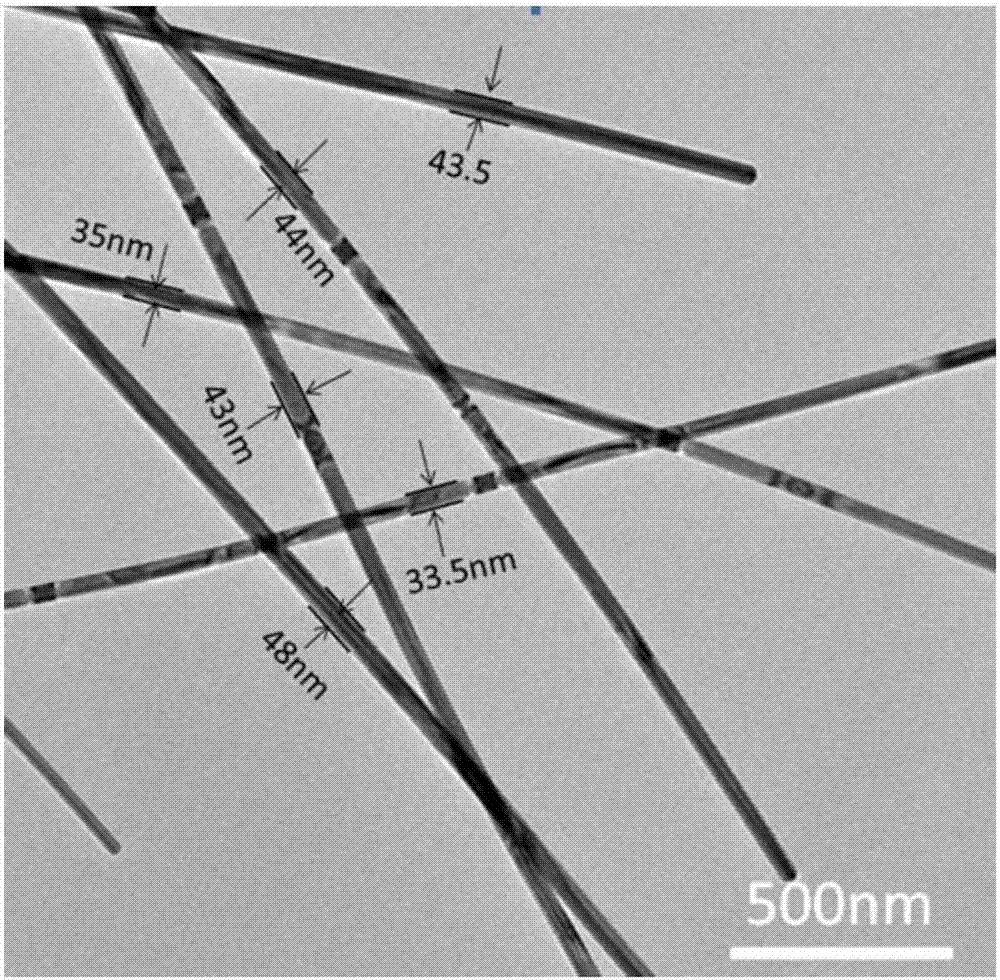
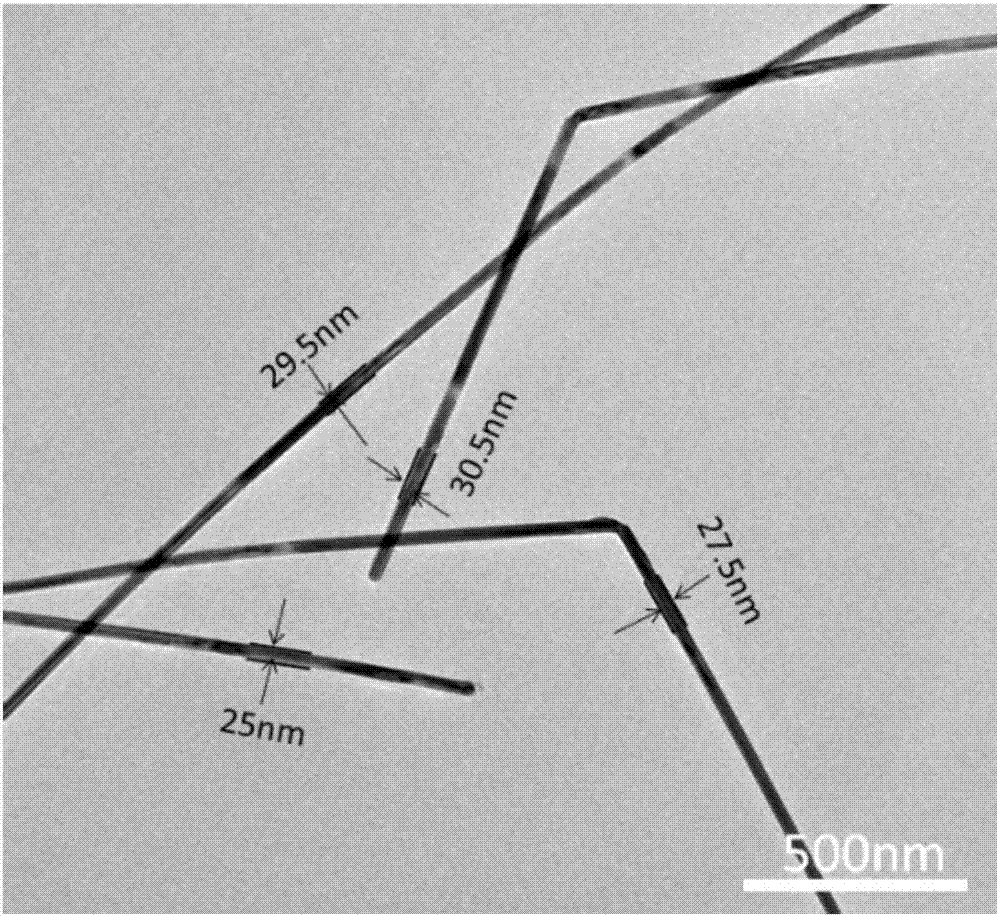
Method for preparing silver nanowires with smaller diameter through mixing polyhydric alcohols
A silver nanowire and small-diameter technology, which is applied in the field of nanomaterial preparation, can solve the problems of difficult control of nanowire diameter uniformity, failure to meet market demand, and difficulty in large-scale preparation, and achieve easy implementation and promotion, uniform diameter Easy to control and reproducible results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Step1: Prepare the solution for the reaction
[0039] Heat and stir 0.1330 g of polyvinylpyrrolidone (PVP-1300000) in an oil bath at 150° C. and dissolve in 6 ml of diethylene glycol to form solution A.
[0040] Solution B was formed by dissolving 0.135 g of silver salt in 2 ml of ethylene glycol at room temperature.
[0041] Solution C was formed by dissolving 0.0016 g of the chloride salt in 10 ml of ethylene glycol at room temperature.
[0042] The total volume of solution A, solution B and solution C is 18 ml.
[0043] Step2: The reaction generates a mixed solution
[0044] Pour solution C into solution A, heat and stir at 150°C to mix evenly to form mixed solution D.
[0045] Step3: Generate silver nanowires
[0046] Slowly add solution B to mixed solution D at a rate of 10ml / h through a microflow pump, heat (150°C) and stir in an oil bath while adding dropwise, then condense and reflux, and finally react at 150°C for 18 minutes , that is, to generate silver n...
Embodiment 2
[0052] Step1: Prepare the solution for the reaction
[0053] 0.1330 g of polyvinylpyrrolidone (PVP-1300000) was heated and stirred in an oil bath at 150° C. and dissolved in 8 ml of diethylene glycol to form solution A.
[0054] Solution B was formed by dissolving 0.135 g of silver salt in 2 ml of ethylene glycol at room temperature.
[0055] Solution C was formed by dissolving 0.0016 g of the chloride salt in 8 ml of ethylene glycol at room temperature.
[0056] The total volume of solution A, solution B and solution C is 18 ml.
[0057] Step2: The reaction generates a mixed solution
[0058] Pour solution C into solution A, heat and stir at 150°C to mix evenly to form mixed solution D.
[0059] Step3: Generate silver nanowires
[0060] Slowly add solution B to mixed solution D at a rate of 10ml / h through a microflow pump, heat (150°C) and stir in an oil bath while adding dropwise, then condense and reflux, and finally react at 150°C for 18 minutes , that is, to generate...
Embodiment 3
[0066] Step1: Prepare the solution for the reaction
[0067] Heat and stir 0.1330 g of polyvinylpyrrolidone (PVP-1300000) in an oil bath at 150° C. and dissolve in 10 ml of diethylene glycol to form solution A.
[0068] Solution B was formed by dissolving 0.135 g of silver salt in 2 ml of ethylene glycol at room temperature.
[0069] Solution C was formed by dissolving 0.0016 g of the chloride salt in 6 ml of ethylene glycol at room temperature.
[0070] The total volume of solution A, solution B and solution C is 18 ml.
[0071] Step2: The reaction generates a mixed solution
[0072] Pour solution C into solution A, heat and stir at 150°C to mix evenly to form mixed solution D.
[0073] Step3: Generate silver nanowires
[0074] Slowly add solution B to mixed solution D at a rate of 10ml / h through a microflow pump, heat (150°C) and stir in an oil bath while adding dropwise, then condense and reflux, and finally react at 150°C for 18 minutes , that is, to generate silver n...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com