Method for producing p-vinylphenols
A technology of vinyl phenol and phenol, applied in the field of biocatalysis for preparing p-vinyl phenol, can solve problems such as unreported tyrosine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 to 4
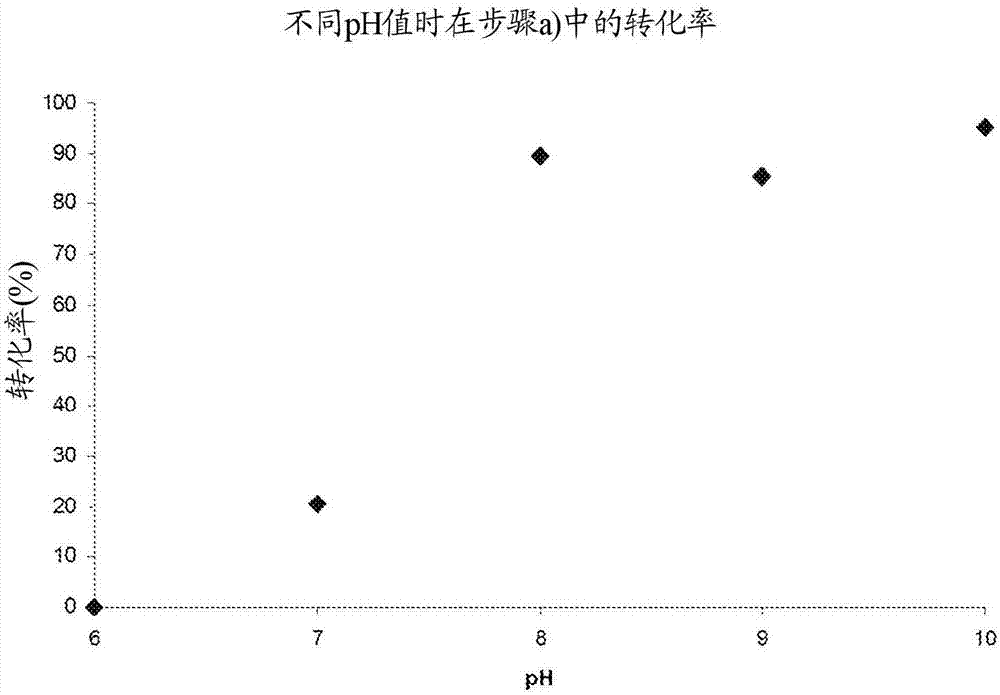
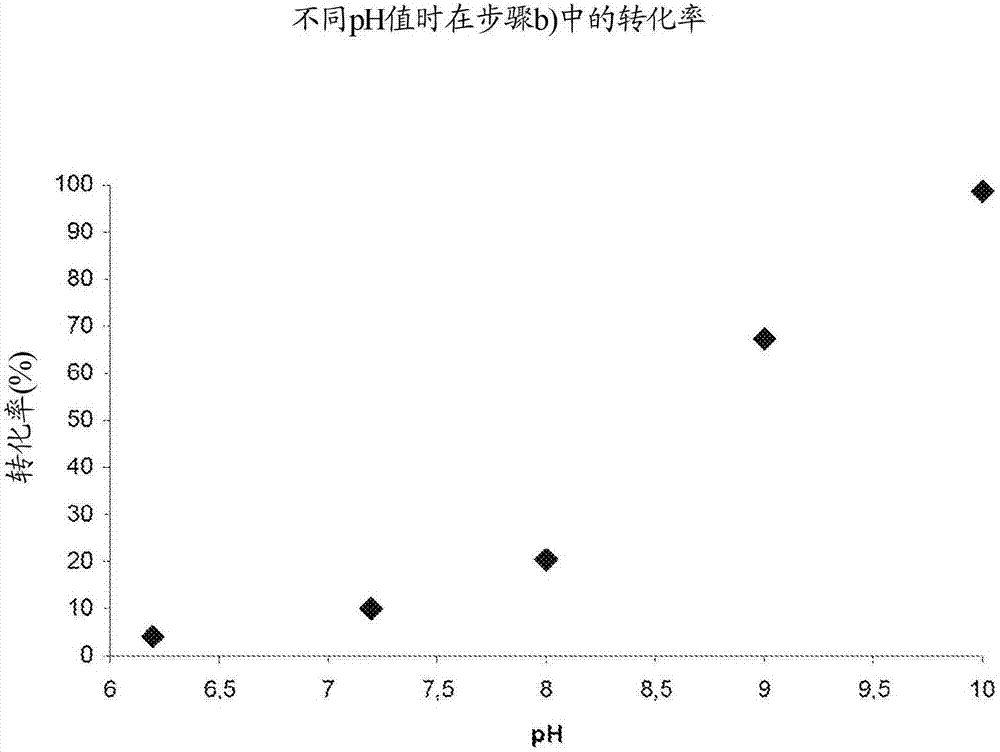
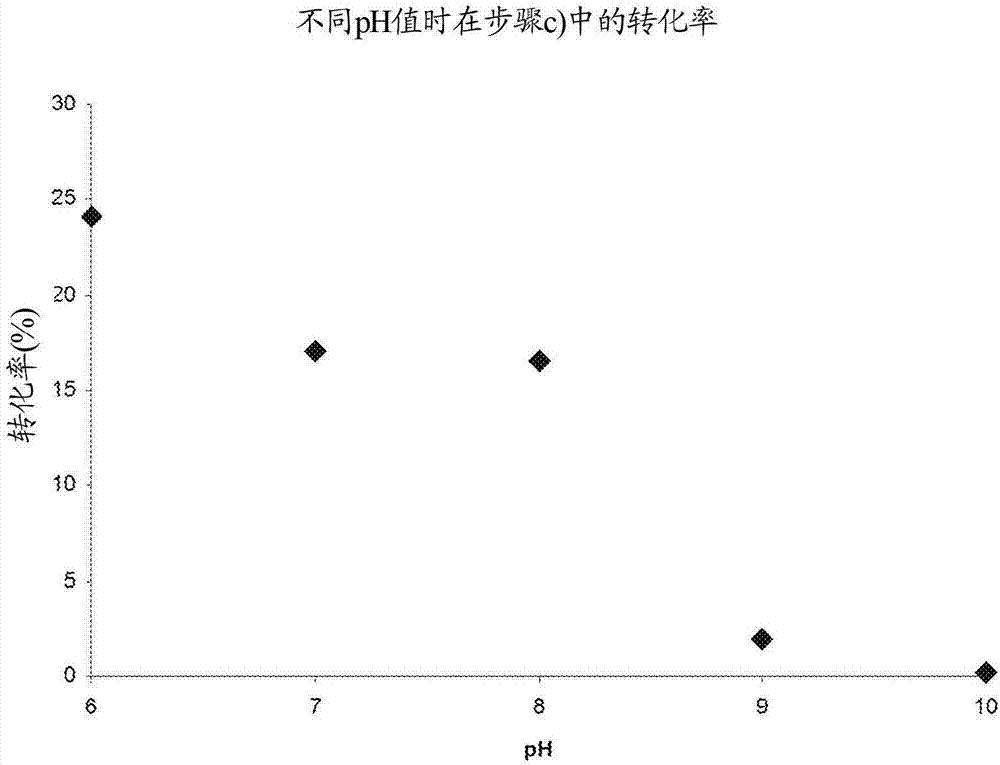
[0076] One-pot reaction of steps a) to c) at different pH values
[0077]
[0078] Using the three enzymes in the artificial synthesis examples 1 to 3, the three-step one-pot reaction using 2-fluorophenol 1a (R=F) was carried out under the following conditions: 1a (23mM); BTS (pyruvate) (46mM) ; TPL (4mg); TAL (20mg); FAD (5mg); Aqueous buffer (50mM): KPi is used for pH 7-8, CHES is used for pH 9-10; Time: 6 hours; 20 ° C; with 850 rpm / min shaking. The pH was varied between 7 and 10, samples were taken at time intervals and analyzed by means of HPLC for the content of 2-fluoro-4-vinylphenol 4a (R=F).
[0079] Figure 4 The results of these reactions are shown. The results show that the conversion at pH 7 (Example 1; about 30%) or at pH 10 (Example 4; about 45%) is significantly lower than at pH 8 (Example 2; about 85%) and pH 9 (Example 3; about 80%), almost the same conversion was obtained for them, this aspect can be attributed to the two enzymes TPL and TAL at pH 7 ...
Embodiment 5 to 8
[0084] One-pot reaction with co-solvent at pH 8
[0085] Therefore, Example 2 above was repeated at pH 8 with varying amounts of co-solvent mixed in and the amount of final product, p-vinylphenol 4a determined. In Example 5 Example 2 was repeated without a co-solvent for comparative purposes, while in Example 6 DMSO was used as a representative of the water-miscible solvent (5 Vol.-%), in Example 7 Diethyl ether was used as a representative of the water-immiscible solvent (5 Vol.-%), and in Example 8, a double amount of diethyl ether (10 Vol.-%) was added based on the volume of the aqueous buffer solution, respectively.
[0086] Figure 7 The results of these experiments are shown. It can be seen that the presence of water-miscible DMSO made practically no difference in the conversion of product 4a, whereas the non-water-miscible diethyl ether increased the conversion by more than 10 at 10 Vol.-% after 6 h reaction time percentage points, but even 20 percentage points at 5 ...
Embodiment 9
[0089] Optimal conditions for a one-pot reaction
[0090] After successful optimization of pH and co-solvent, the experiment in Example 7 was repeated under the following conditions: 1a (23 mM); pyruvate (46 mM); TPL (4 mg); TAL (20 mg); FAD (5 mg); KPi buffer Agent (50mM), pH 8; 5Vol.-%Et 2 O, time: 6 h; 30° C.; shaking at 850 rpm; and here the amounts of reactant 1 a and products 2 a to 4 a were determined at time intervals by means of HPLC.
[0091] Figure 8 The results of this experiment are shown, in which the amount of reactant 1a drops very rapidly at the beginning, and after 6 hours a conversion of more than 95% to the final product 4a is obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com