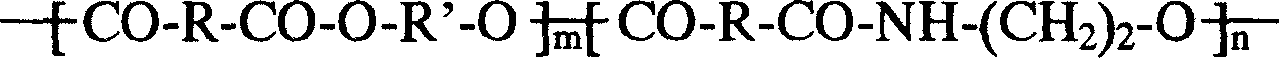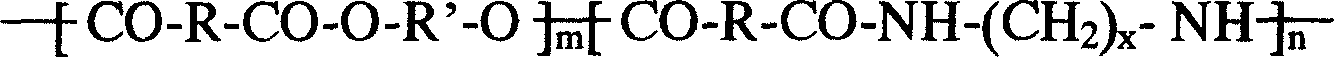Patents
Literature
621 results about "Ethylene glycol toxicity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Diethylene glycol has moderate acute toxicity in animal experiments. The LD50 for small mammals has been tested at between 2 and 25 g/kg, less toxic than its relative ethylene glycol, but still capable of causing toxicity in humans.
Articles derived from compositions containing modified polybutylene terephthalate (PBT) random copolymers derived from polyethylene terephthalate (PET)
ActiveUS20070275242A1Useful performance propertyPlastic recyclingSpecial tyresPolytetramethylene terephthalatePolyethylene terephthalate glycol
Compositions of matter including articles derived from (a) from 5 to 99.99 wt % of a modified polybutylene terephthalate random copolymer that (1) is derived from polyethylene terephthalate and (2) contains a at least one residue derived from polyethylene terephthalate selected from the group consisting of antimony, germanium, diethylene glycol groups, isophthalic acid groups, cis isomer of cyclohexane dimethanol, trans isomer of cyclohexane dimethanol, sodium benzoate, alkali salts, napthalane dicarboxylic acids, 1,3-propane diols, cobalt, cobalt-containing compounds, and combinations thereof, and (b) from 0.01 to 95 wt. % of a member selected from the group consisting of (1) fillers, (2) a carboxy reactive component, (3) polyethyelene terephthalate, (4) a component including a polycarbonate and an impact modifier. The articles may be derived from various conversion processes, e.g., injection molding processes, extrusion processes, thermoforming processes, melt-blown process.
Owner:SHPP GLOBAL TECH BV
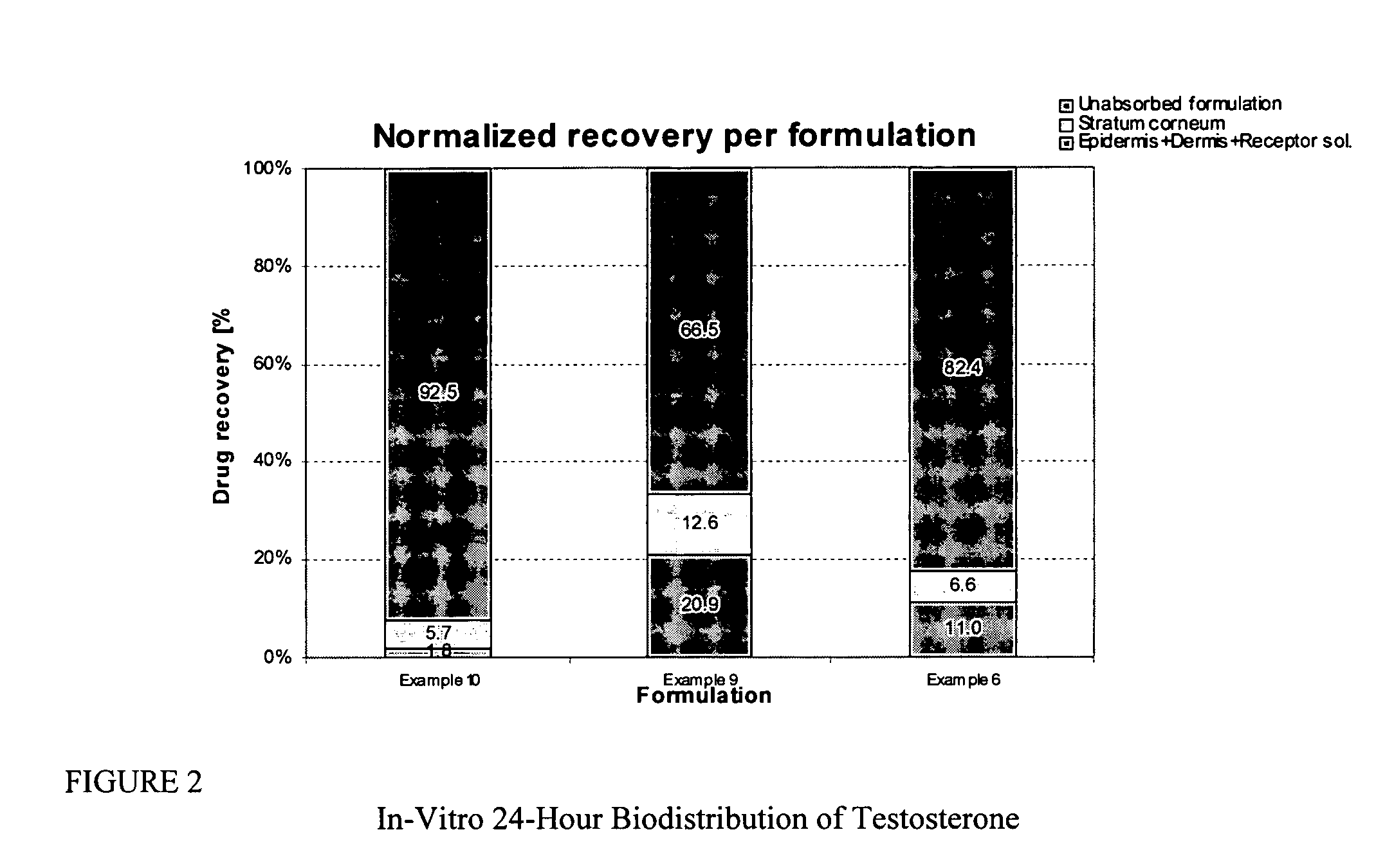
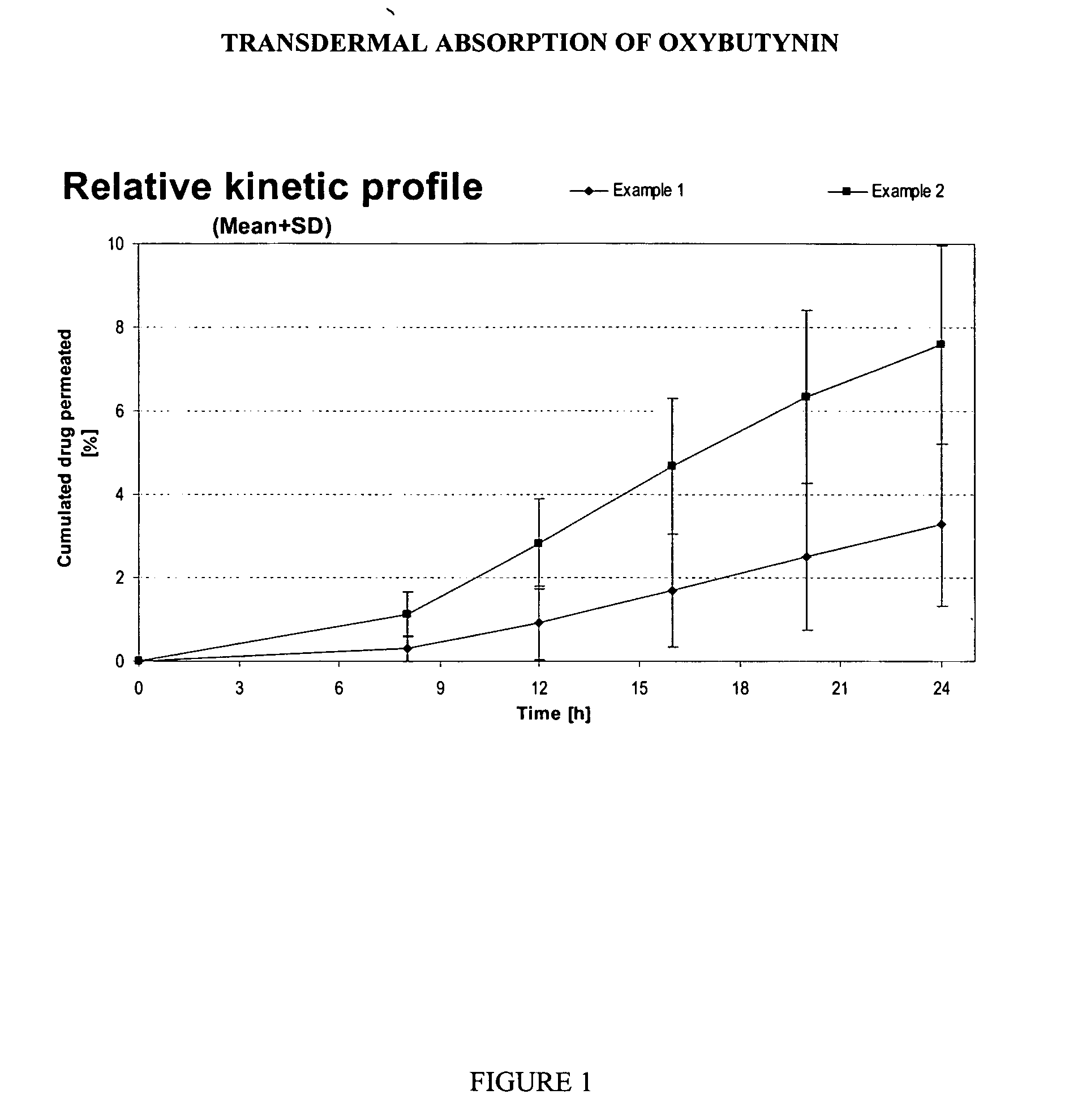
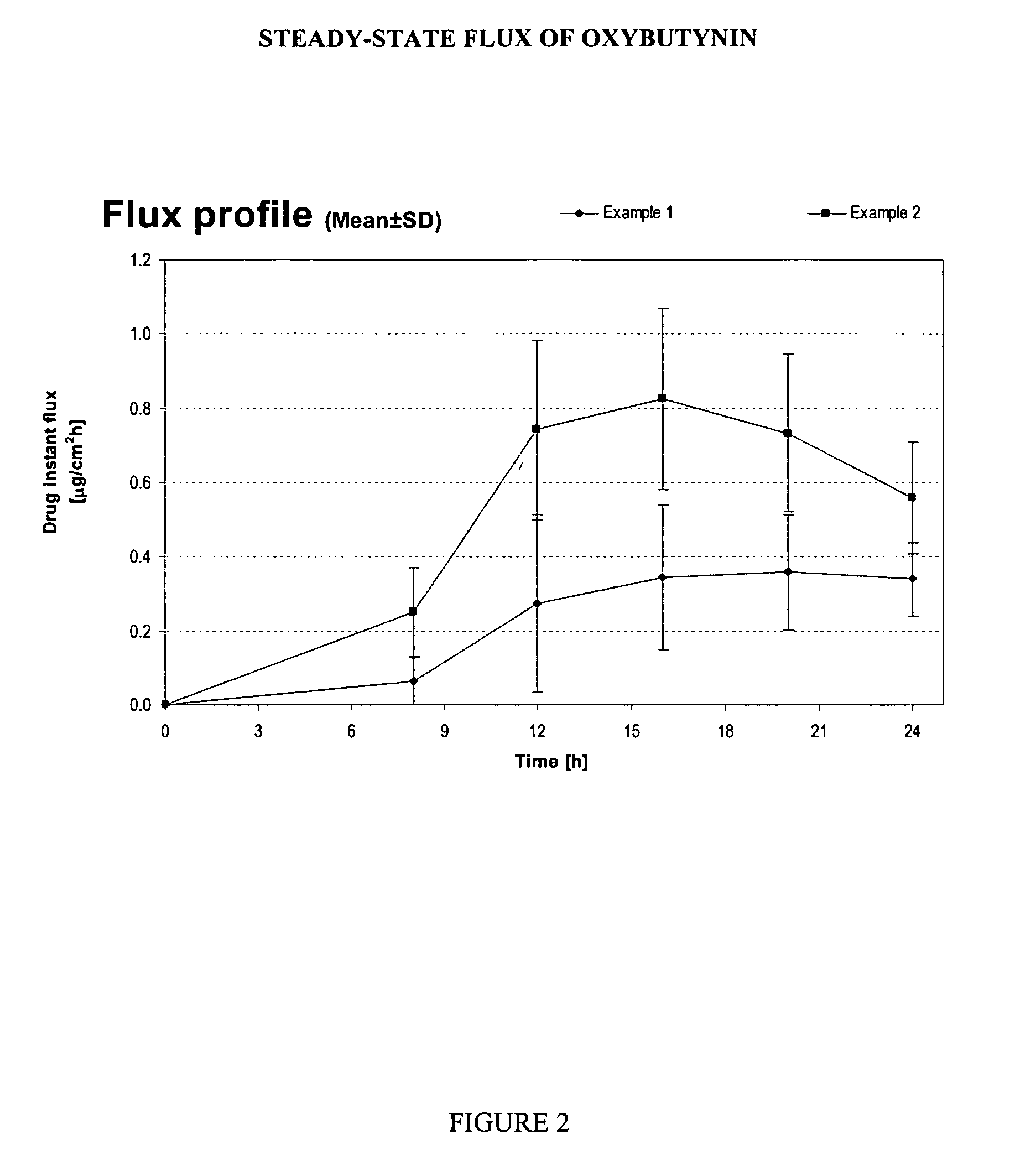
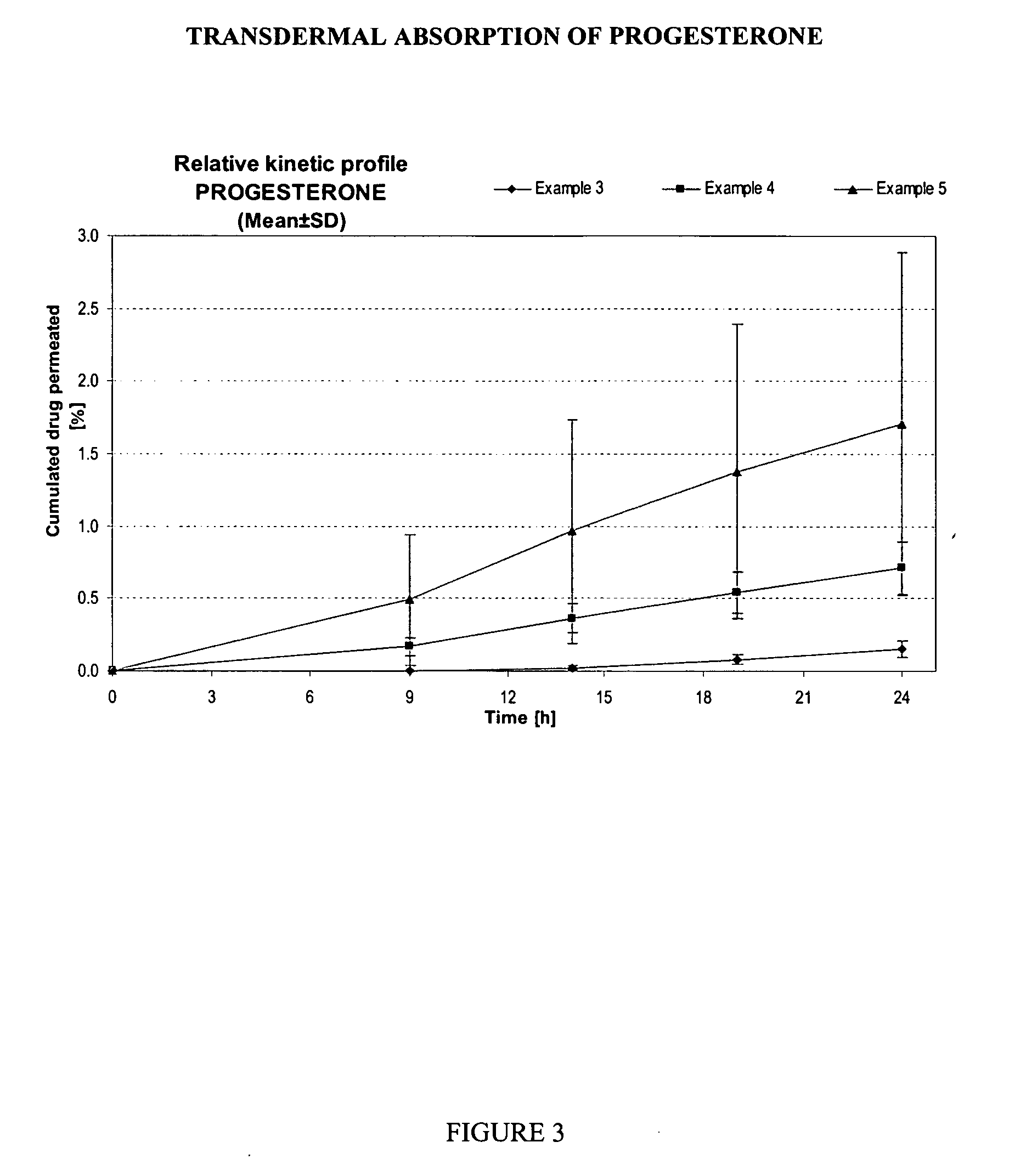
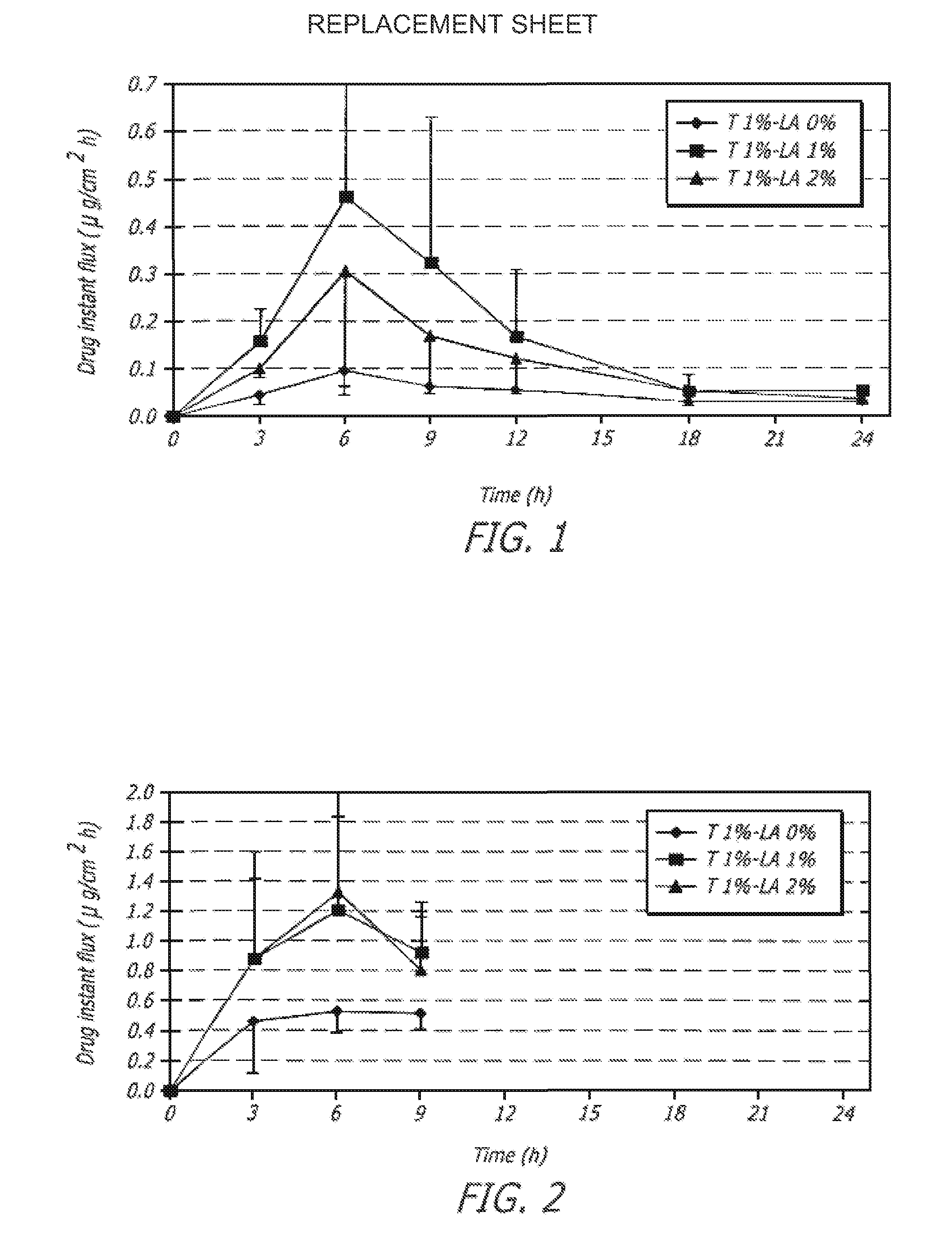
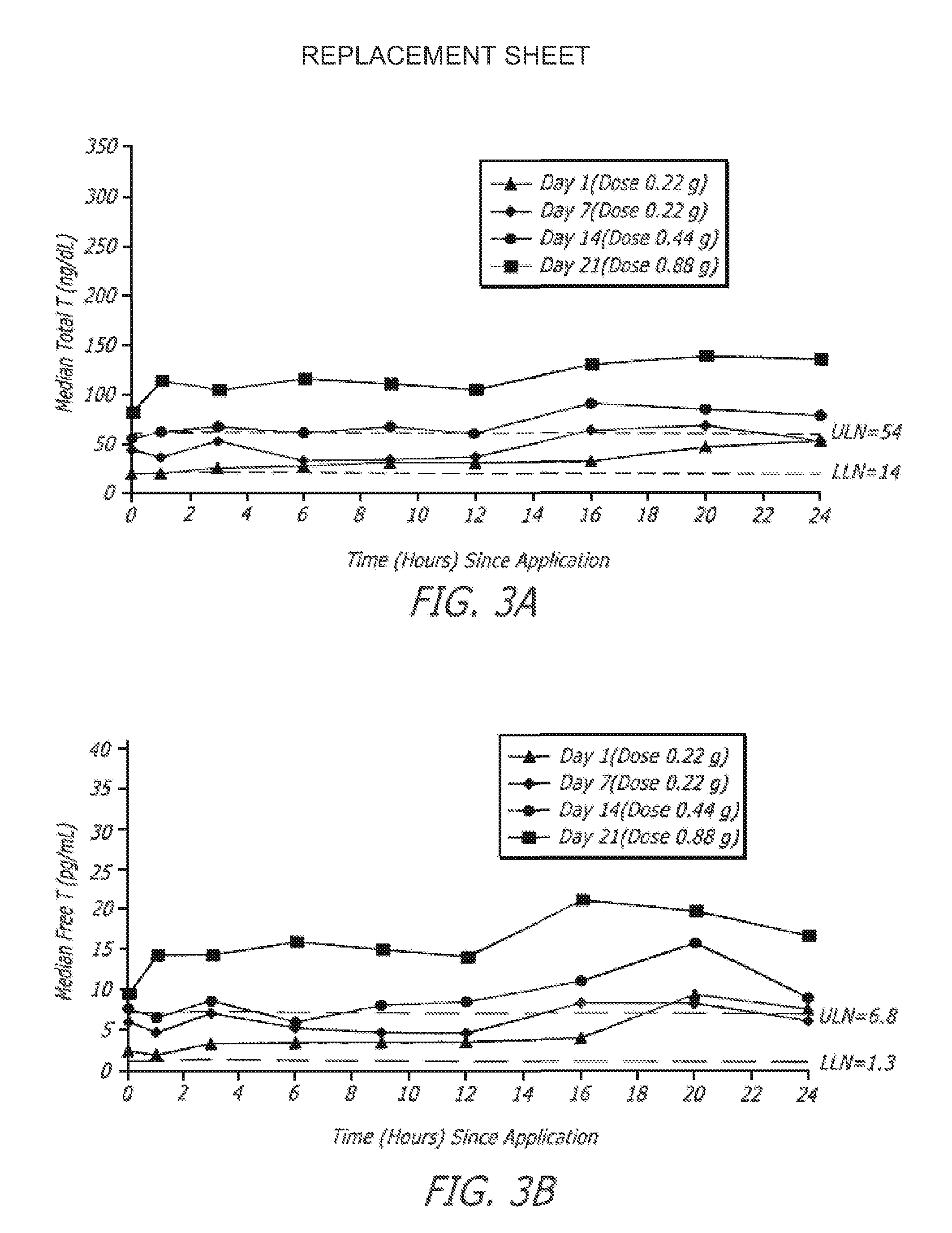
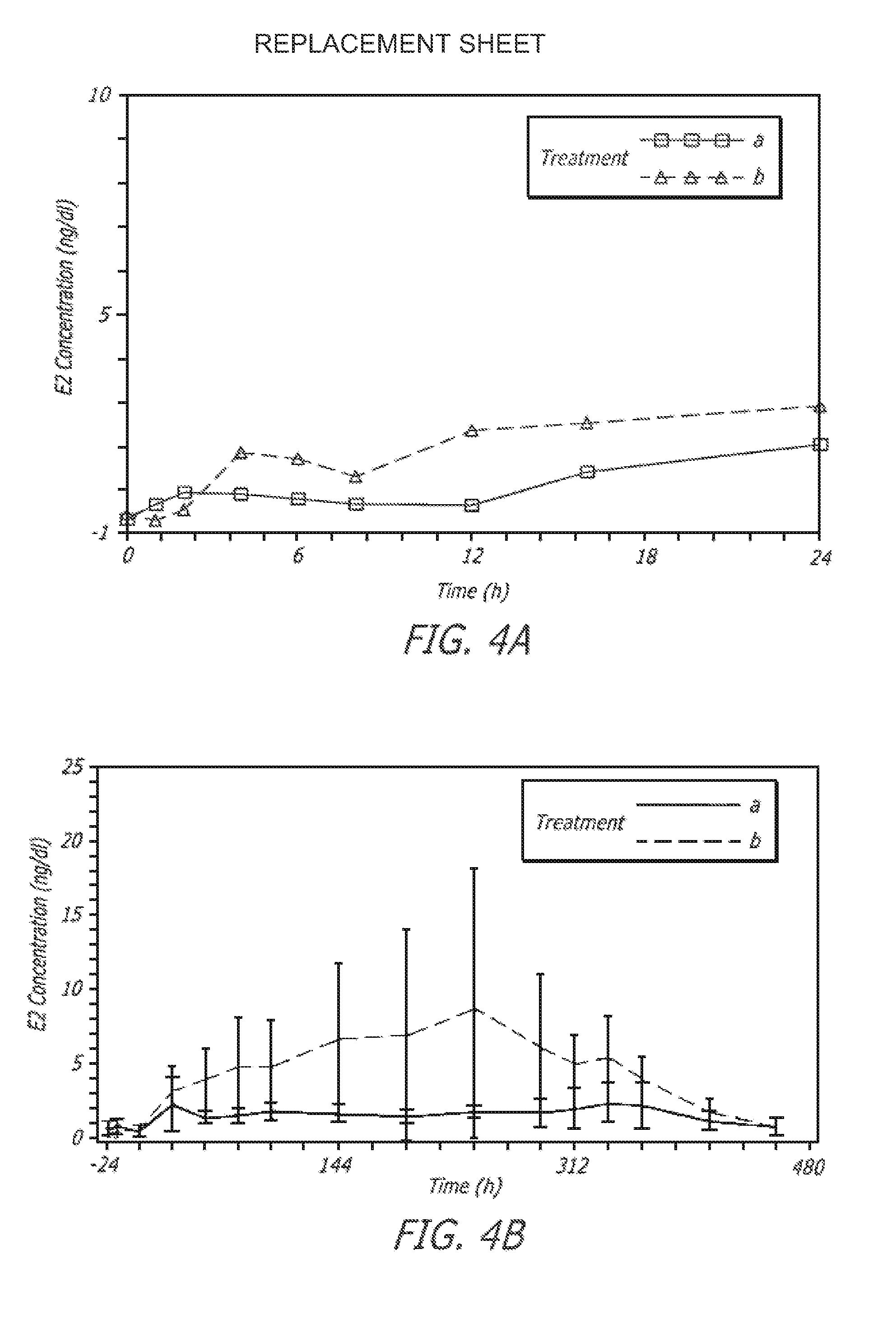
Transdermal pharmaceutical formulation for minimizing skin residues
InactiveUS20060153905A1Reduce transferLoss of therapyOrganic active ingredientsNervous disorderActive agentBULK ACTIVE INGREDIENT
This invention relates to novel transdermal or transmucosal pharmaceutical formulation which reduces the occurrences of contamination of other individuals and the transference to clothing of the user. The novel formulation includes at least one pharmacologically active ingredient, and a solvent system having a monoalkylether of diethylene glycol and a glycol present in specified ratios, and a mixture of water and alcohol. The invention also relates to a method for inhibiting or delaying crystallization of an active agent in a pharmaceutical formulation.
Owner:ANTARES PHARMA IPL
Transdermal pharmaceutical formulation for minimizing skin residues
InactiveUS7335379B2Reducing and preventing transferMinimize contaminationOrganic active ingredientsNervous disorderAlcoholActive agent
This invention relates to novel transdermal or transmucosal pharmaceutical formulation which reduces the occurrences of contamination of other individuals and the transference to clothing of the user. The novel formulation includes at least one pharmacologically active ingredient, and a solvent system having a monoalkylether of diethylene glycol and a glycol present in specified ratios, and a mixture of water and alcohol. The invention also relates to a method for inhibiting or delaying crystallization of an active agent in a pharmaceutical formulation.
Owner:ANTARES PHARMA IPL
Permeation enhancer comprising genus Curcuma or germacrone for transdermal and topical administration of active agents
InactiveUS20050244522A1Increase permeationImprove permeabilityBiocideOrganic active ingredientsTetraglycolGermacrone
A formulation, method and system for the topical, transdermal or transmucosal administration of a therapeutically effective active agent. Particularly, the invention provides a formulation, system and method for enhancing the permeation or penetration of active agents across the dermal or mucosal surfaces of a mammalian subject. The formulation includes a plant extract of the genus Curcuma of the family Zingiberaceae, a germacrone, or a natural or synthetic constituent thereof, which has been found to increase penetration of the active agent across the dermal or mucosal surface. If desired, a secondary permeation enhancer of a polyalcohol, a monoalkyl ether of diethylene glycol, a tetraglycol, or a mixture thereof can be used for certain active agents for optimal permeation enhancement.
Owner:ANTARES PHARMA IPL
Transdermal delivery systems for active agents
InactiveUS20110195114A1Avoid potential undesirable odor and irritation effectsEasy to useAntibacterial agentsBiocideIrritationPatient compliance
A delivery vehicle for topical pharmaceutical formulations that include a C2 to C4 alkanol, a polyalcohol, and a monoalkyl ether of diethylene glycol present in relative amounts sufficient to provide permeation enhancement of an active agent through mammalian dermal or mucosal surfaces. Preferably, the delivery vehicle as well as the formulations that contain it are substantially free of long-chain fatty alcohols, long-chain fatty acids and long-chain fatty esters in order to avoid potential undesirable odor and irritation effects caused by such compounds during use of the formulation. Without these additives, use of the formulations is facilitated and patient compliance is greater
Owner:ANTARES PHARMA IPL
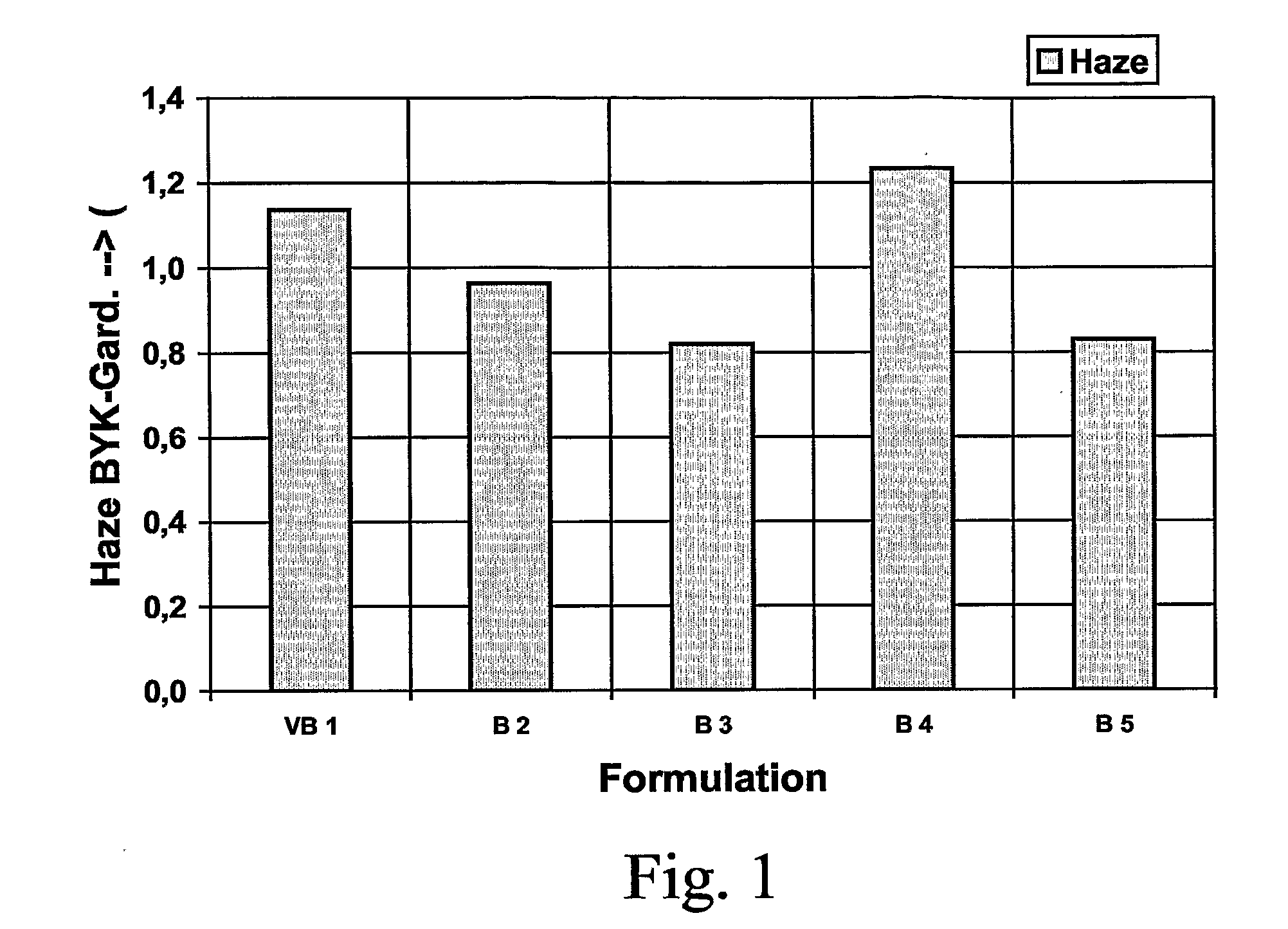
Materials composites of a moulded article of transparent or translucent dyeable plastics moulding compounds
InactiveUS20070128442A1Large flow lengthPerfect purityLayered productsThin material handlingPolymer scienceStearic acid
The present invention relates to materials composites of a moulded article of at least one transparent or translucent dyeable plastics moulding compound which moulded article is bonded to at least one transparent or translucent surface layer and / or to decorative films, functional films or coats or rubbers or other plastics, wherein the plastics moulding compound used for the manufacture of the moulded article, the surface layer or the other plastics contains in an amount of 0.01 to 5.0% by weight, preferably 0.01 to 2.0% by weight, each related to the total weight of the moulding compound, at least one lubricant selected from the group consisting of sorbitan esters, sebacic acid esters, dodecanedioic acid esters, docosanoic acid esters, glycerine, glycol, diethylene glycol, stearoyl amide, stearyl stearate, ethylene bissteroyl amide, octane pyrrolidone, and from the group consisting of non-polar paraffin oils and of tetracosanes, and wherein a permanent adhesion to the other plastics layers and / or sheets or coats or rubbers or other plastics is achieved.
Owner:EMS CHEM AG
Conductive composition, conductive film, and process for the formation of the film
ActiveUS20050116203A1Conductive materialNon-conductive material with dispersed conductive materialParticulatesSilver carbonate
A conductive composition capable of producing a conductive paint with excellent flexibility and a high conductivity comparable to that of metallic silver, without using high temperatures as film forming conditions. The conductive composition includes a particulate silver compound and a binder, and optionally a reducing agent and a binder. Silver oxide, silver carbonate and silver acetate and the like are used as the particulate silver compound. Ethylene glycol, diethylene glycol, and ethylene glycol diacetate and the like are used as the reducing agent, and a fine powder of a thermosetting resin such as a polyvalent phenol compound, phenol resin, alkyd resin or polyester resin, or a thermoplastic resin such as a styrene resin or polyethylene terephthalate, with an average particle diameter from 20 nm to 5 μm is used as the binder. Furthermore, the average particle diameter of the particulate silver compound is preferably from 0.01 to 10 μm.
Owner:THE FUJIKURA CABLE WORKS LTD +1
Silicate cement grinding aid and preparation method thereof
The invention discloses a silicate cement grinding aid and a preparation method thereof. The silicate cement grinding aid is prepared from the following main raw materials by weight: 3-6 parts of sodium hexametaphosphate, 2-5 parts of aluminum sulfate, 5-8 parts of tetra sodium salt of amino trimethylene phosphonic acid (ATMP.Na4), 4-6 parts of ethylene diamine tetra (methylene phosphonic acid) sodium, 38-45 parts of polymeric alkylol amine, 34-42 parts of triisopropanolamine, 8-12 parts of waste engine oil, 2-5 parts of sodium alpha-olefin sulfonate, 10-16 parts of sorbitol, 15-20 parts of diethylene glycol, 8-13 parts of polypropylene wax, 6-10 parts of oxidized polyethlene wax, 4-8 parts of lignite wax, 20-30 parts of odium thiosulfate, 12-18 parts of sodium thiosulfate and 15-20 parts of quicklime powder. The prepared silicate cement grinding aid has the advantages that the quality is stable, the effect is remarkable, the adding amount is small, the use is simple, and the adding is more convenient and reliable to control.
Owner:王金奎
Liquid benzoate ester compositions and aqueous polymer compositions containing same as plasticizers
Owner:EASTMAN SPECIALITIES HLDG CORP
Ultralight high-elastic rubber sole material and preparation method thereof
InactiveCN103205026AIncrease elasticityImprove bending resistanceSolesSpecific gravityButadiene-styrene rubber
The invention discloses an ultralight high-elastic rubber sole material and a preparation method thereof. The ultralight high-elastic rubber sole material comprises the following raw materials in parts by weight: 40-60 parts of natural rubber, 30-40 parts of synthetic rubber, 10-20 parts of SEBS (Styrene-Butadiene-Styrene Block Copolymer) rubber, 10-20 parts of synthetic EVA (Ethylene Vinyl-Acetate Copolymer), 30-40 parts of ultralight white carbon black, 6-10 parts of naphthenic oil, 3-5 parts of high-activity zinc oxide, 3-5 parts of activating agents, 2-3 parts of vulcanization accelerators, 1-2 parts of stearic acid, 2-3 parts of diethylene glycol and 1-3 parts of anti-aging agents. The preparation method of the ultralight high-elastic rubber sole material comprises four steps. The ultralight high-elastic sole rubber prepared through the method disclosed by the invention has the advantages of scientificity in formula, low cost, simple preparation process and lighter specific gravity of the sole material and effectively enhances the elasticity and bending resistant degree of a sole.
Owner:南平市天时雨投资合伙企业(有限合伙)
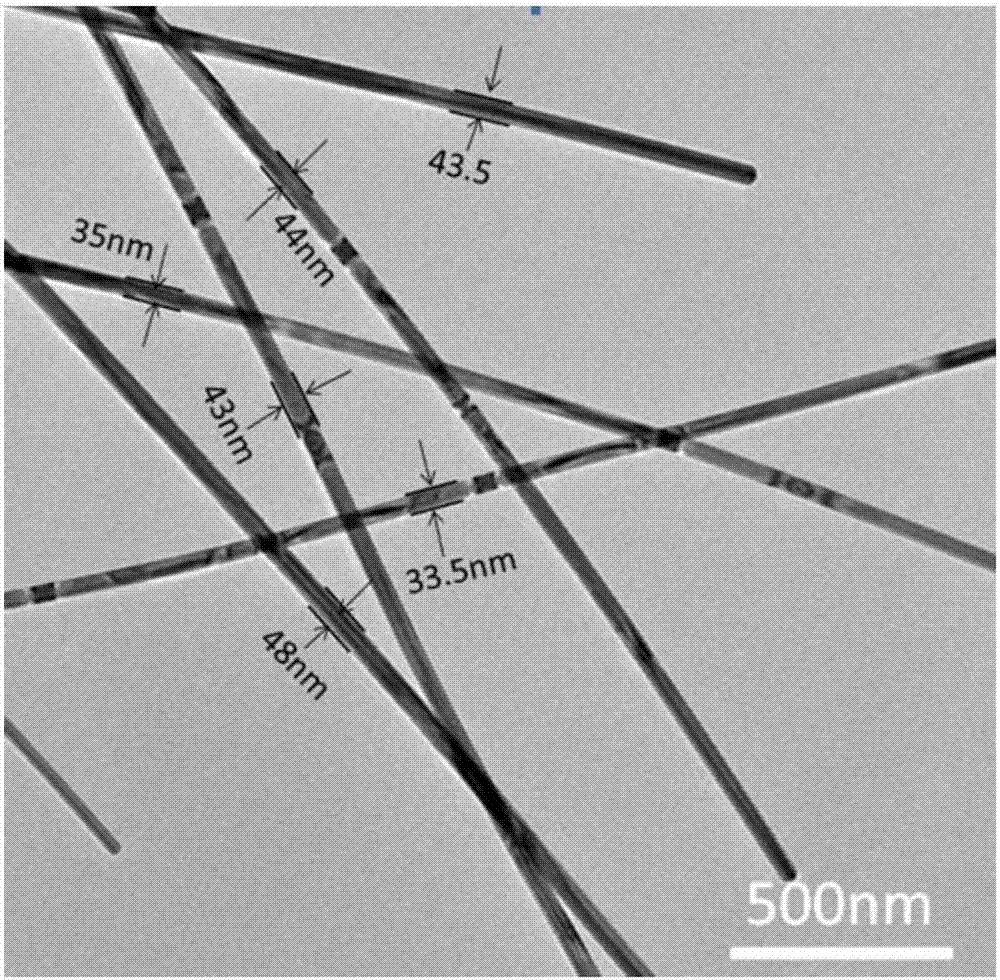
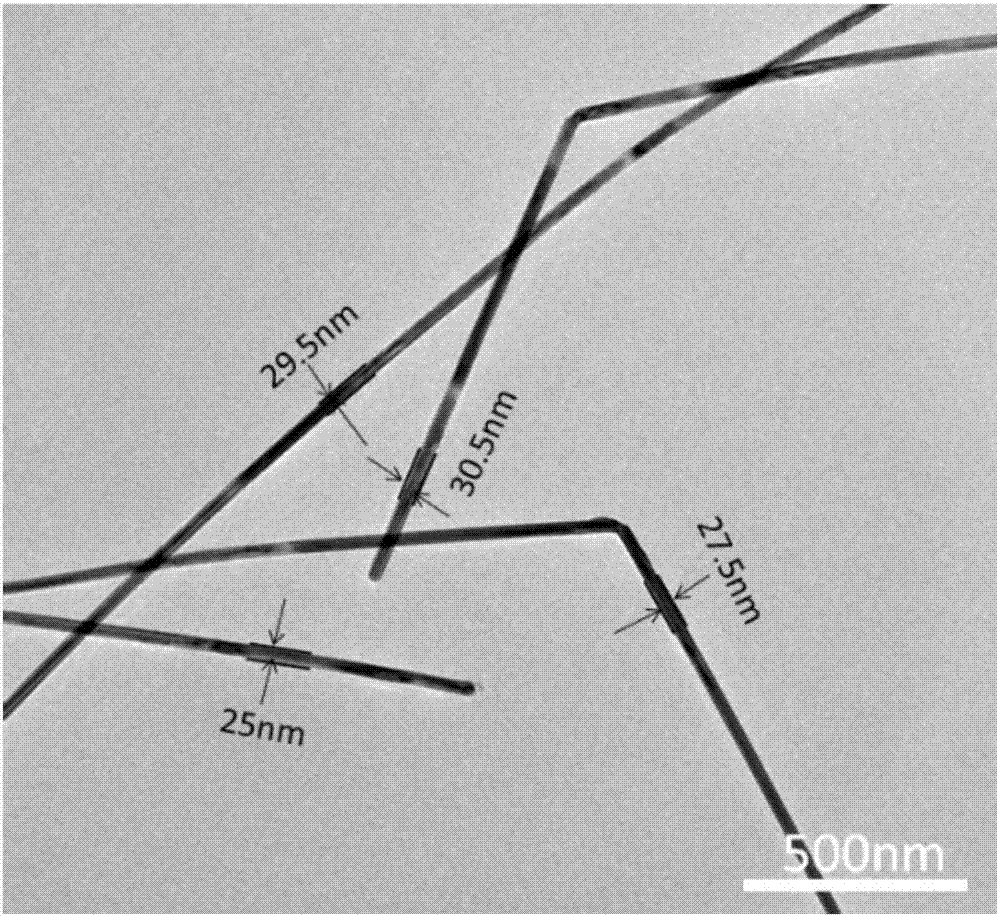
Method for preparing silver nanowires with smaller diameter through mixing polyhydric alcohols
ActiveCN106938341ASave powerAchieve purificationMaterial nanotechnologyTransportation and packagingPolyolAlcohol
The invention discloses a method for preparing silver nanowires with smaller diameters through mixing polyhydric alcohol, in which, silver nanowires with different diameters, different lengths and smaller diameters can be obtained by regulating the volume ratio, reaction temperature, and reaction time of ethanediol and diethylene glycol. The beneficial effects of the invention are: (1) the silver nanowires with different diameters and different lengths can be obtained, and the diameters of the silver nanowires may be controlled to be 40 nm or lower, even may be reduced to about 20 nm; (2) the reaction condition is not required to be accurately controlled, the uniformity of the diameters of the silver nanowires is easily controlled, the purity is relatively high, and can be prepared in a large scale; and (3) no expensive experiment apparatus is needed and the preparing cost is low.
Owner:NORTHWEST UNIV
Degradable unsaturated polyesteramide resin and synthesis method thereof
The invention adopts a melt polycondensation method. A few of monomers of C2-5 aliphatic dibasic alcohol, diethylene glycol, polyglycol, fumaric acid, maleic anhydride, lactic acid, glycolic acid, ethanolamine, C2 to C12 aliphatic diamine, glutamic acid, lysine, glycine, etc. are taken as basic materials to be synthesized to obtain non toxic unsaturated polyester-amide resin with adjustable degradation rate and lower cost; wherein, the partial fumaric acid or maleic anhydride can be replaced with phthalic anhydride, isophthalic acid, or adipic acid. The resin yearns for being used as matrix resin of medical bone internal fixation material, tissue engineering scaffold material, bone tissue temporary substitutes, environmental protection type bonding agent, environmental protection type fiberglass reinforced plastics, environmental protection type coating material, disposable tableware, packing material, shopping bags, disposable bags, drug coating or capsule, drug delivery (controlled-release) material, agricultural mulching films, etc., and can be recovered to be utilized.
Owner:HUNAN UNIV
Neutral blockage removing agent composition used for oil recovery formation in oilfield and preparation method thereof
ActiveCN104194759AImprove cleanlinessNo pollutionDrilling compositionActivated attapulgitePolythylene glycol
The invention relates to a neutral blockage removing agent composition used for an oil recovery formation in an oilfield. The neutral blockage removing agent composition is prepared from the following raw materials in parts by weight: 30-36 parts of disodium polyepoxysuccinicate, 18-25 parts of sodium gluconate, 2-3 parts of polyethylene glycol, 2-4 parts of triethanolamine, 9-12 parts of ethyl lactate, 7-10 parts of fatty alcohol polyoxyethylene ether sodium sulfate, 8-12 parts of amino acid sodium, 10-15 parts of activated attapulgite, 0.1-0.2 part of vanadium pentoxide, 5-7 parts of lipase, 5-8 parts of sorbitan fatty acid ester, 15-18 parts of P-hydroxy sodium sulfonate, 3-7 parts of tert-butyl hydroperoxide and 0.1-0.3 part of diethylene glycol monolaurate. The neutral blockage removing agent composition has high blockage removing speed, is neutral, is free of corrosion and can quickly dissolve material scales, asphalt sediment scales, carbonate scales, silicate scales and the like generated by an ASP flooding system; waste liquid for blockage removal can be degraded and does not need to be discharged onto the ground to be subjected to sewage treatment, the formation in the oilfield is cleaned to remove the blockage, no corrosion, dead angle, precipitation or secondary well blockage is generated, and the blockage removal time does not exceed 24 hours.
Owner:GANSU HEIMA PETROCHEM ENG
Liquid benzoate ester compositions and aqueous polymer compositions containing same as plasticizers
InactiveUS20030050372A1Antifouling/underwater paintsOther chemical processesPolymer scienceDiethylene glycol
The addition of at least about 30 weight percent of diethylene glycol (DEG) monobenzoate or dipropylene glycol (DPG) monobenzoate to diethylene glycol (DEG) dibenzoate, which is a solid at 28° C., results in a mixture that is unexpectedly a liquid at this temperature. The resultant mixtures are effective plasticizers for aqueous polymer compositions, including adhesives and caulks.
Owner:EASTMAN SPECIALITIES HLDG CORP
Plastic lens and process for preparing the lens
ActiveUS20050068492A1Inhibit coloringImprove scratch resistanceOrganic chemistryOptical articlesUltraviolet lightsDiethylene glycol
A plastic lens which absorbs ultraviolet light having wavelength of about 400 nm and suppresses coloring and a process for producing the lens. A plastic lens may be made from a composition which comprises (A) a lens material monomer comprising diethylene glycol bisallylcarbonate, (B) an organic peroxide-based polymerization initiator, (C) a cobalt compound represented by at least one of CoO.Al2O3 and Co.Al2O4, and (D) at least one ultraviolet light absorbent selected from 2-hydroxy-4-octyloxy-benzophenone, 2,2′,4,4′-tetrahydroxy-4-octyloxybenzophenone and 2,2′,4′-trihydroxy-4-octyloxybenzophenone. A process for producing a plastic lens comprises mixing component (A), component (B), a cobalt fluid comprising component (C) in a dispersant and component (D) and a step of casting the mixed fluid into a mold and polymerizing the fluid to obtain a plastic lens.
Owner:HOYA CORP
Ink jet black ink, ink set, ink jet recording method, ink cartridge, recording unit, and ink jet recording apparatus
InactiveUS20060146108A1Good storage stabilityHigh sticking recovery propertyMeasurement apparatus componentsDuplicating/marking methodsDecompositionDiethylene glycol
To provide an ink jet black ink which has a favorable tint for ink jet black ink, provides high ozone fastness, a high image density, and high sticking recovery property, and is capable of suppressing the decomposition of a compound represented by the following general formula (I) or a salt thereof at the time of storage for a long time period. The ink jet black ink is an ink jet black ink comprising at least a compound represented by the following general formula (I) or a salt thereof as a coloring material, and ethylene glycol or diethylene glycol, wherein the content of ethylene glycol or diethylene glycol is 15 mass % or more and 30 mass % or less with respect to the total mass of the ink jet black ink. General formula (I)
Owner:CANON KK
Holographic recording medium
Disclosed is a holographic optical recording medium, comprising a recording layer containing a three dimensionally cross-linked polymer matrix, a radically polymerizable compound, and a photo radical polymerization initiator, the three dimensionally cross-linked polymer matrix comprising a cured material of a reaction product between a diglycidyl ether and an amine, the diglycidyl ether being selected from the group consisting of 1,6-hexanediol diglycidyl ether and diethylene glycol diglycidyl ether.
Owner:KK TOSHIBA
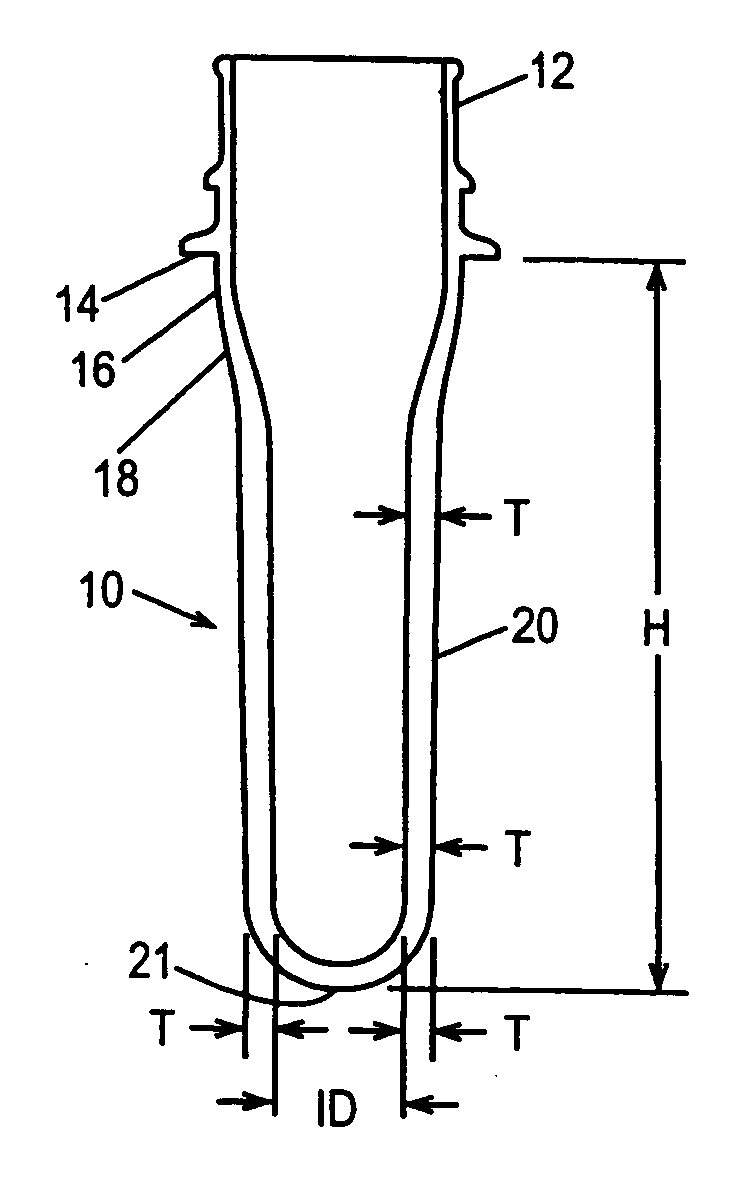
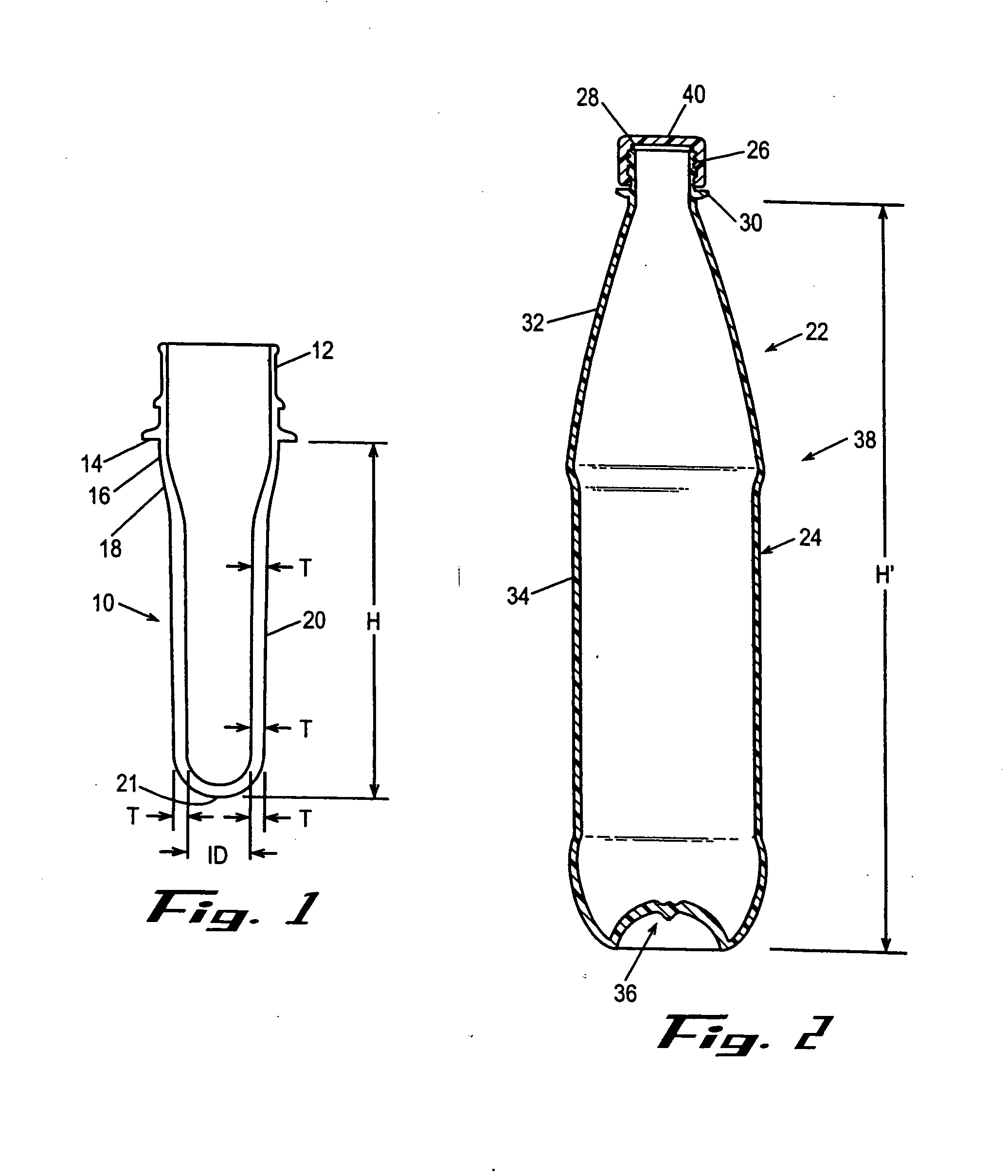
Copolyester composition for manufacturing large volume polyester bottle
A copolyester composition which includes polyethylene terephthalate (PET) is used to manufacture bottle embryos having weight greater than 600 grams and thickness greater than 7.0 millimeters. The copolyester composition of this invention is used to manufacture containers having an inner volume greater than 10 liters. The components of this copolyester composition are polyethylene terephthalate resin, X mole % of isophthalic acid (IPA), Y mole % of diethylene glycol (DEG) and Z mole % of 2, 6 napthalene dicarboxylic acid where X, Y, and Z conform to the following conditions:2.5≦X<6.0,2.5≦Y≦5.0,0≦Z≦5.0; andthe copolyester composition has an intrinsic viscosity of between 0.75 and 0.85 dl / g.
Owner:NANYA PLASTICS CORP
Tin cobalt alloy/ graphene composite material and preparation method thereof
InactiveCN104064739AInhibition of volume changeAlleviate volume changesCell electrodesSecondary cellsSolventGlycol synthesis
The invention discloses a tin cobalt alloy / graphene composite material and a preparation method of the tin cobalt alloy / graphene composite material. The preparation method of the tin cobalt alloy / graphene composite material includes the following steps that firstly, graphene oxide is dispersed into methyl alcohol or ethylalcohol or ethylene glycol or diethylene glycol, and then suspension liquid is prepared; secondly, cobalt salt and tin slat of which the mass ratio of the sum of mass to the mass of graphene oxide ranges from 60:1 to 30:1 dissolve in the suspension liquid prepared through the first step, wherein the molar ratio of a cobalt element to a tin element ranges from 1: 0.66 to 1:3; thirdly, a sodium borohydride alcoholic solution of which the concentration ranges from 1 mol / L to 2.5 mol / L is prepared, alcohol used in the first step is used in this step, the sodium borohydride alcoholic solution is mixed with the suspension liquid obtained through the second step, nitrogen is injected, and after 10 minutes of bubbling, solvent heat treatment is conducted, wherein solvent heat treatment refers to the process that a mixture reacts for 6 h to 12 h at the temperature of 100 DEG C to 160 DEG C in a closed reaction kettle and then is cooled to the room temperature, and the molar ratio of the cobalt element in sodium borohydride to the cobalt element in the cobalt salt is 6-13 to 1; fourthly, a sample obtained in the third step is repeatedly washed centrifugally in ethyl alcohol and deionized water, and a product is obtained after vacuum drying.
Owner:CHANGSHA GUORONG NEW ENERGY
Liquid glycol benzoate compositions and polymer compositions containing same
InactiveUS6184278B1Group 5/15 element organic compoundsPaper coatingDiethylene glycolOrganic polymer
The present invention relates to organic polymer compositions, containing liquid ester compositions as plastisizers, said compositions comprising mixtures of esters derived from (a) diethylene glycol and triethylene glycol and (b) benzoic or toluic acid, wherein the freezing point of the ester mixture is below the freezing points of the constituent esters when the relative concentrations of the constituent esters are within specified limits.
Owner:EASTMAN SPECIALITIES HLDG CORP
Pigmented polymerizable compositions and optical articles prepared therefrom
Provided is a polymerizable composition including: (a) a polymerizable component; and (b) a pigment component the pigment component includes: (i) an ionic or amphoteric dispersant material; and (ii) pigment nanoparticles uniformly dispersed in the dispersant material (i). The nanoparticles have a particle size of up to 500 nanometers. Also provided is a polymerizable composition of: (a) a polymerizable component including: (i) diethylene glycol bis(allyl carbonate); and (ii) a radical initiator; and (b) a pigment component which includes: (i) an ionic or amphoteric dispersant material derived from polycaprolactone; and (ii) pigment nanoparticles of ultramarine blue having an average particle size of up to 500 nanometers, uniformly dispersed in the dispersant material.
Owner:PPG IND OHIO INC
Matt, thermoformable, IR-reflective polyester film
InactiveUS20060008641A1Produced cost-effectivelyGood orientationSynthetic resin layered productsCellulosic plastic layered productsPolyesterPolyethylene terephthalate glycol
Biaxially oriented polyester films formed from a crystallizable polyester having increased diethylene glycol content and / or increased polyethylene glycol content, and / or increased isophthalic acid content, preferably polyethylene terephthalate. The films of the invention further include at least one IR-reflective pigment and one UV stabilizer. The inventive films feature adjustable mattness, diffuse scattering power for visible light, high light transmittance and IR reflectance and good thermoformability, and are suitable for thermally protective coatings or thermally protective packaging.
Owner:MITSUBISHI POLYESTER FILM
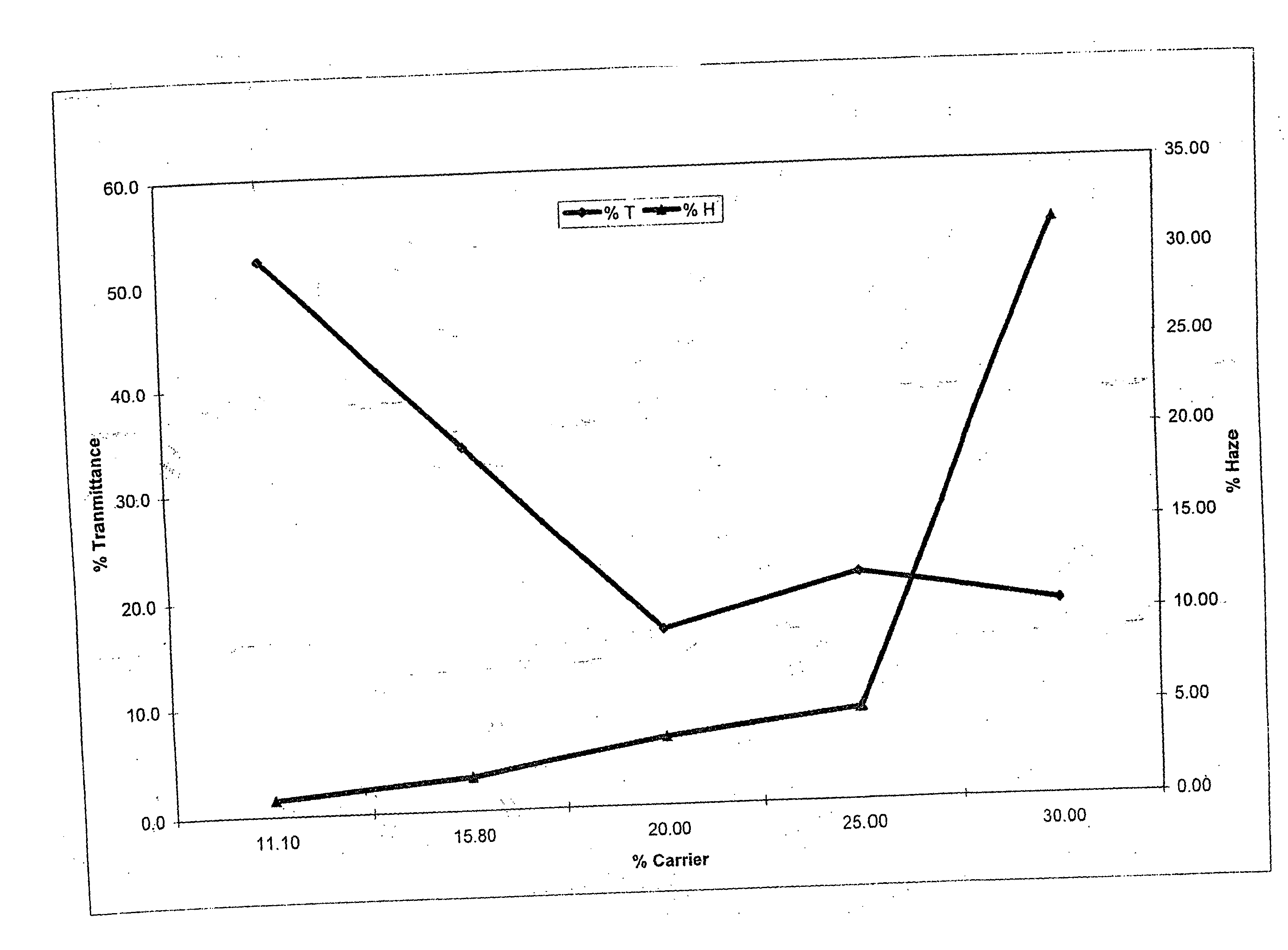
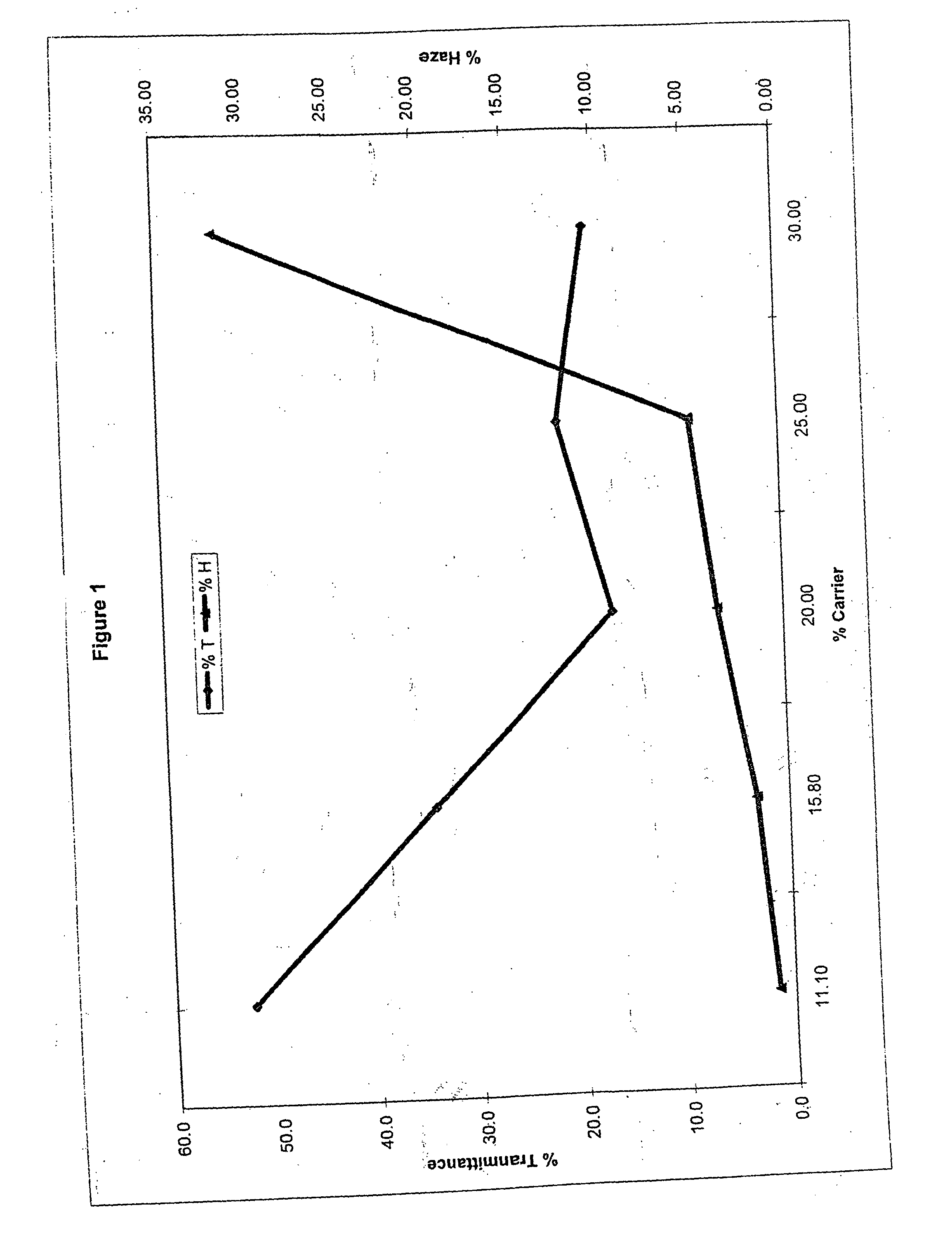
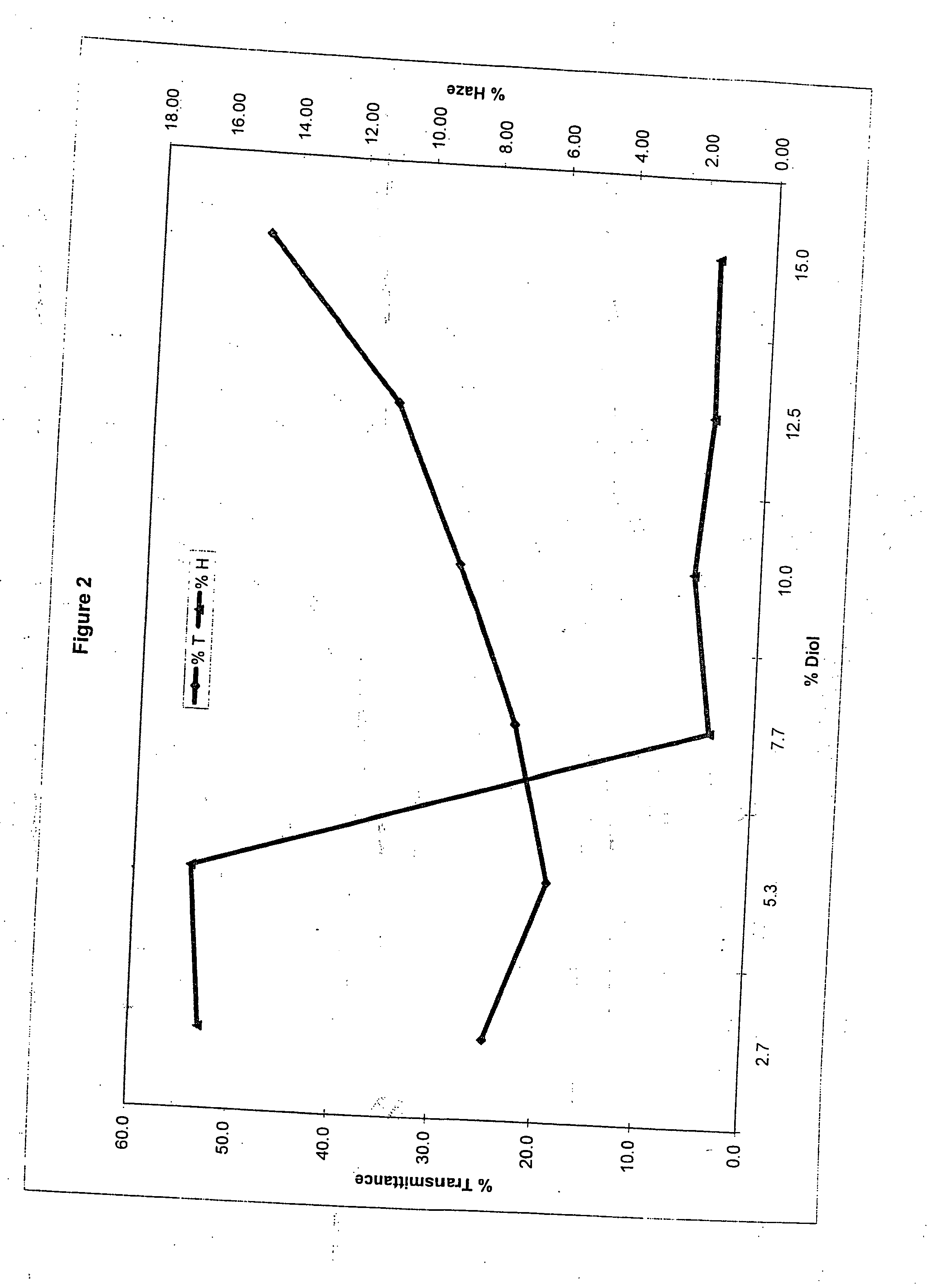
Method of dyeing a plastic article
In the method of the present invention a plastic article (e.g., a molded article of thermoplastic polycarbonate) is immersed at least partially in a dye bath which includes one or more dyes, water, at least one carrier, and at least one diol. The dye bath contains: (i) at least one dye (e.g., a static and / or photochromic dye); (ii) water; (iii) at least one carrier represented by the following general formula I, R—O—(CH2)n—OH I wherein R is a radical selected from linear or branched C1-C18 alkyl, benzyl, benzoyl and phenyl, and n is 2 or 3; and (iv) a diol selected from at least one of linear or branched C2-C20 aliphatic diols, poly(C2-C4 alkylene glycol), cycloaliphatic diols having from 5 to 8 carbon atoms in the cyclic ring, monocyclic aromatic diols, bisphenols and hydrogenated bisphenols. In an embodiment of the present invention, the carrier is ethyleneglycol butyl ether, and the diol is diethylene glycol. The present invention also relates to a method of separating the dye from the water, carrier and diol components of the dye bath, by contacting the dye bath with particulate activated carbon.
Owner:COVESTRO LLC
Low IV pet based copolymer preform with enhanced mechanical properties and cycle time, container made therewith and methods
InactiveUS20060257602A1Low IVRaise the draw ratioSynthetic resin layered productsThin material handlingDiethylene glycolDiol
A preform having a low IV and comprising a PET Copolymer comprising a diol component having repeat units from ethylene glycol and diethylene glycol and a diacid component having repeat units from terephthalic acid and naphthalenedicarboxylic acid. The total amount of diethylene glycol and naphthalenedicarboxylic acid is present in the poly(ethylene terephthalate) copolymer in an amount from about 0.1 mole percent to less than 2.8 mole percent. The preform is useful in making containers and corresponding methods are disclosed.
Owner:THE COCA-COLA CO
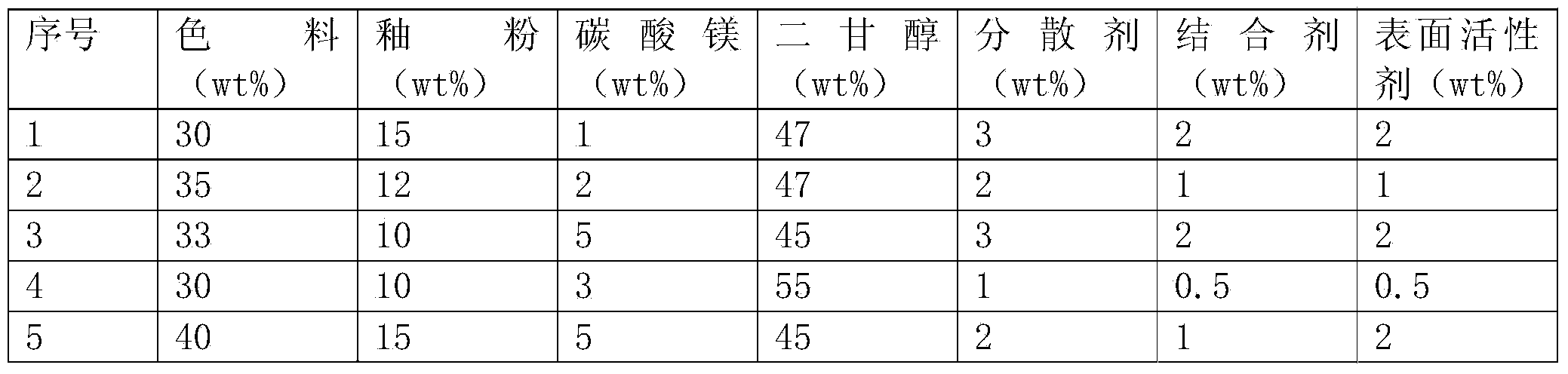
Colored glaze mixed type ink for ceramic ink-jet printing and preparation method thereof
The invention provides colored glaze mixed type ink for ceramic ink-jet printing and a preparation method thereof, belonging to the technology of ceramic tile decoration. The ink comprises the following components by mass percent: 30%-40% of pigment, 10%-15% of glaze powder, 1%-5% of magnesium carbonate, 45%-55% of diethylene glycol, 1%-3% of dispersing agent, 0.5%-2% of binding agent, and 0.5%-2% of surfactant. When the colored glaze mixed type ink for ceramic ink-jet printing is used for producing ceramic tiles for wall and floor, after ink-jet printing, the ceramic tiles can be directly sintered at high temperature without being applied with transparent protective glaze; the glaze in the ink is melted in sintering, so that the color development component of the ink is uniformly dispersed in the melted glaze, the formed ink-jet printing layer has good abrasion resistance, the production technology of the ink-jet printed ceramic tiles is greatly simplified, the production cost is lowered, and the production efficiency is improved.
Owner:LIXIAN XINPENG CERAMIC +1
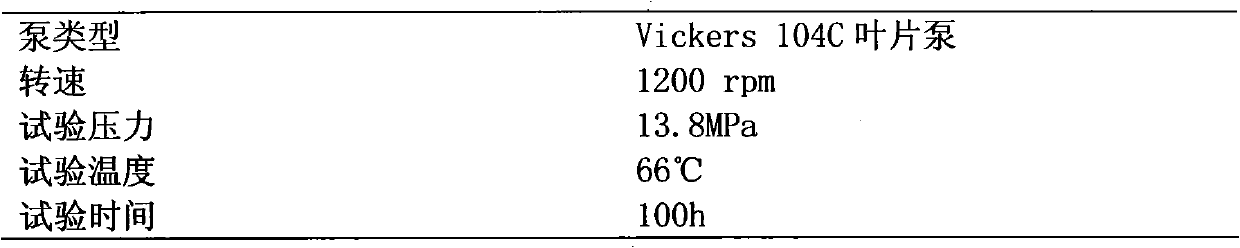
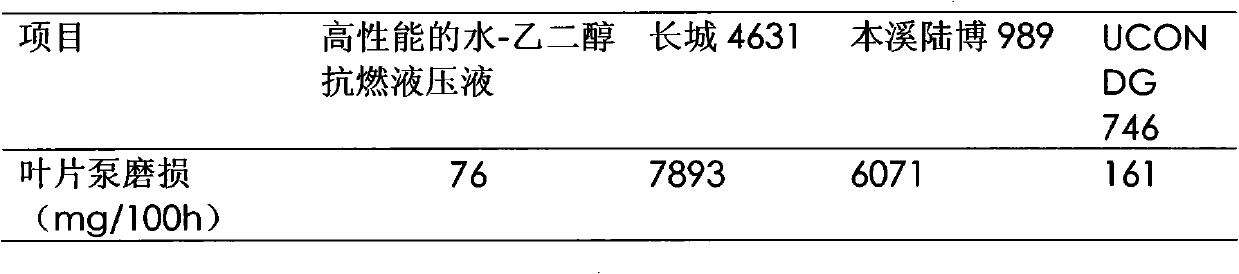
Water-glycol fire-resistant hydraulic fluid
InactiveCN102086422AEnhanced high-pressure anti-wear propertiesImprove the lubrication effectLubricant compositionBenzoic acidCapric Acid
The invention relates to a water-glycol fire-resistant hydraulic fluid. The water-glycol fire-resistant hydraulic fluid comprises the following components in percentage by weight: 37 to 42 percent of water, 12 to 15 percent of the balance of glycol or diethylene glycol and water-soluble polyether tackifier, 0.1 to 0.2 percent of sulfur-containing extreme pressure antiwear additive, 1.0 to 3.0 percent of antiwear additive formed by capric acid or the compounding of the capric acid and caprylic acid, 1.5 to 3.0 percent of at least one amine type antirust agent, 0.1 to 0.3 percent of metal passivator, 0.01 to 0.02 percent of ethylenediaminetetraacetic acid disodium, 0.005 to 0.02 percent of benzoic acid, and 0.002 to 0.005 percent of defoaming agent. The water-glycol fire-resistant hydraulic fluid has the characteristics that: the product has strong high-pressure abrasion resistance, and still has good lubricating property under high-pressure working conditions; and a Vickers 104C vane pump bench test (ASTM D7043) with test pressure of 14MPa prove that the abrasion of a vane pump is less than 100mg.
Owner:PETROCHINA CO LTD
Cleaning Compositions for Microelectronic Substrates
ActiveUS20080051308A1Organic detergent compounding agentsAnionic surface-active compoundsDiethylene glycolNitrogen
A stripping and cleaning composition for cleaning microelectronics substrates, the composition comprising: at least one organic stripping solvent, at least one nucleophilic amine, at least one non-nitrogen containing weak acid in an amount sufficient to neutralize from about 3% to about 75% by weight of the nucleophilic amine such that the stripping composition has an aqueous pH of from about 9.6 to about 10.9, said weak acid having a pK value in aqueous solution of 2.0 or greater and an equivalent weight of less than 140, at least one metal-removing compound selected from the group consisting of diethylene glycol and diethylene glycolamine, and water, and method for cleaning microelectronic substrates with these compositions.
Owner:AVANTOR PERFORMANCE MATERIALS LLC +1
Preparation method for non-crystalline low-melting point polyester hot melt adhesive
The present invention discloses a preparation method for a non-crystalline low-melting point polyester hot melt adhesive. The method comprises the following steps: (1) adopting neopentyl glycol, diethylene glycol, ethylene glycol, adipic acid, sebacic acid and m-phthalic acid as raw materials, and carrying out an esterification reaction under an effect of a catalyst; and (2) adding a stabilizer, a condensation polymerization catalyst and an antioxidant to the product from the step (1), and carrying out a reduced pressure condensation polymerization reaction to obtain a copolyester with a melting point less than 120 DEG C. The polyester hot melt adhesive of the present invention shows a transparent state at the high temperature and the room temperature, does not crystallize, has a good fluidity in low temperature use, and has excellent wettability.
Owner:SHANGHAI TIANYANG HOT MELT ADHESIVE CO LTD +2
Dimer acid modified unsaturated polyester resin and synthetic method thereof
The invention uses a melt phase polycondensation method, uses dimer acid as a reaction monomer for the first time, and performs copolymerization on the dimer acid and several monomers in the group consisting of ethylene glycol, propylene glycol, diethylene glycol, neopentyl glycol, polyethylene glycol, fumaric acid, maleic anhydride, adipic acid, phthalic anhydride and m-phthalic acid to obtain an unsaturated polyester resin with excellent combination properties and low cost. The resin is expected to serve as the matrix resin of medical intrabony fixing materials, tissue engineering support materials, bone tissue temporary substitutes, environment-friendly adhesives, environment-friendly fiber glass reinforced plastics, environment-friendly paints, disposable tableware, packaging materials, shopping bags, garbage bags, pharmaceutical coatings, or capsules, pharmaceutical slow-release (controlled-release) materials, agricultural mulching film and the like.
Owner:HUNAN UNIV
Non-flammable topical anesthetic liquid aerosols
A topical liquid aerosol formulation for accurate metered dose delivery has been developed which includes a concentrate comprising a local anesthetic in a non-alcohol solvent and a hydrofluorocarbon (HFC) propellant. In the preferred embodiment, the concentration of the non-alcohol solvent in the concentrate is between about 75% and 85% by weight of the formulation. In the most preferred embodiment, the non-alcohol solvent is a water-soluble polyol such as ethylene glycol, propylene glycol, glycerol, diethylene glycol, dipropylene glycol, oligoalkylene glycols, liquid polyalkylene glycols, or combinations thereof. In one embodiment, the concentration of the local anesthetic in the concentrate is between about 15% and 25% by weight. In the preferred embodiment, the hydrofluorocarbon propellant is 1,1,1,2-tetrafluoroethane 1,1,1,2,3,3,3-heptafluoropropane or combinations thereof, in a concentration between about 35% and 65% by weight of the final formulation, more preferably between about 45% and 55% by weight of the final formulation. A particularly preferred formulation includes benzocaine, tetracaine, and butylaminobenzoate, wherein the concentration of benzocaine in the concentrate is 14% by weight, the concentration of tetracaine in the concentrate is 2% by weight, and the concentration of butylaminobenzoate in the concentrate is 2% by weight. It has been found that the formulation is more stable in the substantial absence of oxygen. The formulation is preferably administered using a metered dose device for release of a controlled amount of the local anesthetic.
Owner:PRECISION DERMATOLOGY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com