Method for preparing high-purity isobutene from raffinate C4 by means of separation
An isobutene, high-purity technology, applied in the direction of chemical change purification/separation, sustainable manufacturing/processing, organic chemistry, etc., can solve the problems of limited conversion rate, low equipment investment, and low single-pass conversion rate of tert-butanol dehydration reaction , to achieve the effect of improving conversion rate and selectivity, simplifying process flow, and facilitating separation and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
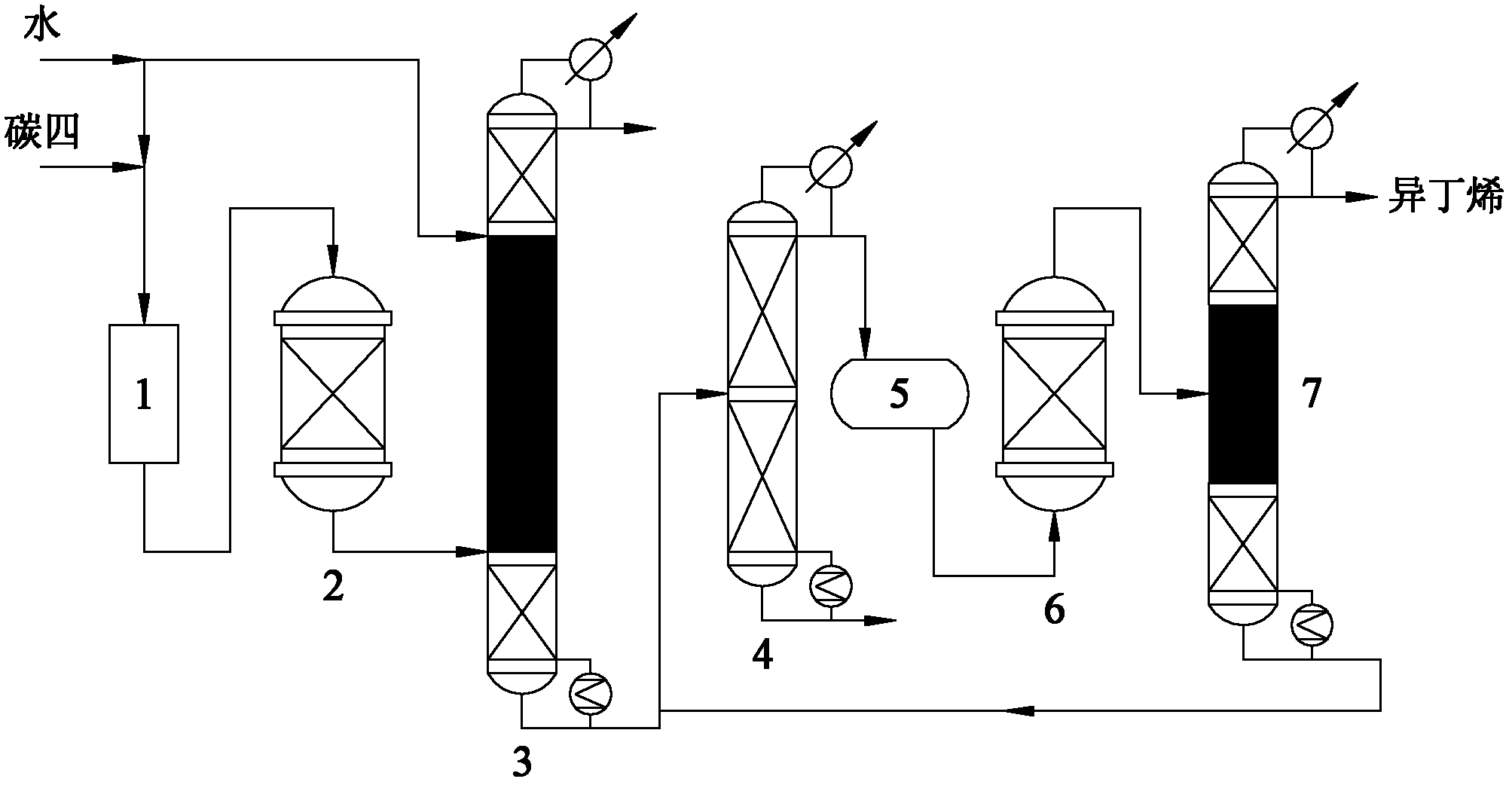
Image
Examples
Embodiment 1
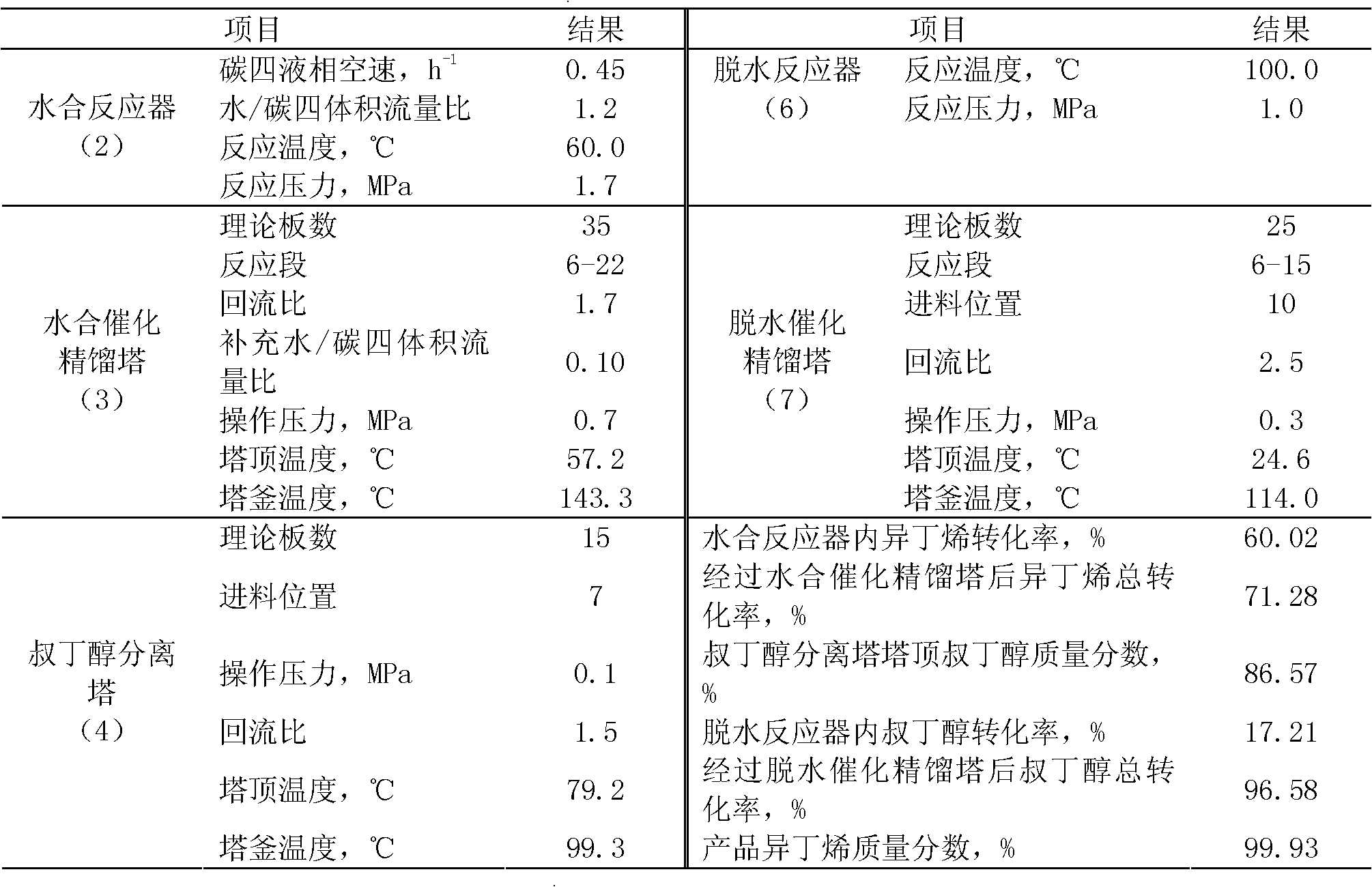
[0033] High-purity isobutene is prepared by separating and preparing high-purity isobutene from the by-product C4 fraction of the refinery fluidized catalytic cracking (FCC) unit after extracting butadiene, and the mass percentage content of isobutene in the raffinate C4 is 25.52%. The operating conditions and results are shown in Table 1, and the mass fraction of the product isobutene reaches 99.93%.
Embodiment 2
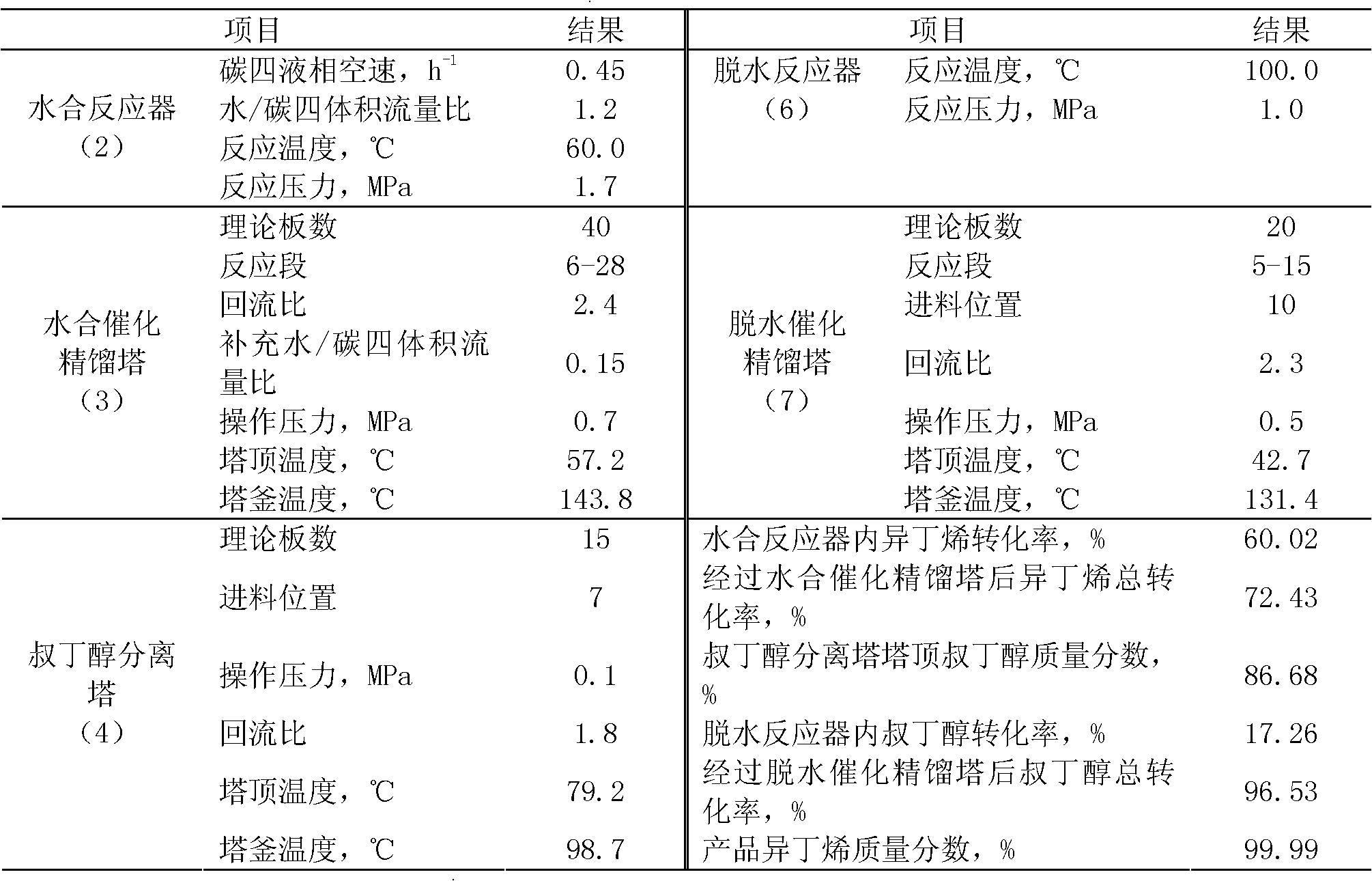
[0035] High-purity isobutene is prepared by separating and preparing high-purity isobutene from the by-product C4 fraction of the refinery fluidized catalytic cracking (FCC) unit after extracting butadiene, and the mass percentage content of isobutene in the raffinate C4 is 25.52%. The operating conditions and results are shown in Table 2, and the mass fraction of the product isobutene reaches 99.99%.
Embodiment 3
[0037] High-purity isobutene is separated and prepared by using the by-product C4 fraction of the steam cracking ethylene plant after extracting butadiene as raw material, and the mass percent content of isobutene in the raffinate C4 is 46.67%. The operating conditions and results are shown in Table 3, and the mass fraction of the product isobutene reaches 99.92%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com