Preparation method of plasticizer diethylene glycol dibenzoate
A technology of diethylene glycol dibenzoate and plasticizer, which is applied in the field of preparation of environment-friendly plastic plasticizer diethylene glycol dibenzoate, and can solve the problems of environmental pollution, short reaction time and high reaction temperature , to achieve the effect of shortening the reaction time, increasing the conversion rate and good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Put 122g of benzoic acid and 55.7g of diethylene glycol into a 500ml four-neck flask, the molar ratio of the feed is 2:1.05, heat up, the benzoic acid is completely dissolved, start stirring, add 1.07g of tetrabutyl titanate at 180°C (for the raw material benzene 0.6% of the total mass of formic acid and diethylene glycol), reflux reaction in the temperature range of 160-180°C for 1-1.5h, turn on the vacuum pump, continue to heat up to 200-220°C, keep the reaction under reduced pressure for 2-3h, and steam out after no water Afterwards, stop reaction, obtain DEDB crude product, esterification rate is 98.13%, after 3%Na 2 CO 3 The aqueous solution is neutralized to remove acid, washed with 90°C hot water for 2 to 3 times, the ester layer is separated, and the plasticizer diethylene glycol dibenzoate (DEDB) product is obtained after vacuum distillation and dehydration.
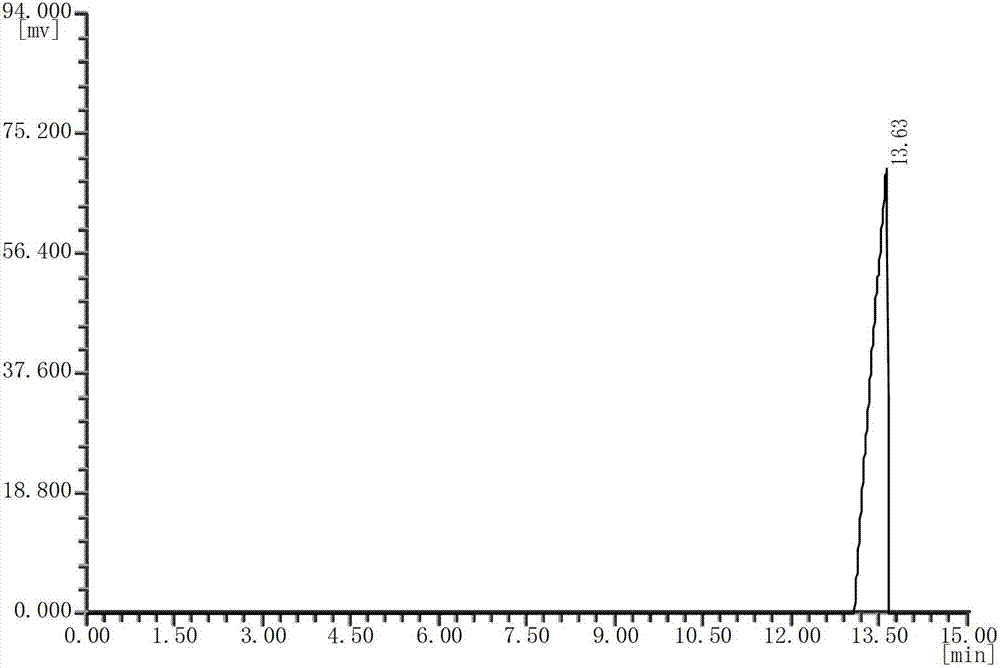
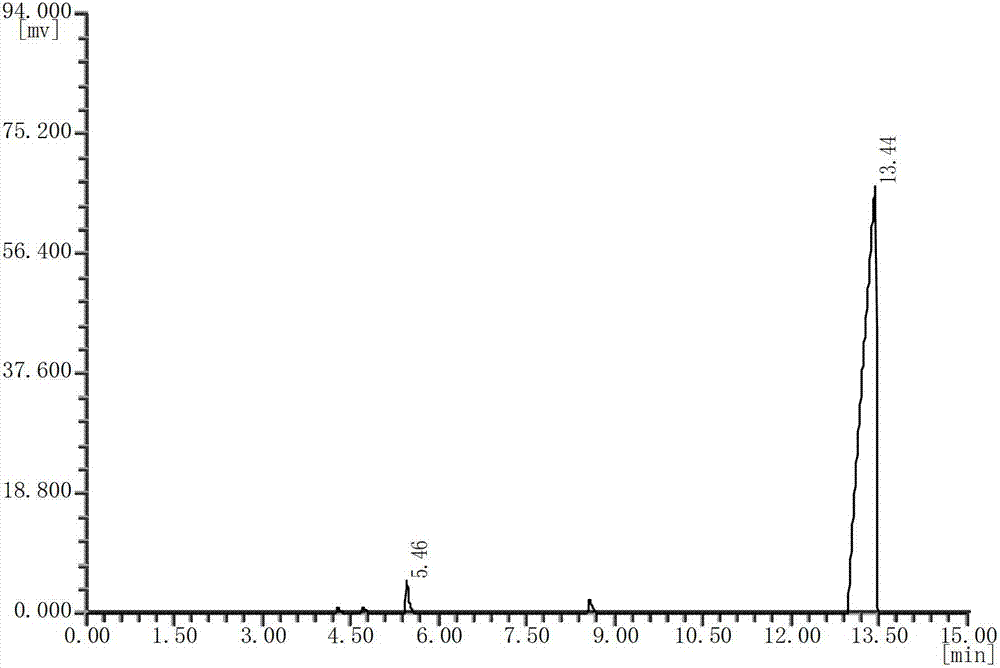
[0024] see figure 2 , with the DEDB standard gas chromatogram (see figure 1 ) comparison, it can b...
Embodiment 2~4
[0026] The operating conditions and steps are the same as in Example 1, the difference is that the molar ratios of benzoic acid and diethylene glycol are (2:1.1), (2:1.15), (2:1.2) respectively, and the amount of catalyst tetrabutyl titanate is equal to It is 0.6% of the total mass of raw materials, and the reaction esterification rates of Examples 2 to 4 are 98.82%, 98.89%, and 99.26%, respectively.
Embodiment 5 and 6
[0028] The operating conditions and steps are the same as in Example 1, the difference is that the molar ratios of benzoic acid and diethylene glycol are (2:1.1) and (2:1.2) respectively, and the catalyst is tin protochloride, and the amount of the catalyst added is the raw material 0.4% of the total mass, the reaction esterification rates of Examples 5 and 6 were 98.16%, 98.46%, respectively.
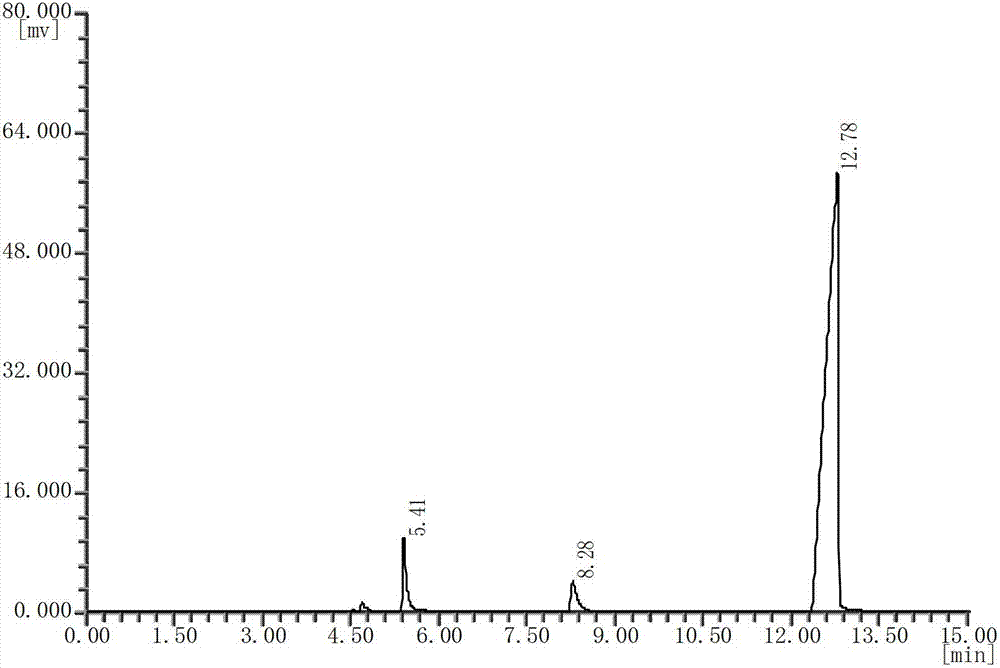
[0029] see image 3 , with the DEDB standard gas chromatogram (see figure 1 ) comparison, it can be seen that the diethylene glycol dibenzoate (DEDB) product prepared in Example 6 has a high purity and basically no other impurities. The peak with a retention time of 12.78 min on the product chromatogram is a DEDB peak with a retention time of 5.41 min The peak is diethylene glycol monobenzoate peak.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com