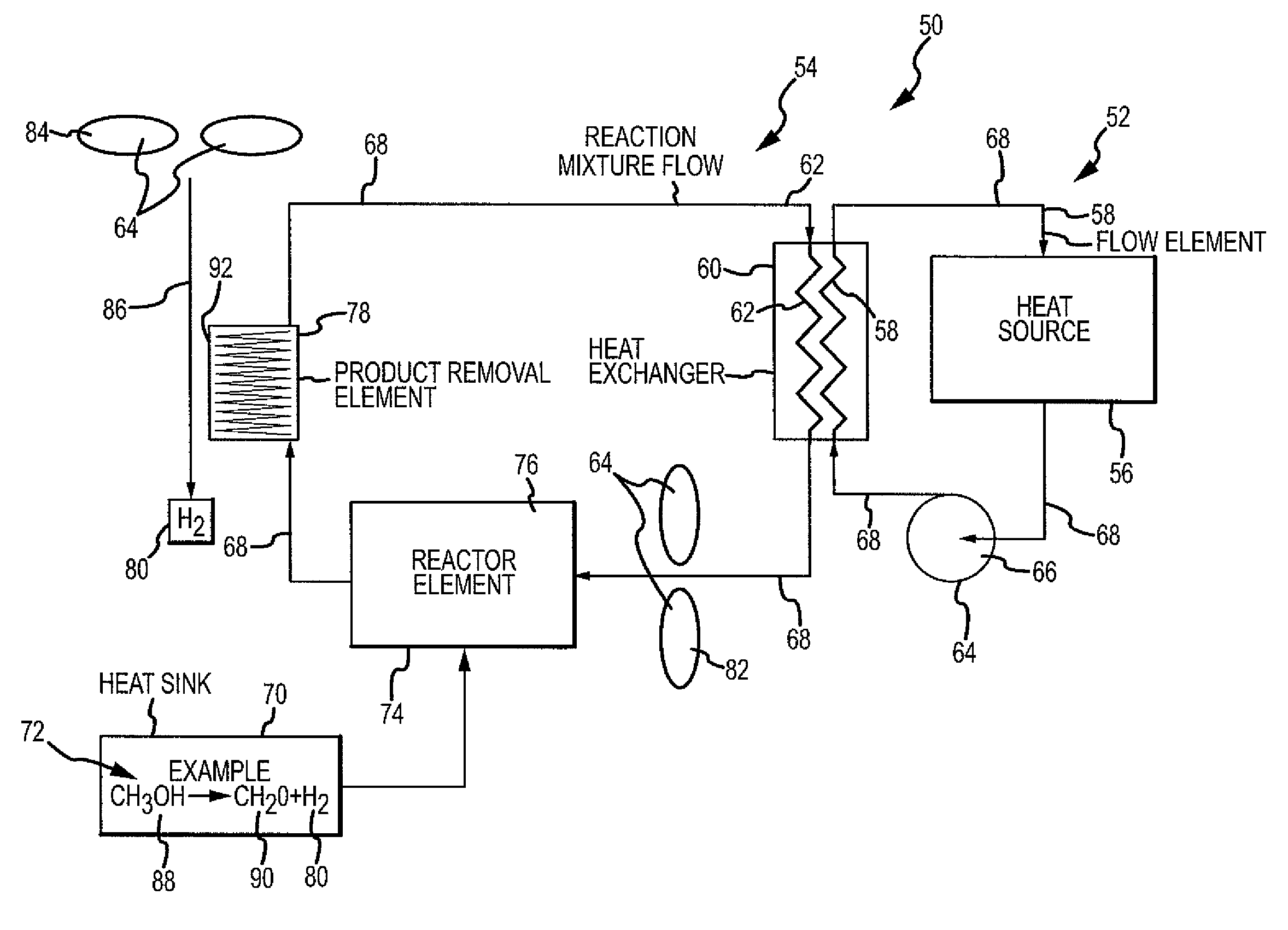
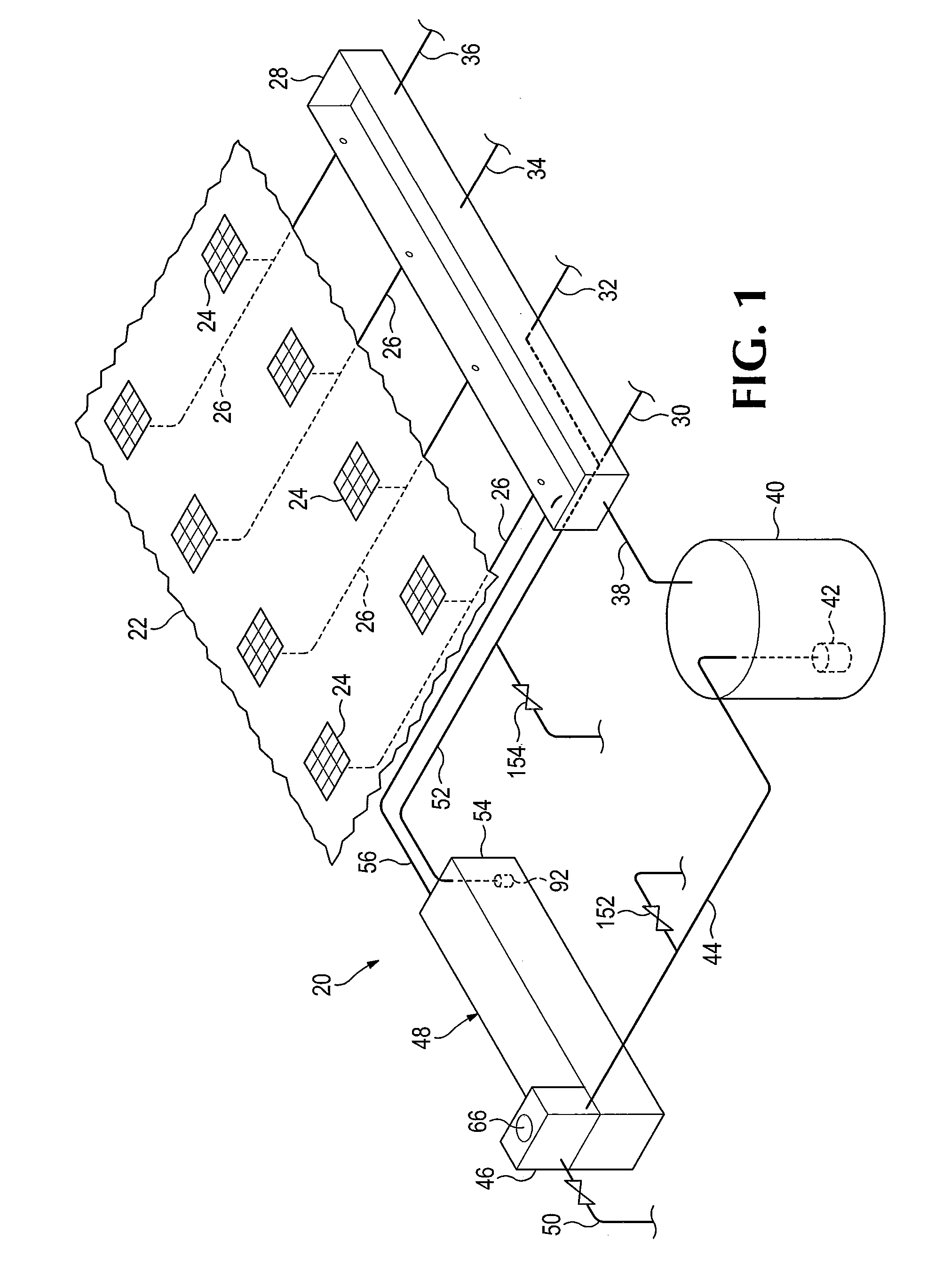
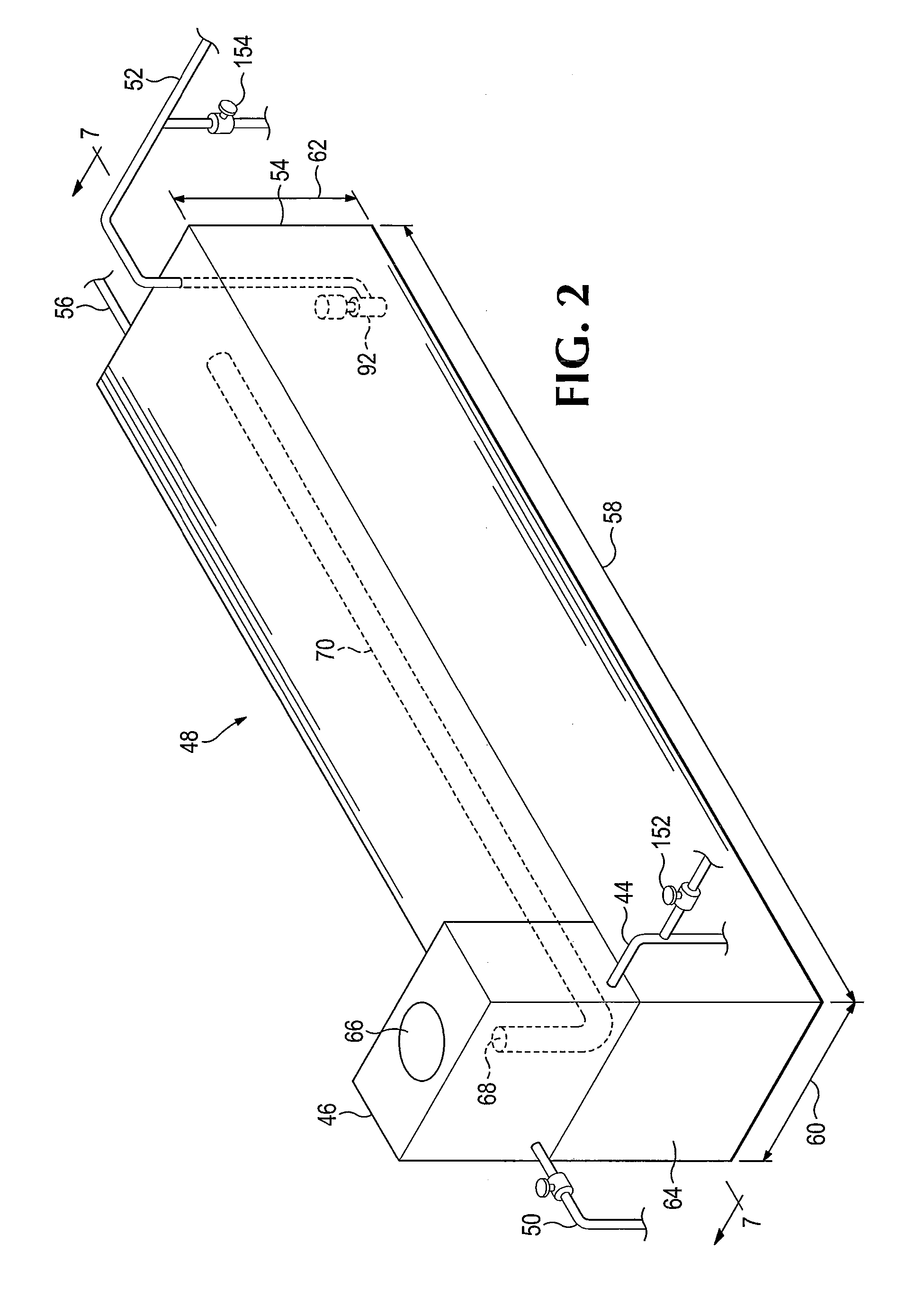
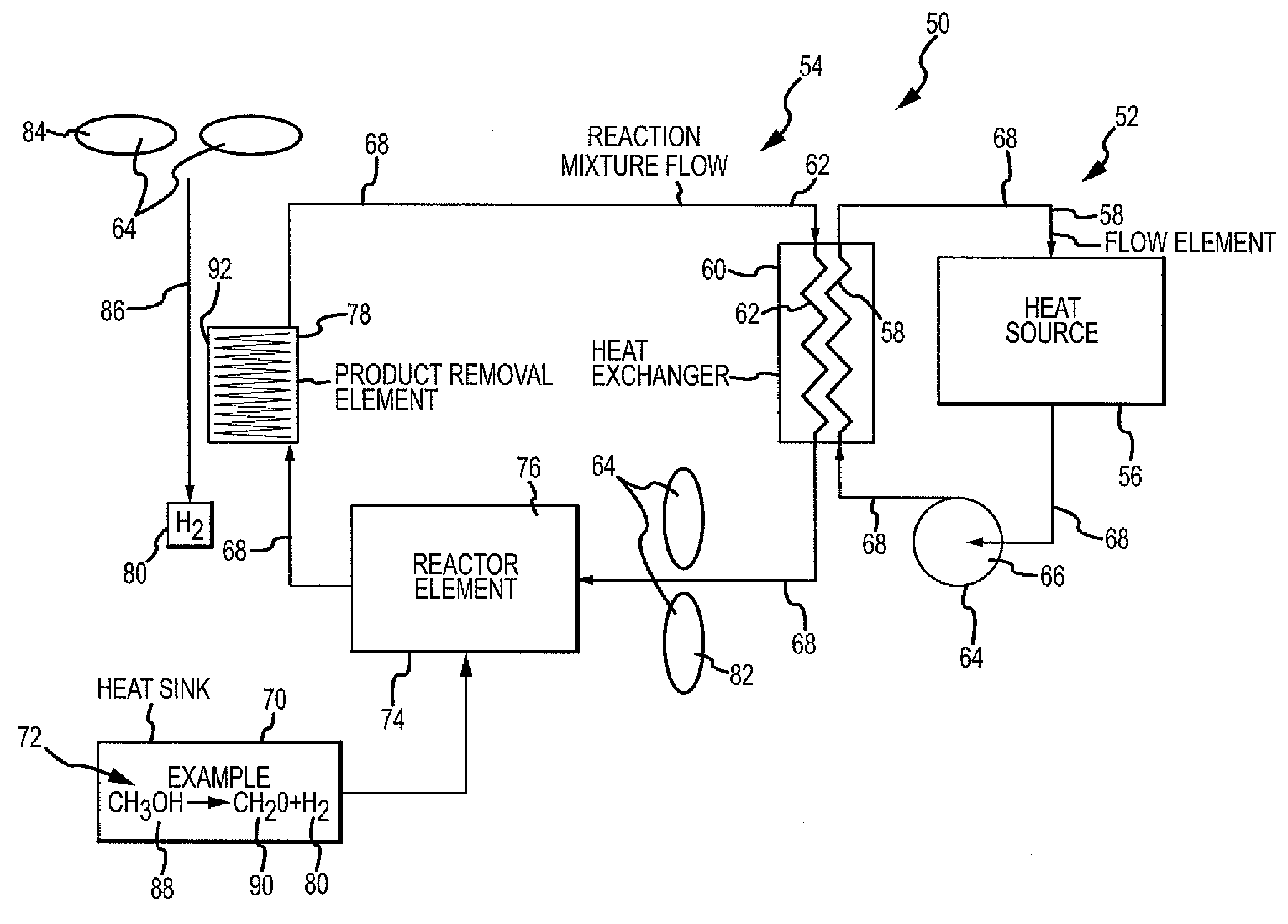
Chemical reaction-based thermal management system and method
ActiveUS7955568B2Improve performanceIncrease heating capacityCosmonautic vehiclesDomestic cooling apparatusChemical reactionProcess engineering
The disclosure provides for a chemical reaction-based thermal management system and method. The system comprises a heat source for heating a first flow element, a heat exchanger for transferring heat from the first flow element to a reaction mixture flow, and a heat sink comprising one or more endothermic chemical reactions to absorb heat from the heat exchanger. The system further comprises a reactor element for approaching chemical equilibrium of the reaction mixture flow, a product removal element for removing one or more products from the one or more endothermic chemical reactions, and a plurality of driver elements for moving the first flow element and for moving the reaction mixture flow.
Owner:THE BOEING CO
Laser-enhanced three-dimensional micro-region electro-deposition method and corresponding device thereof
InactiveCN110565130AImprove electron transfer efficiencyLess stringent requirementsElectrolysis componentsElectrochemistryThermal effect
The invention provides a laser-enhanced three-dimensional micro-region electro-deposition method. the method integrates laser irradiation into a microtubule-based micro-region electro-deposition device to obtain an improved deposition rate. Electrolyte is poured into an electrolyte chamber and a microtubule, a tip of the microtubule is close to a sample surface by the regulation of a translation stage, a droplet at the tip of the microtubule forms a meniscus on the sample surface so that the diffusion of the droplet on the sample surface is limited, and machining accuracy of electro-depositionis beneficially controlled. As the electrolyte in the microtubule is connected with the positive potential and the surface of a conductive sample is the negative potential, the electro-deposition process can occur. The laser is irradiated on the contact surface between the droplet and the sample, and a laser thermal effect improves the electron transfer efficiency in the electrolyte, acceleratesthe positive shift of the electrochemical balance potential and causes local eddy current stirring in the droplet, and therefore accelerates the electro-deposition process. According to the preset pattern, the electronic control transition stage movement is regulated, so the three-dimensional relative movement of the microtubule and the sample surface is realized.
Owner:橙河微系统科技(上海)有限公司
Treatment of Storm Water
ActiveUS20140353225A1Reduce significantly the total copper and total zincReduce flow rateWater cleaningTreatment using aerobic processesFiltrationCompost
A treatment system for collecting storm water from an industrial area and removing suspended and dissolved pollutant materials from the storm water. Storm water is accumulated and delivered to a filtration apparatus in which suspended pollutants are removed by filtration, while dissolved materials, particularly heavy metals including zinc and copper, are removed by chemical chelation in a bed of compost filter media having a high humic acid content and which is kept consistently moist. A sparger provides for wide and generally uniform distribution of the storm water over the bed of filter media and provides for slow passage of the storm water through the filter bed, thus giving a long contact time of the storm water with the filter media in order to promote and enhance chemical chelation progress to chemical equilibrium and removal of a high percentage of dissolved metals, and particularly dissolved copper and zinc.
Owner:GUNDERSON MARINE LLC +1
Chemical reaction-based thermal management system and method
ActiveUS20100236758A1Improve performanceSpeed up the conversion processDomestic cooling apparatusAir-treatment apparatus arrangementsChemical reactionCompound (substance)
The disclosure provides for a chemical reaction-based thermal management system and method. The system comprises a heat source for heating a first flow element, a heat exchanger for transferring heat from the first flow element to a reaction mixture flow, and a heat sink comprising one or more endothermic chemical reactions to absorb heat from the heat exchanger. The system further comprises a reactor element for approaching chemical equilibrium of the reaction mixture flow, a product removal element for removing one or more products from the one or more endothermic chemical reactions, and a plurality of driver elements for moving the first flow element and for moving the reaction mixture flow.
Owner:THE BOEING CO
A method for continuously preparing dichloropropanol with glycerol and hydrochloric acid
InactiveCN102295529ABroaden the range of sourcesReduce manufacturing costPreparation by halogen introductionAlkaline earth metalPretreatment method
The object of the invention is to provide a method for synthesizing dichloropropanol with glycerin and hydrochloric acid. After pretreated industrial hydrochloric acid or pretreated industrial by-product hydrochloric acid or reagent hydrochloric acid is mixed with glycerin and catalyst in a batching tank, continuous Put it into a reactor equipped with a fractionation device on the top, continue heating under normal pressure for chlorination reaction, the temperature is controlled at 100-200°C, while continuously performing chlorination reaction, continuously distill the material in the reactor, and the product dichloro Propanol and water are continuously taken out from the reaction system after being condensed into liquid by the condenser; the same pretreatment method of industrial hydrochloric acid and industrial by-product hydrochloric acid of the present invention is to add one or more phosphorus-containing and / or sulfur-containing hydrochloric acid to the hydrochloric acid to be treated. Alkali metal and / or alkaline earth metal salts of inorganic acids, added in an amount of 0.1 to 15% of the weight of the hydrochloric acid to be treated, stirred at a temperature of 0 to 100°C for 0.5 to 15 hours; And the by-product hydrochloric acid provides a good outlet, which is conducive to reducing the production cost of dichloropropanol.
Owner:江西省化学工业研究所 +1
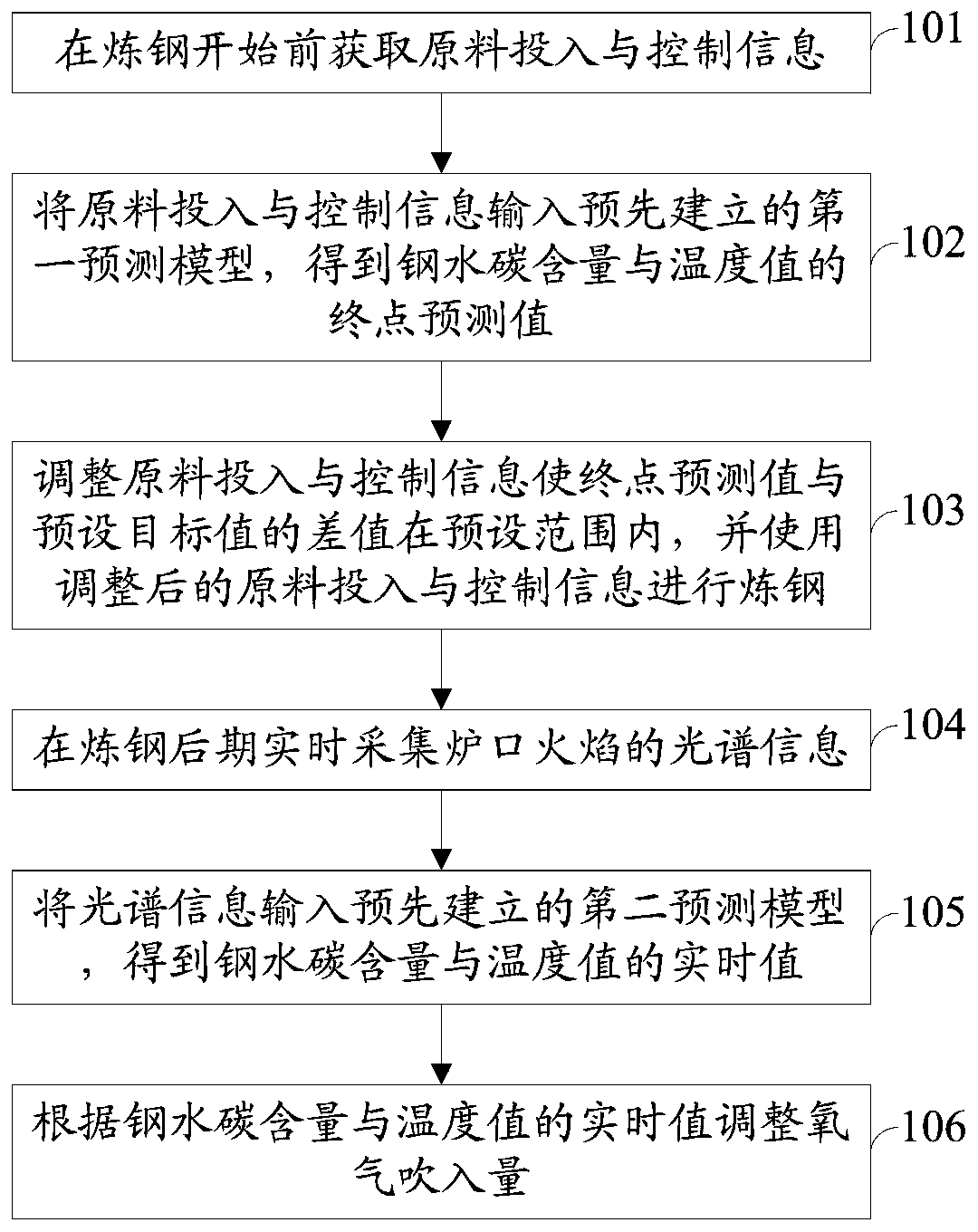
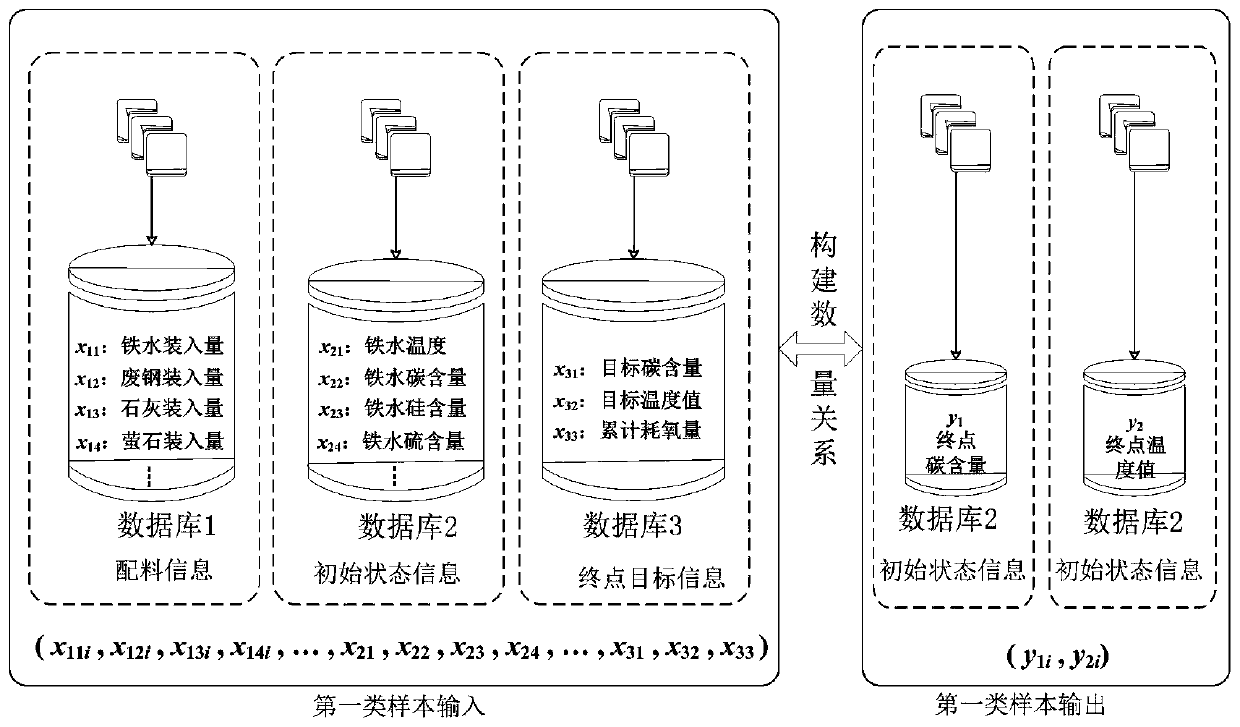
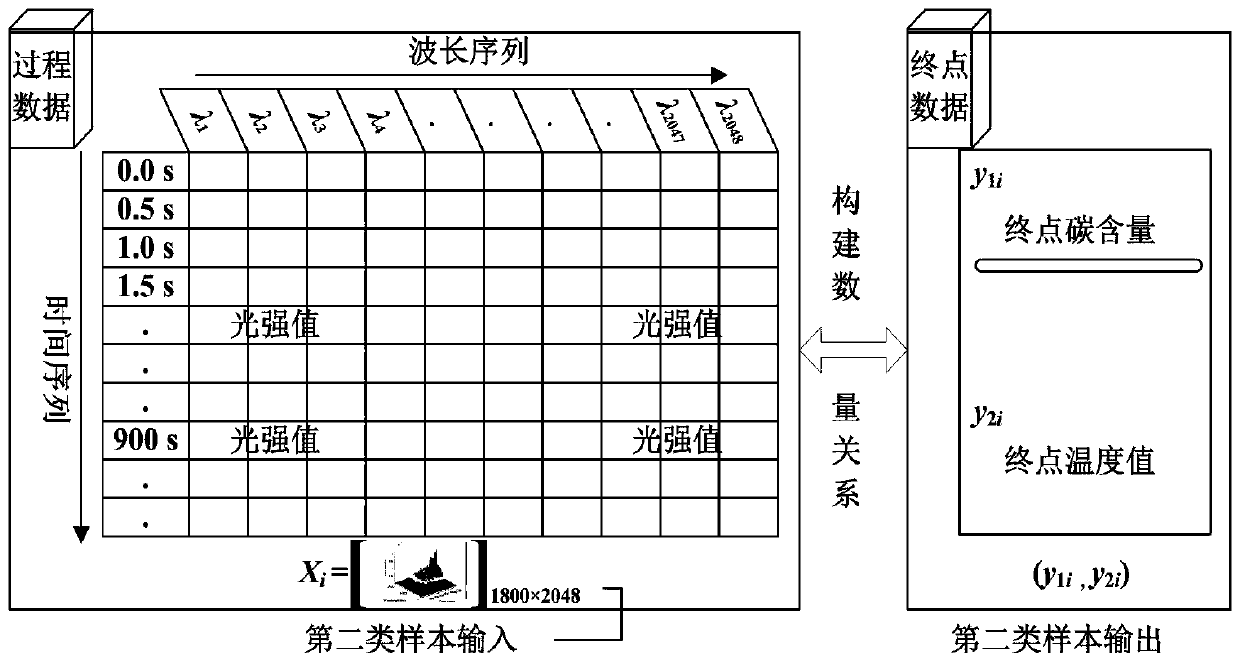
Real-time determining method and system for carbon content and temperature value of molten steel in steelmaking later stage
InactiveCN109975507ARealize real-time monitoringReduce distractionsMaterial analysisSteelmakingMaterial balance
The invention discloses a real-time determining method and system for carbon content and a temperature value of molten steel in a steelmaking later stage. The method comprises the following steps of collecting spectral information of flame at the furnace mouth in real time in the steelmaking later stage; and inputting the spectral information into a pre-established second prediction model to obtain a real-time value of the carbon content and the temperature value of the molten steel, wherein the second prediction model is a real-time prediction model obtained due to training by taking the pre-acquired spectrum information and the carbon content and temperature value of the molten steel corresponding to the spectrum information as samples, taking the spectral information as input, and taking the real-time carbon content and temperature value of the molten steel corresponding to the spectrum information as output; and the real-time carbon content and temperature value of the molten steelcorresponding to the spectrum information is derived by using a material balance and chemical balance principle after obtaining the carbon content and the end temperature value of the end molten steel. The method and system provided by the invention can improve the prediction reliability and accuracy of the carbon content and temperature value of molten steel of small and medium-sized convertersin which the sub-gun cannot be installed.
Owner:NORTH CHINA UNIVERSITY OF SCIENCE AND TECHNOLOGY
Recirculated cooling water nanofiltration ion exchange softening micro-basification method
ActiveCN101353191AIncrease the concentration ratioEmission reductionWater/sewage treatment by ion-exchangeProcess equipmentIon exchange
The invention discloses a method which is suitable for softening and dealing with industrial circulating cooling water by micro-basification. Nanofiltration process equipment is adopted for removing the multivalent ions such as Ca<2+>, Mg<2+>, SO4<2+>, and the like, in the water and some Na<+> ions are preserved, or the combination of cation-exchange resin weak-acid and nanofiltration is adopted for removing the multivalent ions such as Ca<2+>, Mg<2+>, SO4<2+>, and the like, in the water and some Na<+> ions are preserved; anion resin of OH-type / CO3-type / HCO3-type is adopted for removing the left superacid anions in the water, and the superacid anions are exchanged by OH<-> / CO3<2-> / HCO3<->, and the producing water is alkaline. The alkaline producing water reacts with the CO2 in the air to realize chemical equilibrium, and dilute solution with buffering is formed, the pH value of the circulating cooling water is adjusted to alkalescency ranging from 8.3 to 9.2, and softening and micro-basification treatment of the circulating cooling water is realized. The method of the invention can realize that various additionally added chemical agents such as antiseptic, corrosion inhibitor, scale inhibitor, water stabilizer, bactericidal algicide, and the like, are avoided for use in the circulating cooling water system, and has the technical effects of corrosion prevention, scaling prevention and microbial contamination prevention as well as comprehensive economic benefit that discharge of enriched water and chemical substance is reduced.
Owner:WUHAN UNIV +1
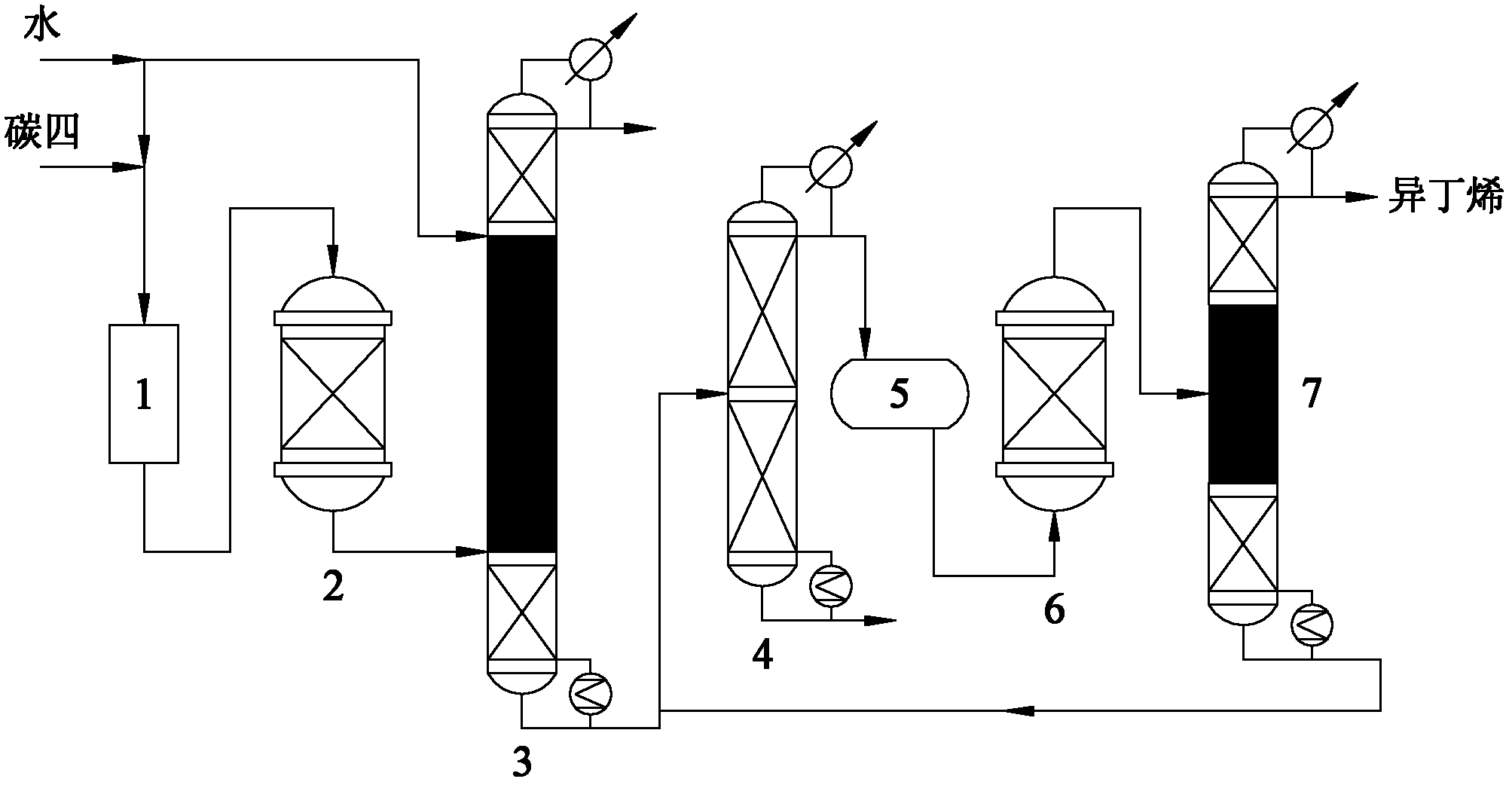
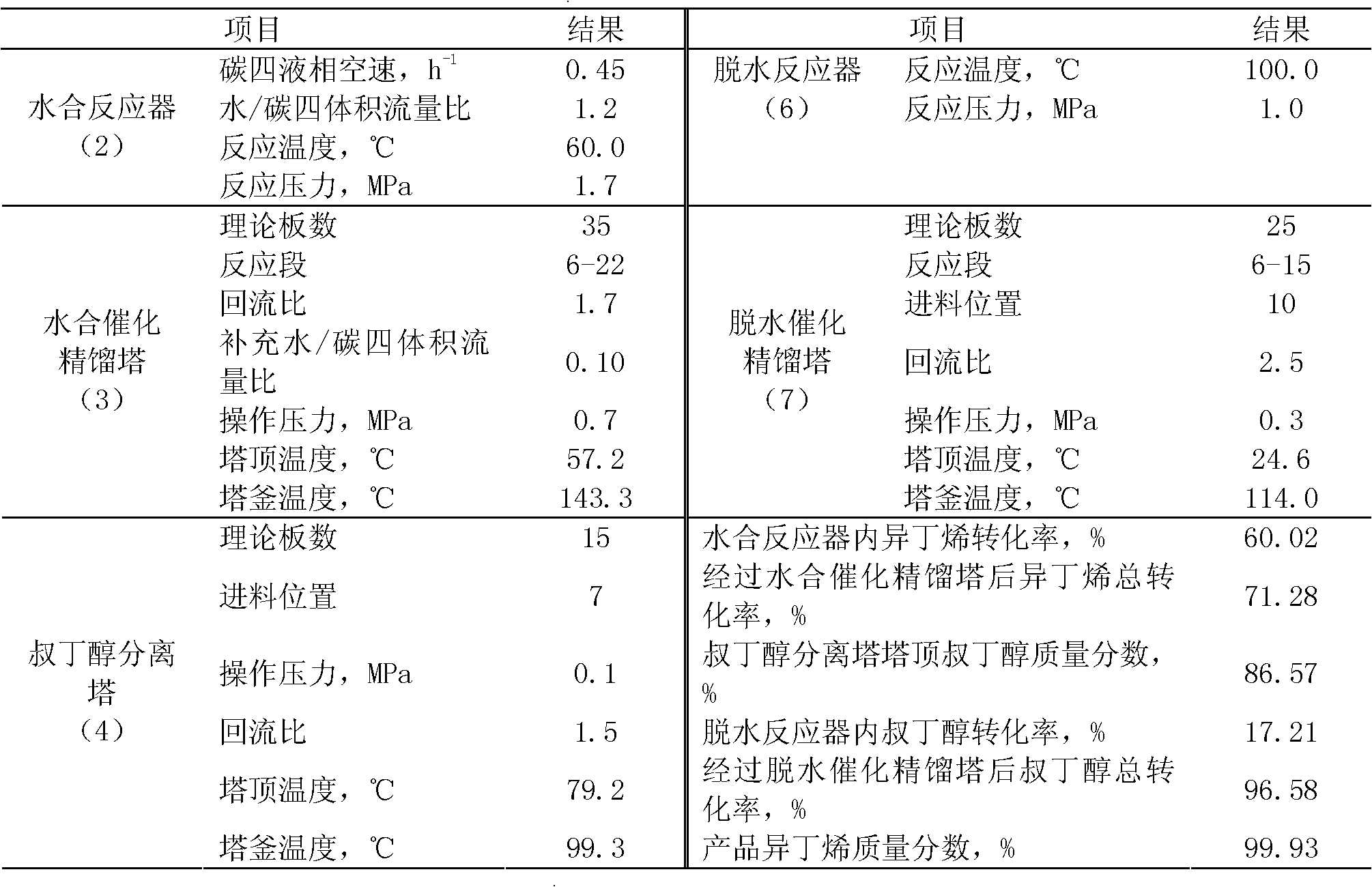
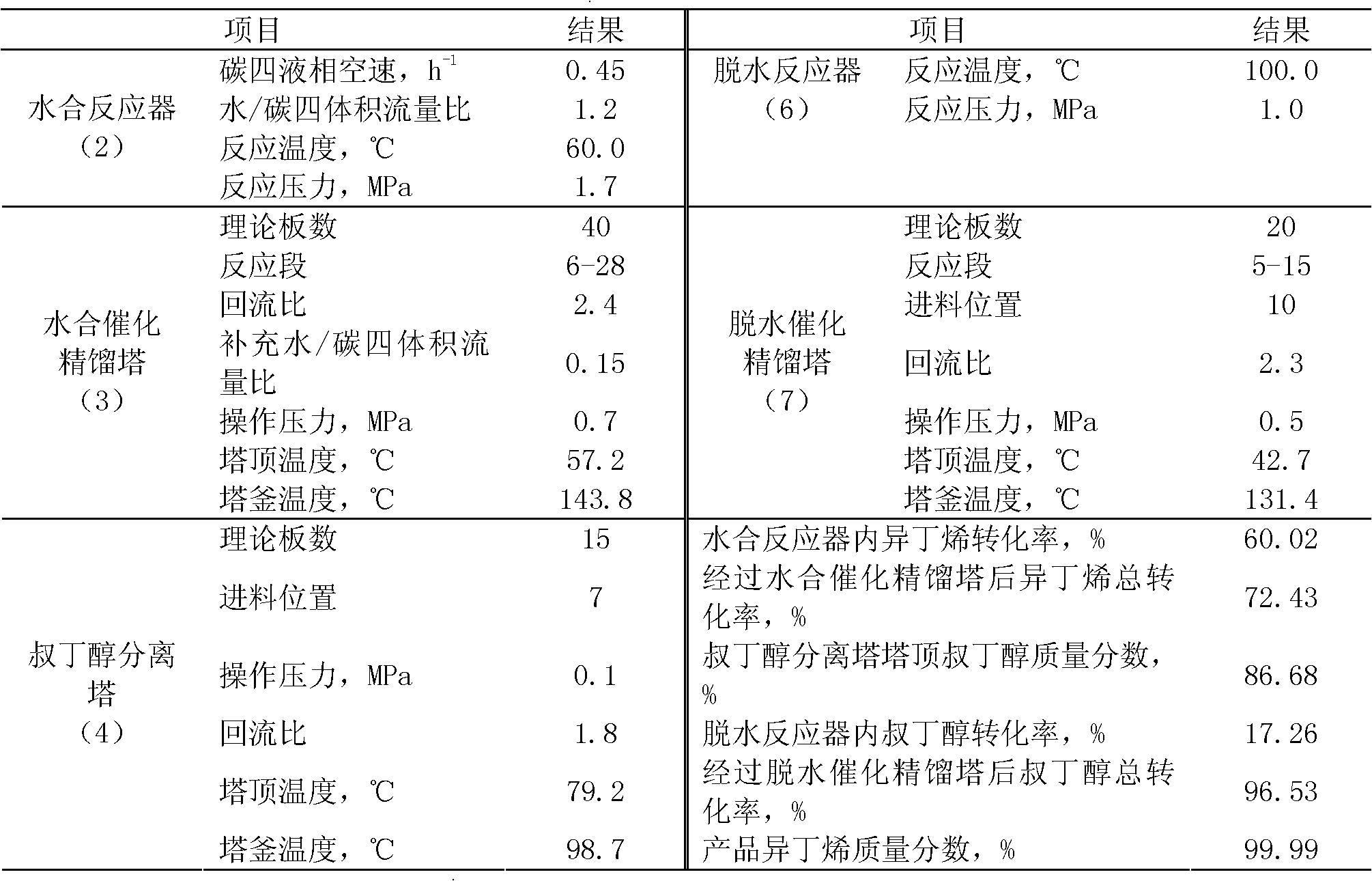
Method for preparing high-purity isobutene from raffinate C4 by means of separation
InactiveCN102633588AReduce generationAvoid pollutionChemical industryChemical modification purification/separationHydration reactionFixed bed
A method for preparing high-purity isobutene from raffinate C4 by means of separation belongs to the technical field of isobutene preparation, and includes the steps: by combining a fixed bed reactor with a catalysis rectifying column and utilizing macro-porous sulfoacid cation exchange resin as catalyst, mixing C4 with water, then sequentially carrying out hydration reaction in a hydration reactor and a hydration rectifying column to generate tert-butyl alcohol, refining tert-butyl alcohol aqueous solution by a tert-butyl alcohol separating column, and sequentially carrying out dehydration reaction in a dehydration reactor and a dehydration catalysis rectifying column to generate the isobutene. The method adopting the catalysis rectifying technology breaks through chemical equilibrium limitations, conversion rate of the isobutene in the hydration reaction and the tert-butyl alcohol in the dehydration reaction is increased, selectivity of the isobutene in the hydration reaction and the tert-butyl alcohol in the dehydration reaction is enhanced, process is simplified, equipment investment cost is lowered, the catalysis reaction and a rectifying process are coupled, energy consumption during production is reduced, and operating cost is lowered.
Owner:BEIJING UNIV OF CHEM TECH
Recirculated cooling water ion exchange softening micro-basification processing method
ActiveCN101353190APrevent scalingAvoid breedingWater/sewage treatment by ion-exchangeIon exchangeEconomic benefits
The invention discloses a method for softening and dealing with circulating cooling water by micro-basification. Cation resin of Na-type is adopted for removing the ions of Ca<2+> and Mg<2+> in the water, and the ions are exchanged by Na<+> ions, or the combination of H-type-Na-type cation resin is adopted for removing the ions of Ca<2+> and Mg<2+ >, and some of the ions are exchanged by Na<+> ions; anion resin of OH-type, CO3-type or HCO3-type is adopted for removing superacid anions in the water, and the superacid anions are exchanged by OH<->, CO3<2-> or HCO3<->. The water out from the ion exchanger reacts with the CO2 in the air to realize chemical equilibrium, and dilute solution with buffering is formed, pH value of the circulating cooling water is adjusted to alkalescency ranging from 8.6 to 9.7, and softening and micro-basification treatment of the circulating cooling water is realized. The method of the invention can realize that the circulating cooling water system has the technical effects of corrosion prevention, scaling prevention and microbe growth prevention and has comprehensive economic benefit that discharge of enriched water and chemical substance is reduced under the condition that various additionally added chemical agents such as antiseptic, corrosion inhibitor, scale inhibitor, water stabilizer, bactericidal algicide, and the like, are avoided for use.
Owner:WUHAN UNIV +1
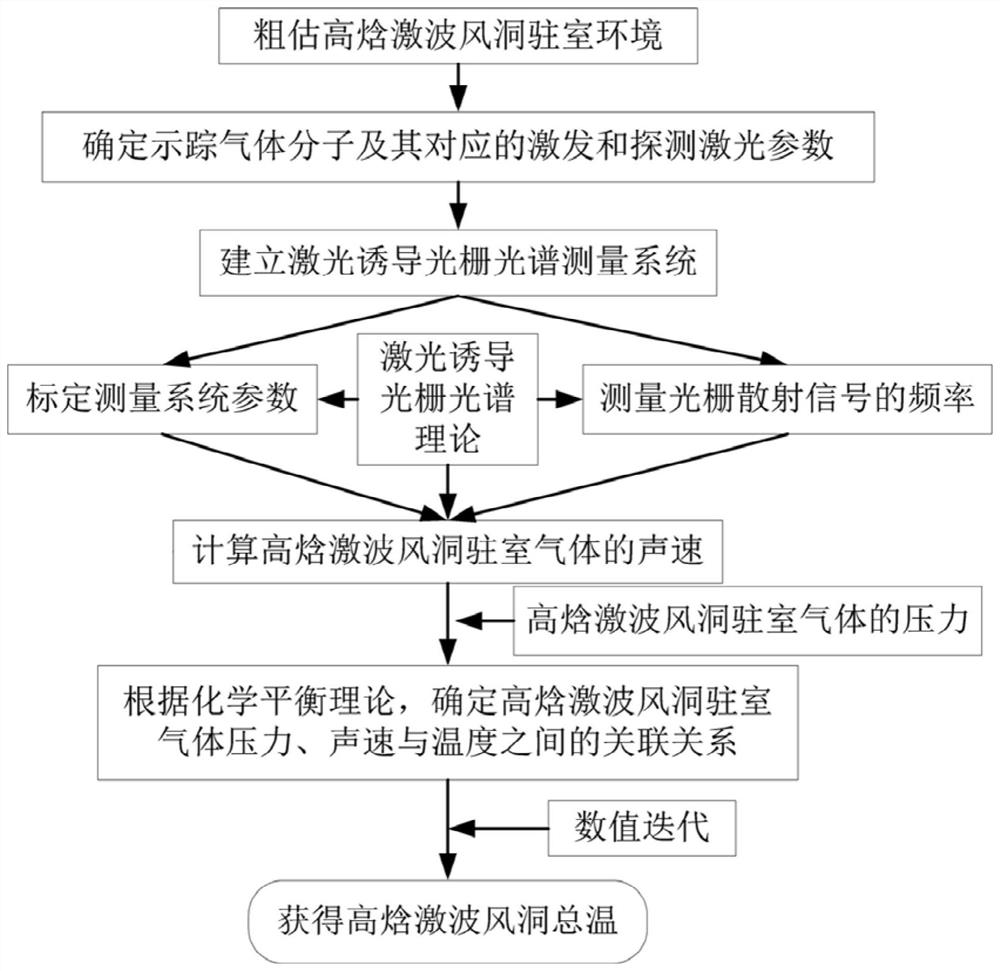
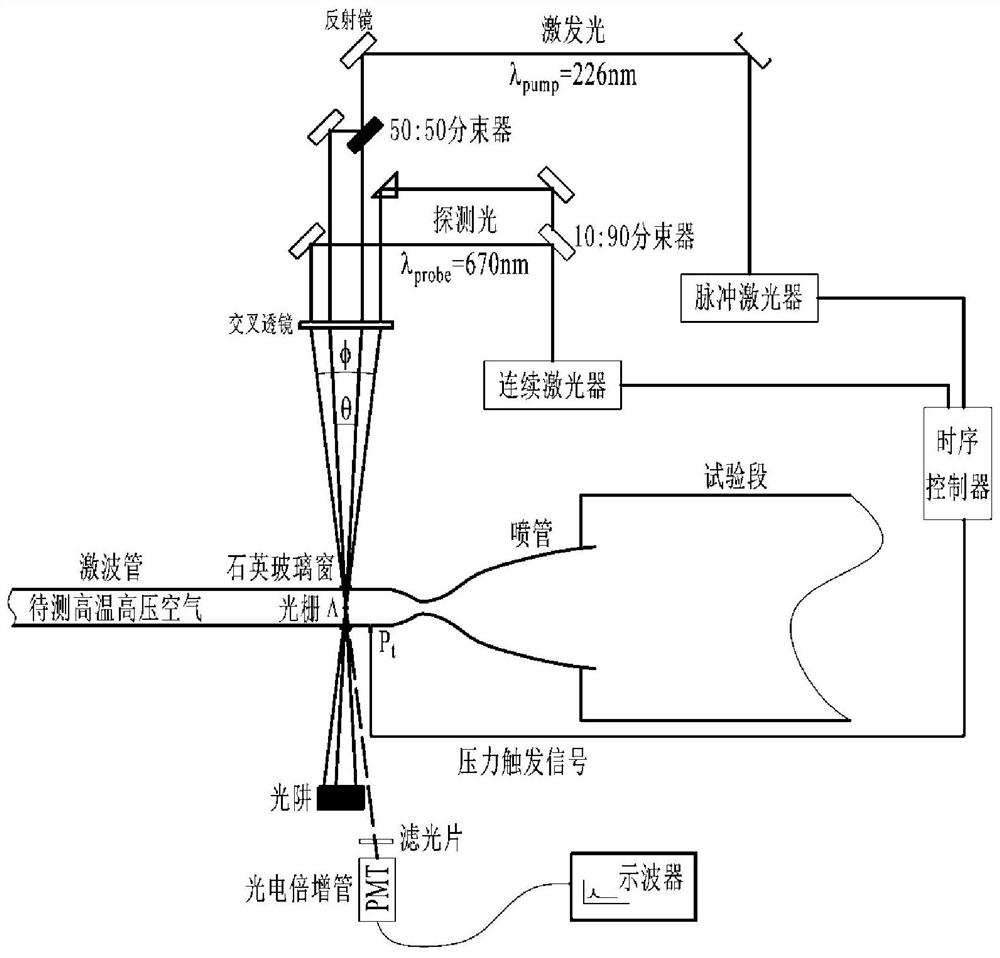
High-enthalpy shock tunnel total temperature measurement method
ActiveCN112067241ARealize measurementSolving Heat Balance ProblemsAerodynamic testingSustainable transportationShock waveGrating
The invention discloses a high-enthalpy shock tunnel total temperature measurement method and belongs to the technical field of wind tunnel tests. According to the invention, the sound velocity of theplenum gas is measured through the photoinduced transient grating spectrum technology, and then the temperature, namely the total temperature, of the plenum gas of the high-enthalpy shock tunnel is calculated through numerical iteration according to the plenum gas pressure of the high-enthalpy shock tunnel and the chemical equilibrium theory.
Owner:CHINA ACAD OF AEROSPACE AERODYNAMICS
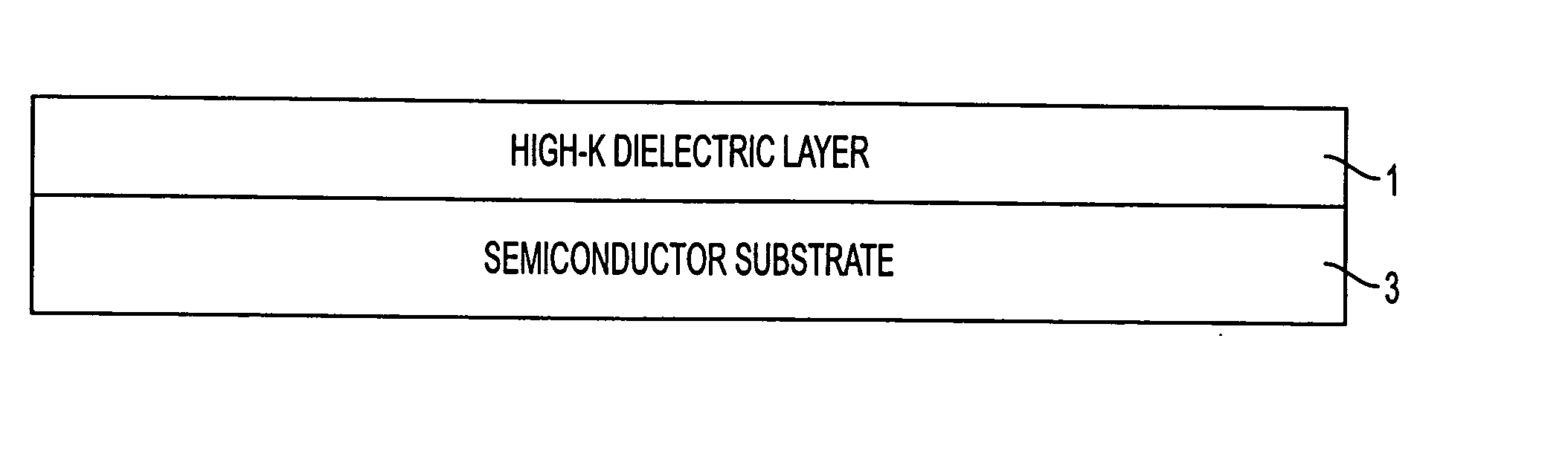
Fabrication process for a semiconductor device having a metal oxide dielectric material with a high dielectric constant, annealed with a buffered anneal process
InactiveUS20050042846A1High carrier mobilityHigh drive currentSemiconductor/solid-state device manufacturingCapacitorsSemiconductorMetal
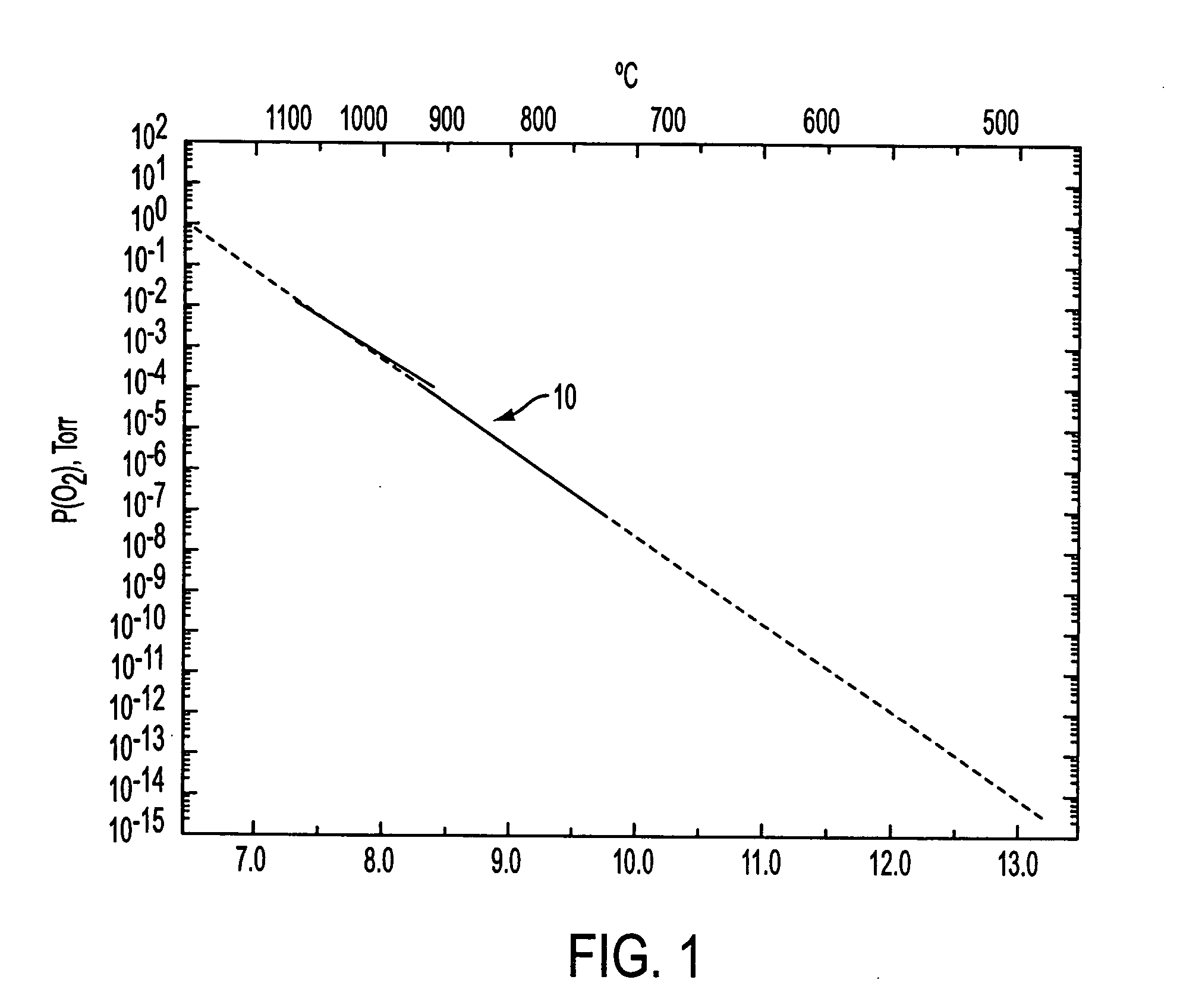
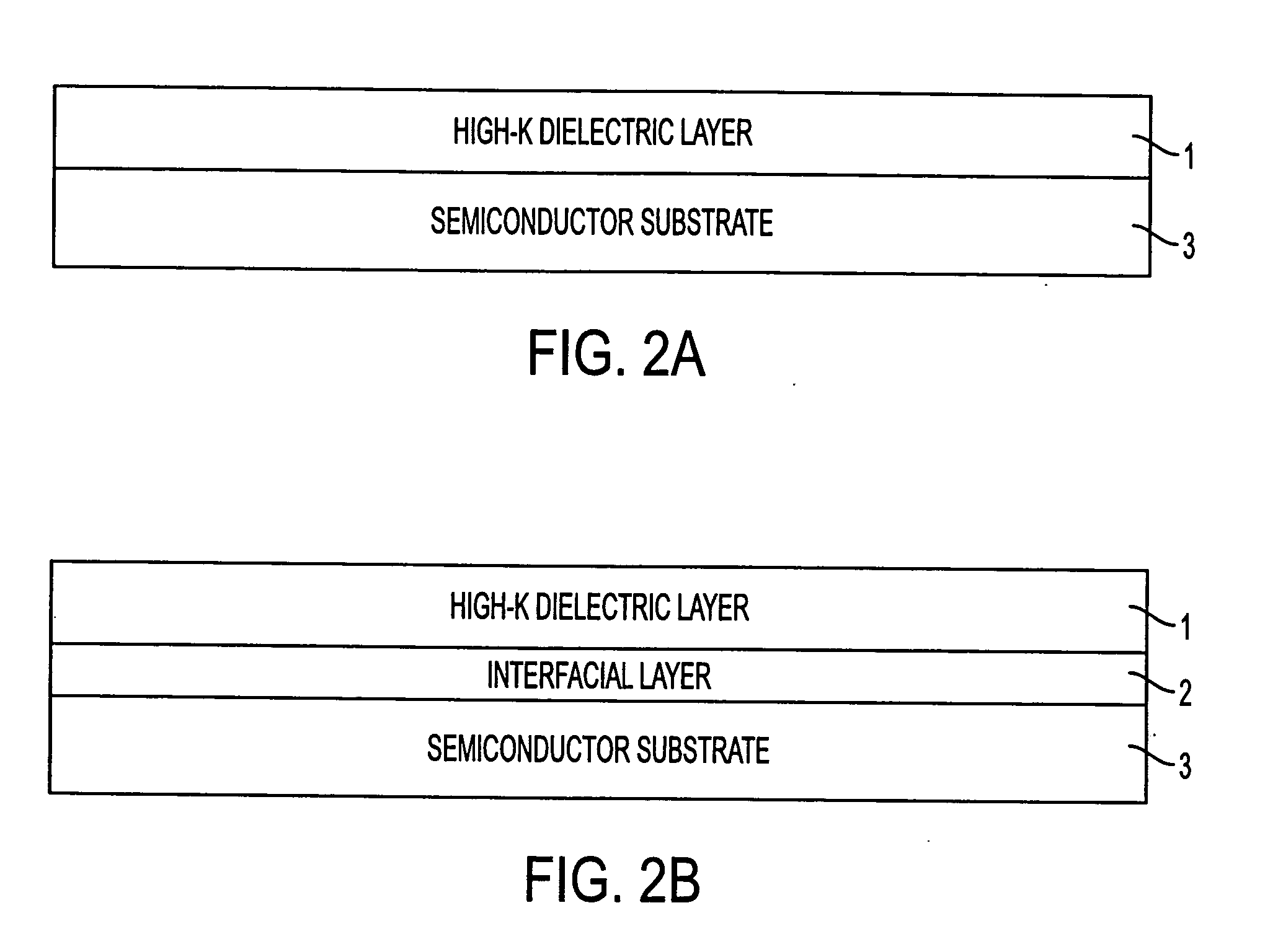
A method of forming an annealed high-K metal oxide transistor gate structure is disclosed. A metal oxide layer is formed over a semiconductor substrate. The metal oxide layer undergoes a buffered annealed process in an oxygen atmosphere to anneal the metal oxide layer at or below the thermodynamic chemical equilibrium of SiO / SiO2 and at or above the thermodynamic chemical equilibrium of the metal oxide layer.
Owner:GREEN MARTIN L +1
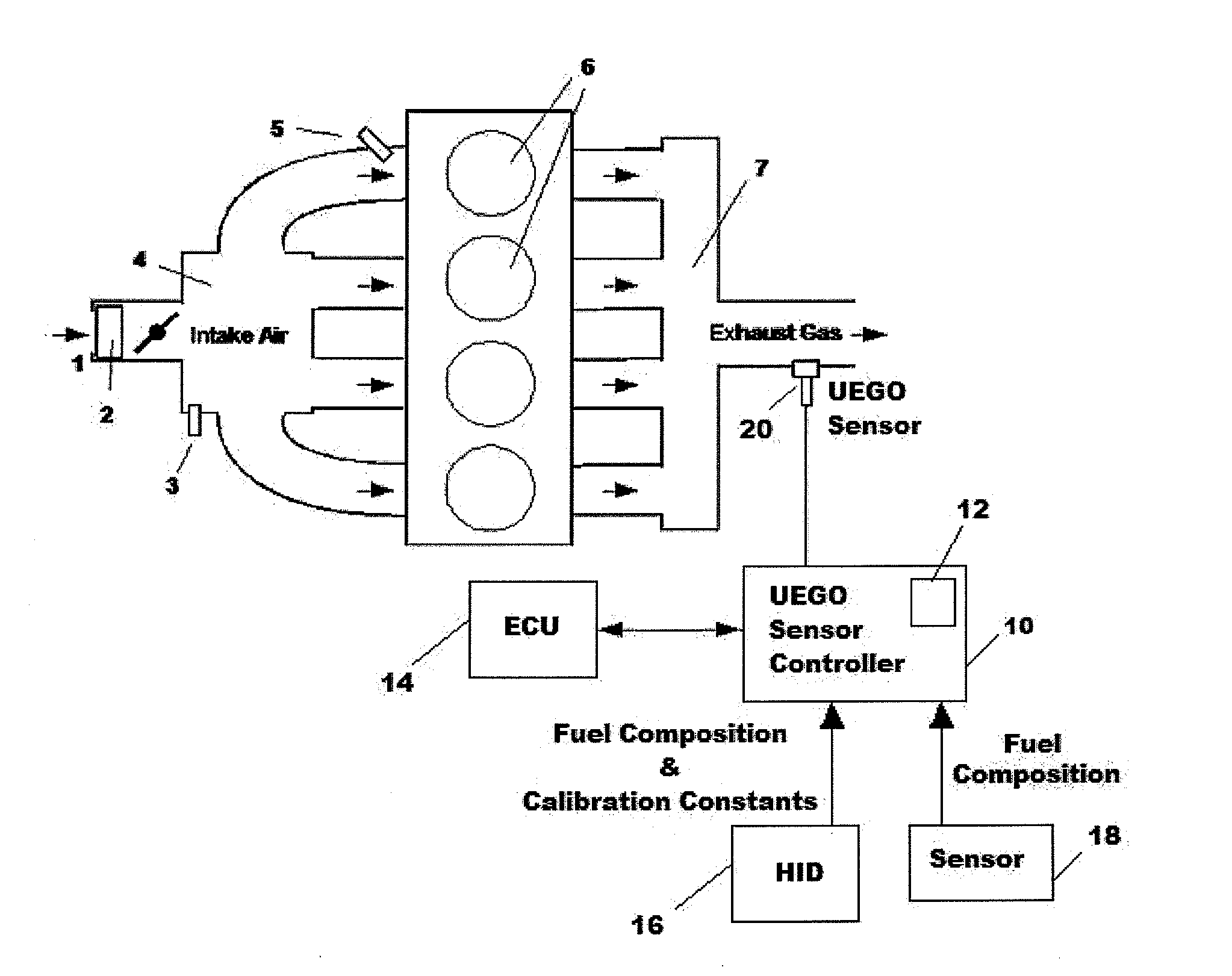
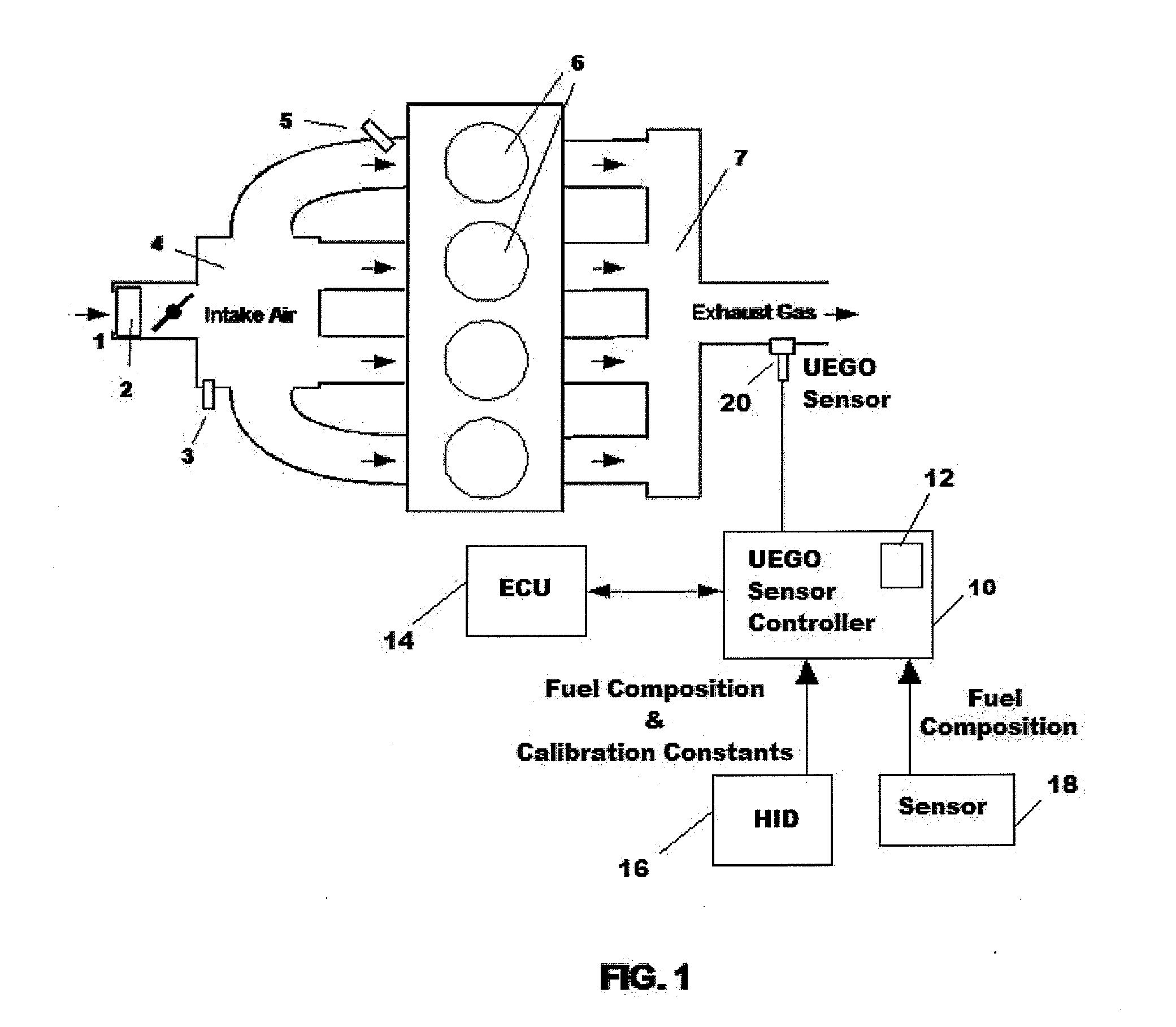
Uego sensor air-fuel ratio determination system and method
A method of determining an air-fuel ratio of an internal combustion engine in real-time includes: calibrating sensitivity of a universal exhaust gas oxygen sensor to a plurality of gases; inputting to a universal exhaust gas oxygen sensor controller a molecular composition of Hydrogen, Carbon, Oxygen, and Nitrogen which comprise a combustion fuel in use in the internal combustion engine; calculating with the universal exhaust gas oxygen sensor controller an air-to-fuel ratio by performing a chemical balance equation calculation based on the universal exhaust gas oxygen sensor sensitivity calibration and the input combustion fuel molecular composition; and transmitting the calculated air-to-fuel ratio to an engine control unit in real-time.
Owner:BG SOFLEX
Simulation and evaluation method and system for under-salt dolomite water-rock reaction
ActiveCN111855715AClear controlImprove efficiencyEarth material testingMaterial analysis using radiation diffractionDolostoneSoil science
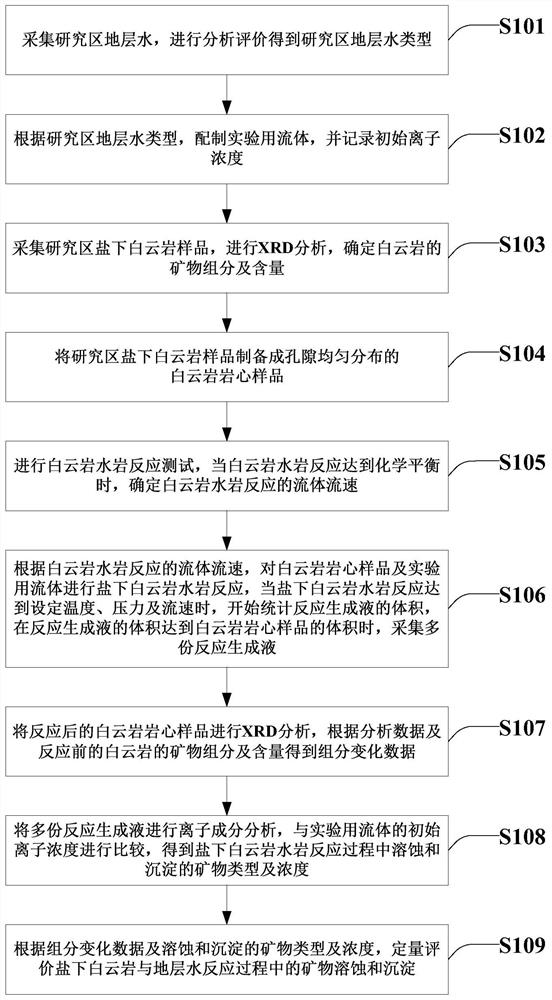
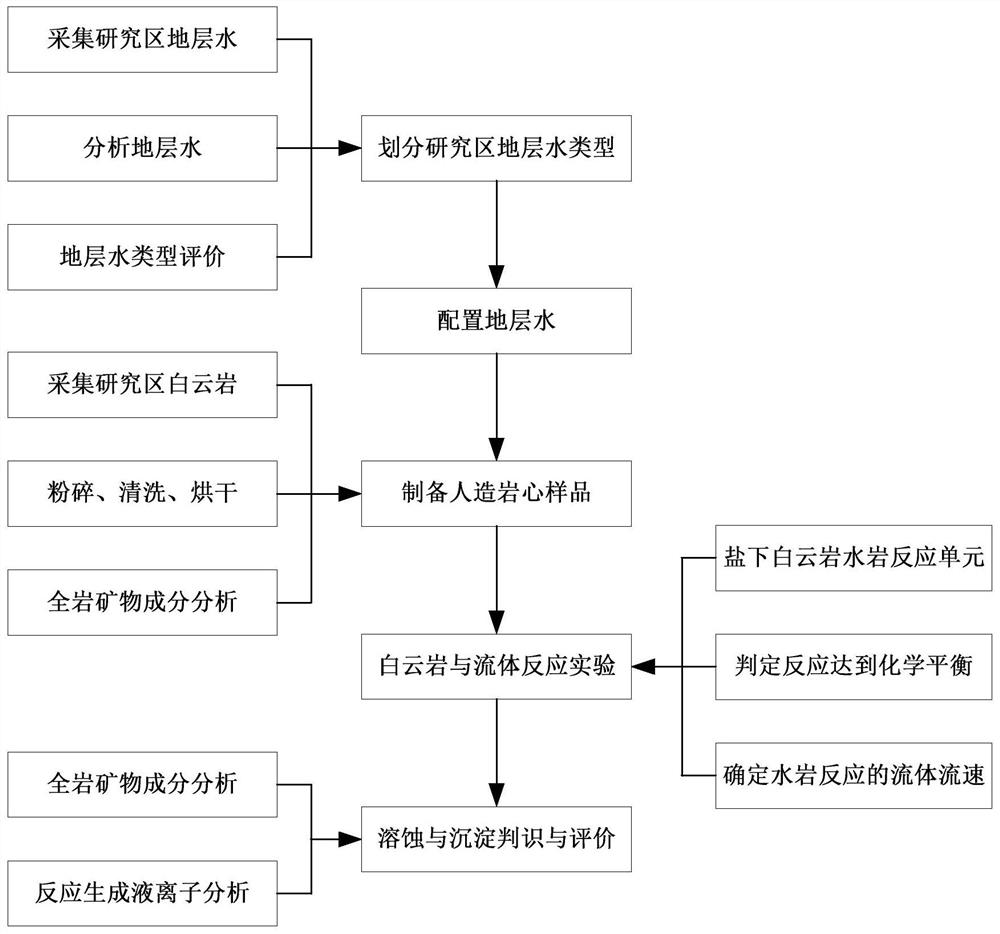
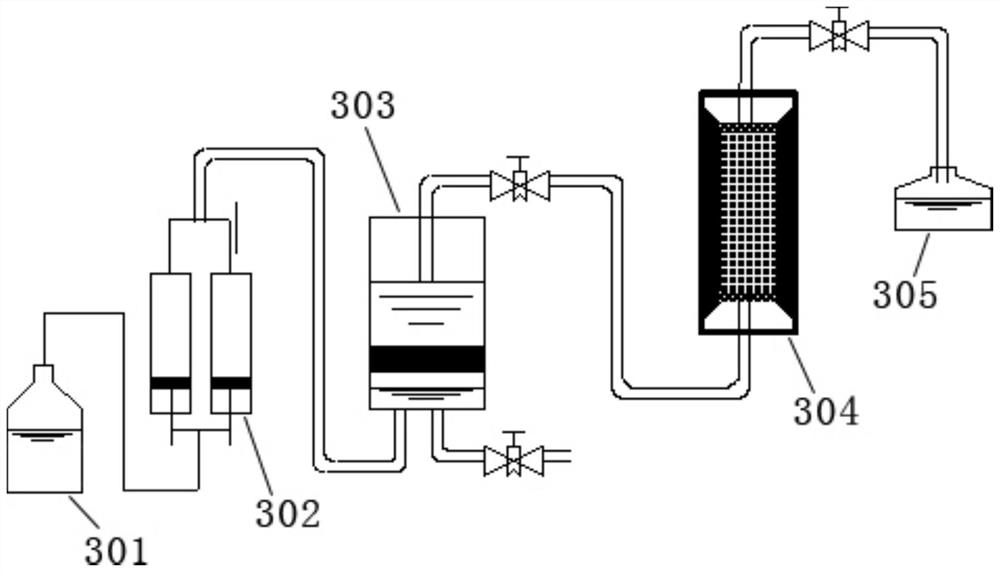
The invention provides a simulation and evaluation method and system for an under-salt dolomite water-rock reaction. The method comprises analyzing stratum water of a research area; stratum water classification is carried out by using a Sulin method; formulating experimental fluids, preparation of dolomite core sample, dolomite water rock reaction test is performed; the dolomite water-rock reaction is judged to reach chemical equilibrium; the method comprises the following steps: determining the fluid flow rate of the dolomite water rock reaction, carrying out the under-salt dolomite water rock reaction by using the prepared experimental fluid and a dolomite rock core sample, analyzing and calculating the changes of mineral components, corrosion and precipitation, and quantitatively evaluating the influence of the under-salt dolomite formation water on the corrosion and precipitation of the dolomite. According to the method, the quantitative evaluation of the reaction between the dolomite and the corresponding formation water under the geological background of the subsalt dolomite reservoir can be realized; the specific influence of stratum water attributes in the dolomite water-rock reaction process can be analyzed, control factors beneficial to formation and storage of under-salt dolomite pores are defined, and an analysis basis is provided for large-scale and efficient under-salt dolomite reservoir distribution and prediction.
Owner:PETROCHINA CO LTD
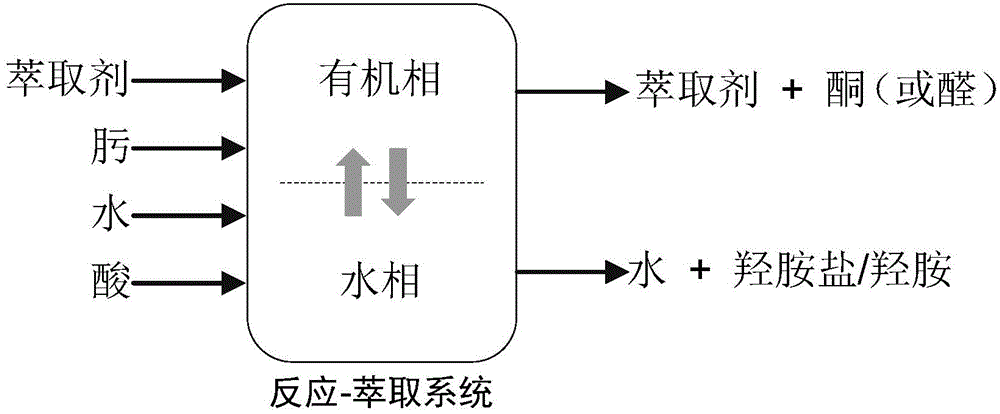
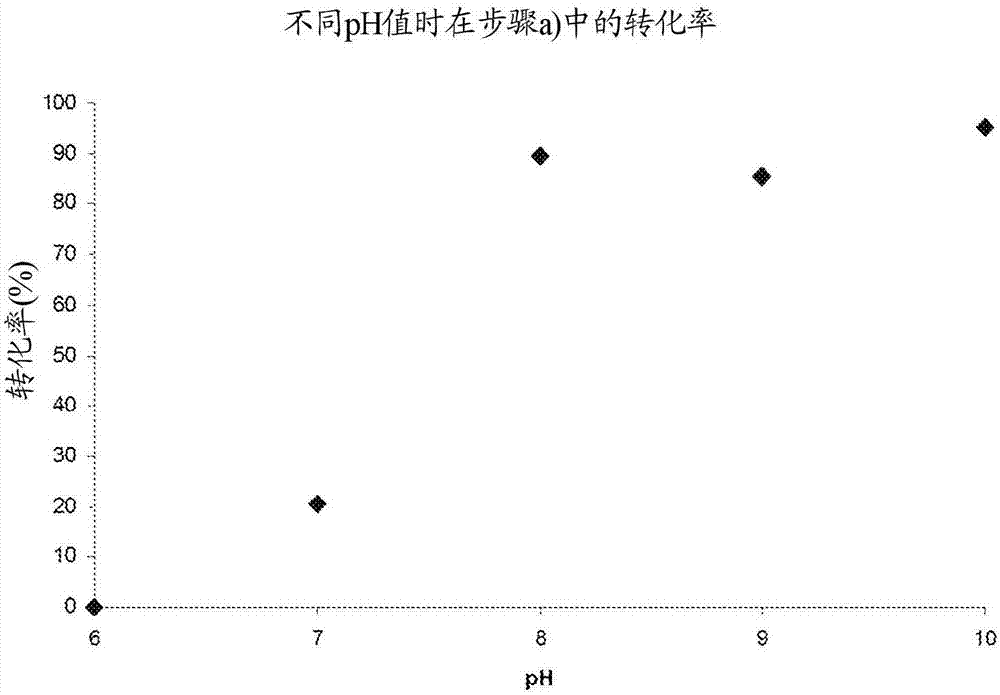
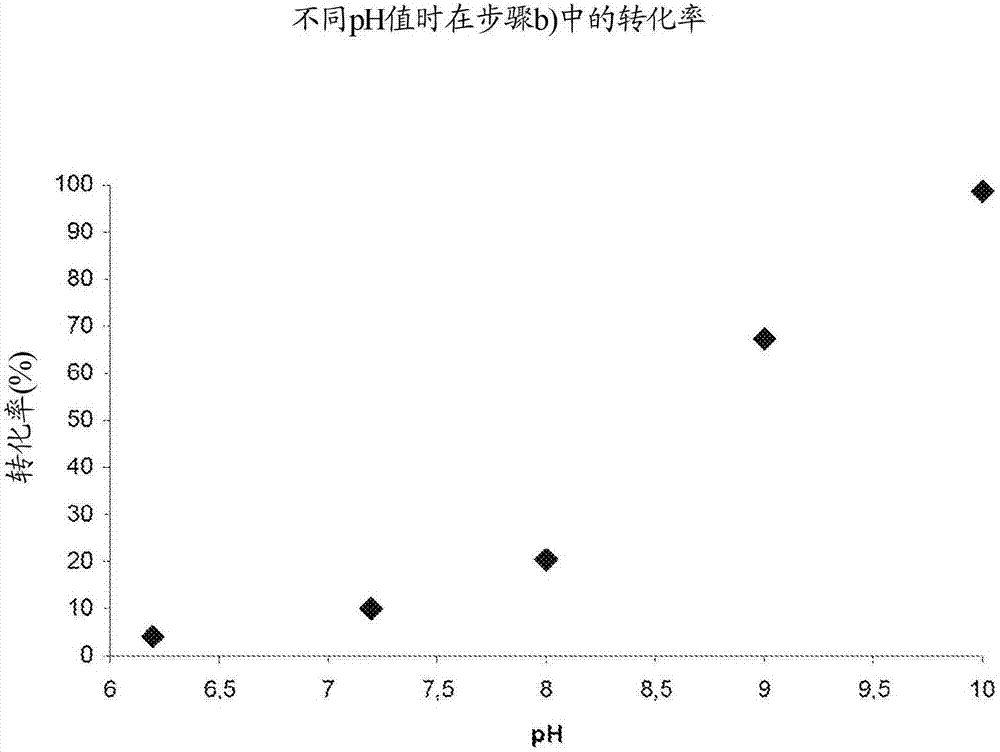
Reaction-extraction coupling method for preparation of hydroxylamine salt / hydroxylamine
A oxime hydrolysis and solvent extraction coupling method for one step preparation of hydroxylamine salt / hydroxylamine. The invention is characterized in that oxime (ketoxime or aldoxime) is used as a raw material, and the hydrolysis reaction of oxime in an acidic solution and organic solvent extraction and separation are coupled, so that an extraction agent extracts the correspondingly generated ketone or aldehyde into an organic phase while the hydrolysis reaction is carried out, thus breaking the restrictions of oxime hydrolysis reaction chemical equilibrium under mild conditions, introducing the reaction to the direction for generation of hydroxylamine salt / hydroxylamine, and improving the reaction conversion rate and yield of hydroxylamine salt / hydroxylamine. The hydroxylamine salt preparation method provided by the invention has the characteristics of simple process and operation, mild reaction conditions, high conversion rate and selectivity, and low energy consumption, etc.
Owner:XIANGTAN UNIV
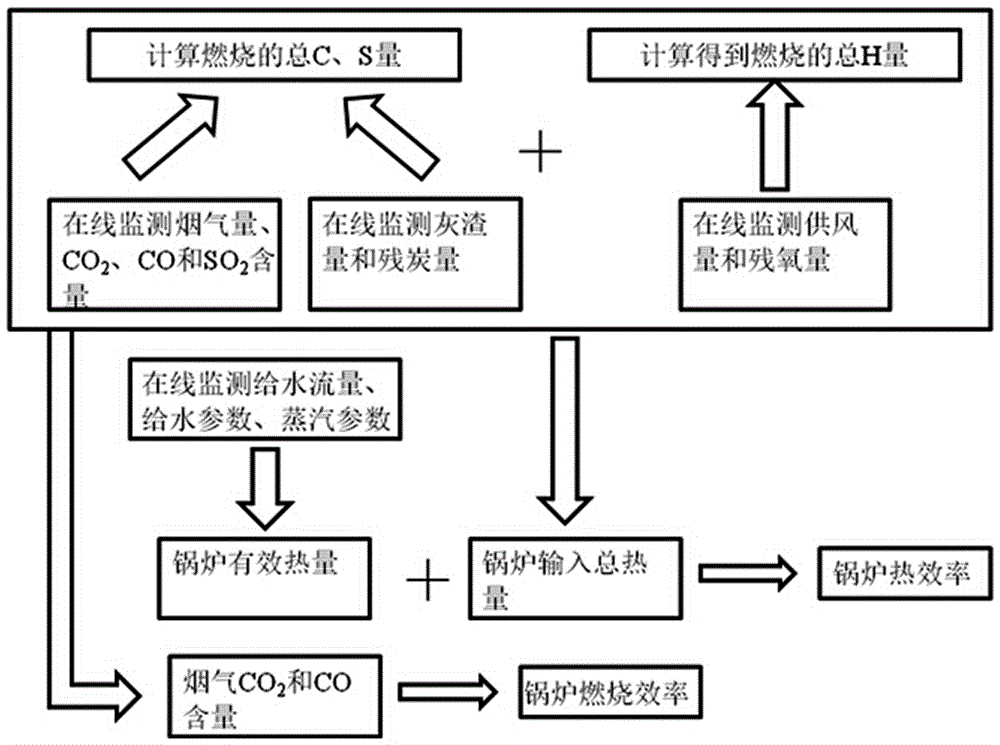
Coal-fired boiler energy efficiency on-line monitoring method based on chemical balance
ActiveCN106323657AReal-time online monitoring of exhaust gas temperatureReal-time online monitoring of flue gas oxygen contentStructural/machines measurementCombustionProcess engineering
The invention discloses a coal-fired boiler energy efficiency on-line monitoring method based on chemical balance. The coal-fired boiler energy efficiency on-line monitoring method comprises steps of monitoring a boiler working medium parameter, calculating to obtain boiler effective heat, monitoring a boiler smoke parameter, an ash parameter and a wind supply parameter, calculating total quantities of C,H and S which are generated through boiler combustion through chemical equilibrium, using combustion heat of C, H and S to calculate total heat inputted into the boiler, calculating heat efficiency of the boiler in real time according to effective heat and total heat of the boiler, and calculating boiler combustion efficiency in real time according to concentration of CO2 and CO. The beneficial effects of the coal-fired boiler energy efficiency on-line monitoring method are that: all data is obtained through testing online in real time, boiler heat efficiency can be calculated in real time through a chemical equilibrium theory, boiler heat efficiency and combustion efficiency can be monitored online, and a boiler real time energy efficiency level and a combustion level can be reflected accurately.
Owner:SHENYANG ACAD OF ENVIRONMENTAL SCI
Method for producing p-vinylphenols
The invention relates to a biocatalytic method for producing p-vinylphenols, comprising a three-stage one-pot reaction according to the following reaction scheme: (A) wherein a) an optionally substituted phenol (1) is bound to pyruvic acid (BTS) to form the optionally substituted tyrosine (2) by means of catalytic action of a tyrosine phenol-lyase (TPL) and in the presence of ammonium ions, b) ammonia is eliminated from the tyrosine (2) by means of catalytic action of a tyrosine-ammonia-lyase (TAL) or phenyl-ammonia-lyase (PAL), in order to produce an optionally substituted p-coumaric acid (3), and c) the p-coumaric acid (3) undergoes a decarboxylation by means of catalytic action of a phenolic acid decarboxylase (PAD), in order to produce the desired, optionally substituted p-vinyl phenol(4); d) wherein the resultant CO2 is removed from the reaction system, in order to move the chemical equilibrium of all three reaction steps in the direction of the products.
Owner:UNIVERSITY OF GRAZ
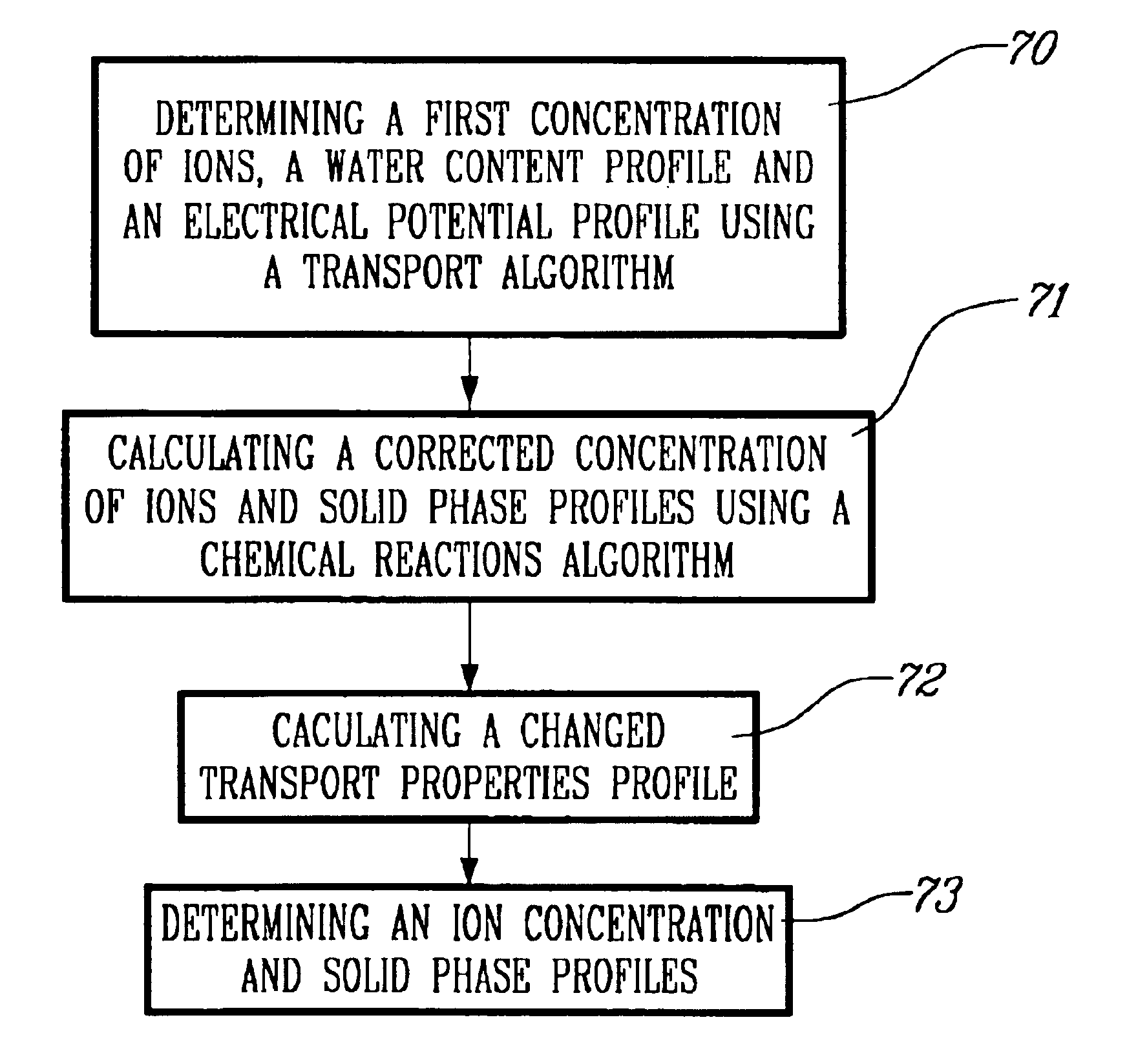
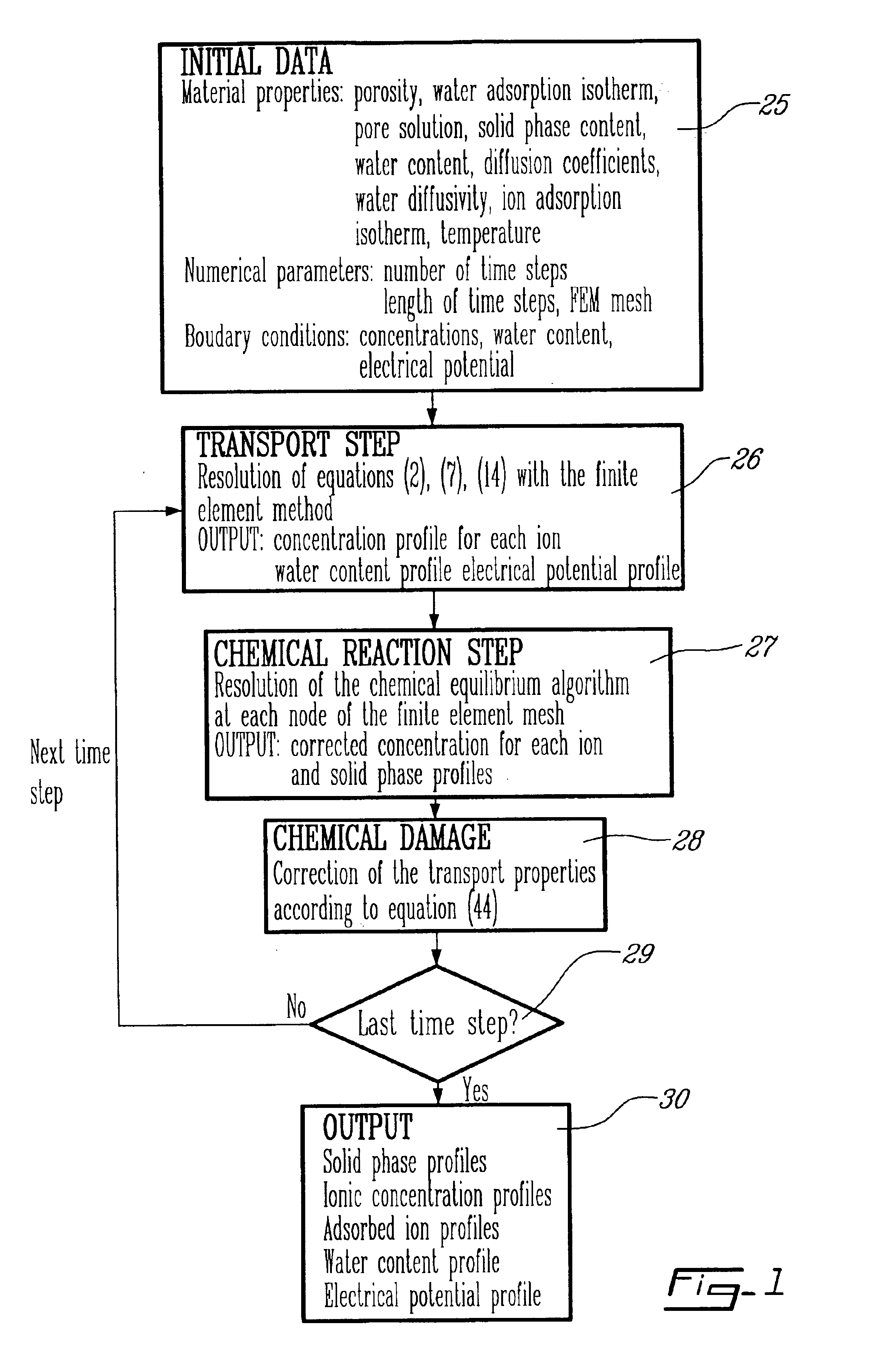
Method for modeling the transport of ions in hydrated cement systems
InactiveUS6959270B2Material analysis by electric/magnetic meansAnalogue computers for chemical processesDiffusionSulfate
The present invention relates to a computational tool that allows to evaluate the degradation of cement-based materials under various types of chemical attacks such as sulfates, chlorides, and plain water. It is based on the physical principles of ionic mass conservation and chemical equilibrium between a solution and different solid phases. The effect of the dissolution or the precipitation of solid phases on the transport coefficients is considered. A method for determining an ion concentration in solution of at least two ions capable of undergoing transport in a cement-based material under a chemical attack and a solid phase profile for at least one component of said cement-based material is provided. A method for determining a diffusion coefficient for each of at least two ions capable of undergoing transport in a cement-based material is also provided.
Owner:UNIV LAVAL +1
Preparation method of dinitrogen trioxide and reaction equipment thereof
ActiveCN101885479AImprove conversion rateThe reaction equipment is simpleNitrogen trioxideDinitrogen trioxideGas blending
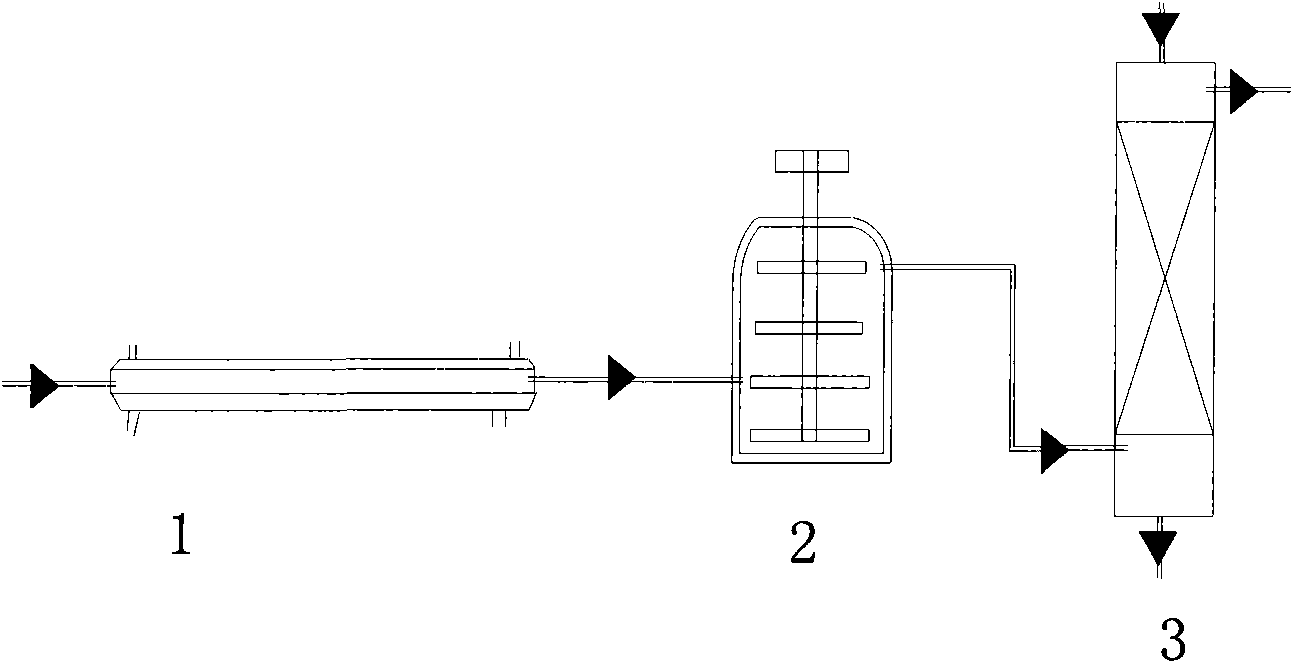
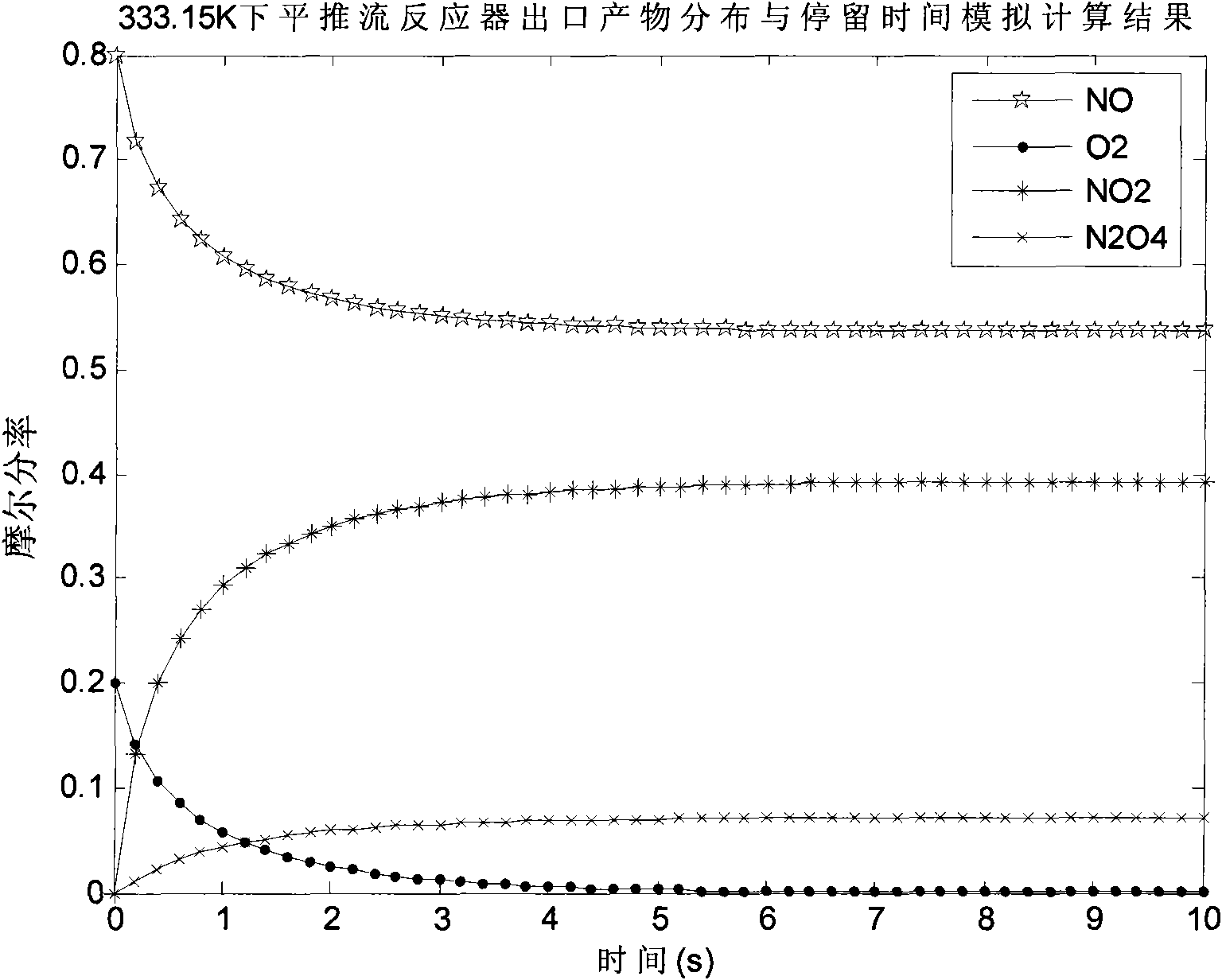
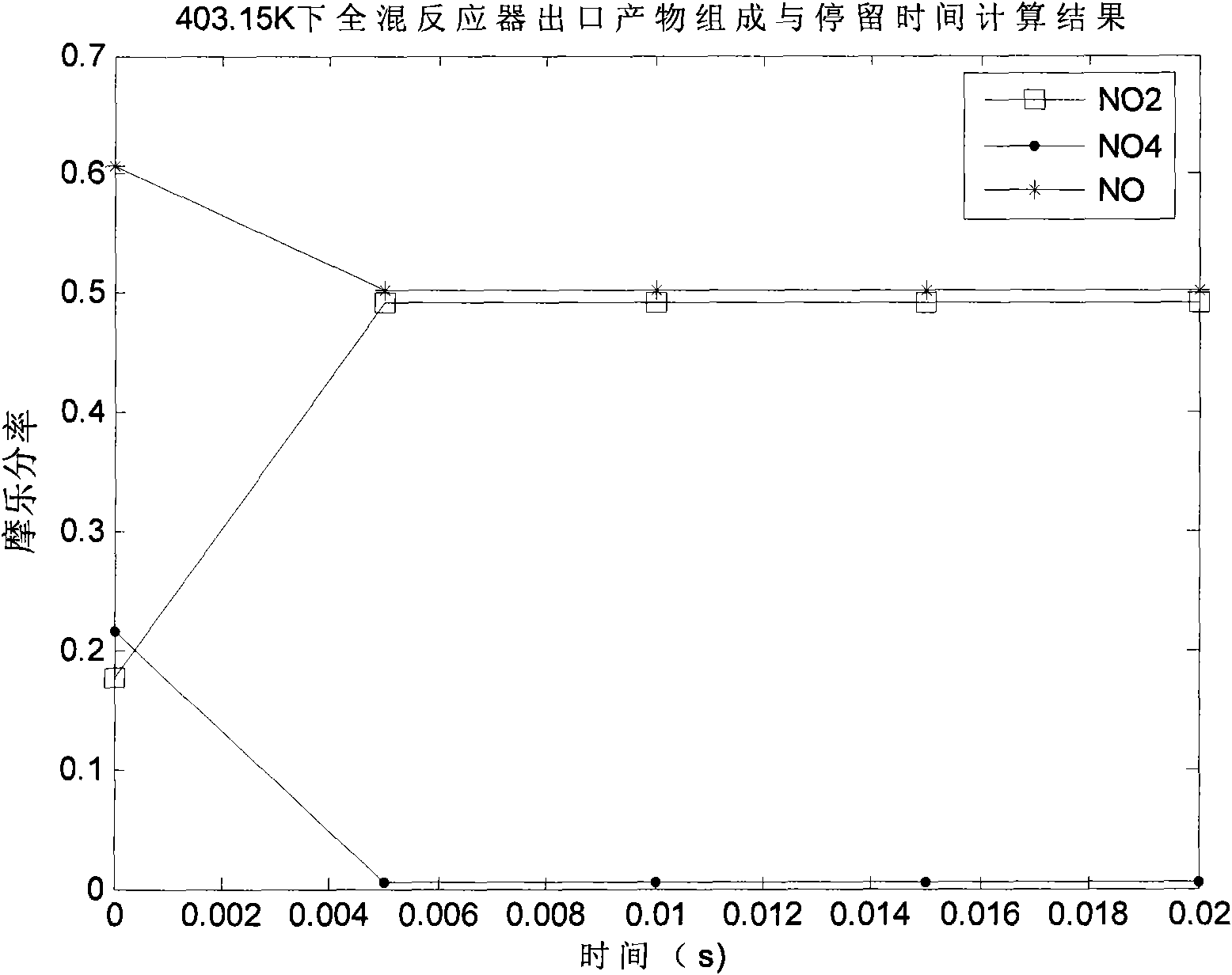
The invention discloses a preparation method of N2O3, comprising the following steps: a) oxidizing the gases containing NO to generate a gas mixture basically formed by N2O4, NO2 and NO; and b) carrying out chemical balance adjustment on the gas mixture generated in the step a) to obtain a gas mixture basically formed by NO and NO2 in molar ratio of 1:1. The invention also provides a reaction device implementing the method, comprising i) a plug flow isothermal reactor and ii) a perfectly mixed isothermal reactor. The invention has the advantages of moderate operation conditions, short reaction time, high product purity, strong suitability to the raw materials, etc. The gas mixture generated by the method of the invention reacts with C1-C4 alkanol to obtain C1-C4 alkyl ester.
Owner:PUJING CHEM IND SHA +1
Method for calculating mass concentration of ammonium sulfate liquid injection for ion-type rare earth in-situ leaching
ActiveCN106939374AThe injection mass concentration is scientific and reasonableProcess efficiency improvementRare earth ionsIn situ leach
The invention relates to a method for calculating the mass concentration of an ammonium sulfate liquid injection for ion-type rare earth in-situ leaching, and the method is suitable for parameter design of a leaching agent solution for in-situ leaching. The method comprises: step 1, carrying out a leaching test in a tube; step 2, calculating the chemical equilibrium constant K of an ore body and the mole ratio n of reactants; step 3, measuring the parameter of a soil body; step 4, calculating the rare earth ion concentration corresponding to the rare earth raw ore grade; step 5, calculating the theoretical mass concentration of the ammonium sulfate liquid injection; and step 6, calculating the actual mass concentration of the ammonium sulfate liquid injection. Through overall consideration of all conditions, taking the chemical equilibrium constant as a starting point and taking the in-situ actual situation into consideration, the method for calculating the mass concentration of an ammonium sulfate liquid injection is provided, and the method provides theoretical basis for the mass concentration of a reasonably-added ammonium sulfate liquid injection which acts as a leaching agent. The mass concentration decided through the method is scientific and reasonable, and compared with a predicted rare earth leaching rate, the error is no more than 5%.
Owner:JIANGXI UNIV OF SCI & TECH
Method for producing biodiesel from grease raw material with high acid value
InactiveCN104194946AEfficient use ofReduce pollutionFatty acid esterificationBiofuelsIsobutanolMonoglyceride
The invention provides a method for producing biodiesel from a grease raw material with a high acid value, which has the advantages of mild conditions, clean process and no corrosion to a device. The method is characterized by reacting glycerol instead of lower alcohols (for example methanol and ethanol) with fatty acid in presence of an acidic ionic liquid without corrosion to the device to obtain a mixture of monoglyceride, diglyceride or triglyceride, removing water generated in the reaction out of the reaction system to break the chemical equilibrium of the reaction, and then under the action of a basic catalyst, carrying out ester change reaction on the esterified products with the low acid values by virtue of a mixture of methanol, isopropanol or isobutanol to produce biodiesel (fatty acid esters) with the low acid value and a low condensation point. The ionic liquid is recycled and the process cleanness is achieved.
Owner:深圳市贝壳能源科技有限公司
Faintly acid suspension liquid for removing anodic oxide film of titanium and titanium alloy
The invention discloses a faintly acid suspension liquid for removing anodic oxide film of titanium and titanium alloy, which is prepared by formula solid powder and distilled water by a mass ratio of 1:(0.2-1),wherein the Potential of Hydrogen (pH) is in a range between 3 and 6, fluorides of the formula solid powder are in an amount of 50wt.% to 80wt.% , solid acid is in an amount of 10wt.% to 50wt.%, stimulating materials are in an amount of 5wt.% to 50wt.%. A trace of Hydrogen Fluoride (HF) retreating film is produced on the basis of the principle of chemical equilibrium mobile on the faintly acid condition, therefore, the preparing method is simple and effective, convenient to refill, and has a continuous controllability , no erosion to matrixes and no harm to human bodies.
Owner:NANCHANG HANGKONG UNIVERSITY
Synthetic method of AMPS (2-Acrylamide-2-methylpropanesulfonic acid)
InactiveCN102452962AHigh yieldReduce energy consumptionSulfonic acid preparationAcrylonitrileSolvent
The invention provides a synthetic method of AMPS (2-Acrylamide-2-methylpropanesulfonic acid), which comprises the following steps of: contacting acrylonitrile and sulfuric acid with isobutene to generate a pasty product; and separating the pasty product to obtain AMPS. The method is characterized in that: at least part of acrylonitrile is from waste liquid produced during the process in which acrylonitrile is taken as a raw material to synthesize AMPS. According to the synthetic method of AMPS, provided by the invention, acrylonitrile waste liquid with a certain proportion is directly taken as a reaction solvent and a reactant for synthesizing AMPS, so that the process of rectification recovery is reduced or completely given up, and the energy consumption is greatly reduced; and simultaneously, as the acrylonitrile waste liquid contains a reaction byproduct, in terms of chemical equilibrium, the side reaction can be prevented from occurring. Thus, compared with the prior art, the method provided by the invention can improve the yield of the product of AMPS.
Owner:CHINA PETROLEUM & CHEM CORP +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com