Method for biosynthesizing high-added-value compound by utilizing lignocellulose derivatives
A lignocellulose, high value-added technology, applied in the field of microorganisms, can solve the problems of long reaction steps, many by-products, and unsuitable for green and sustainable synthesis in chemical synthesis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
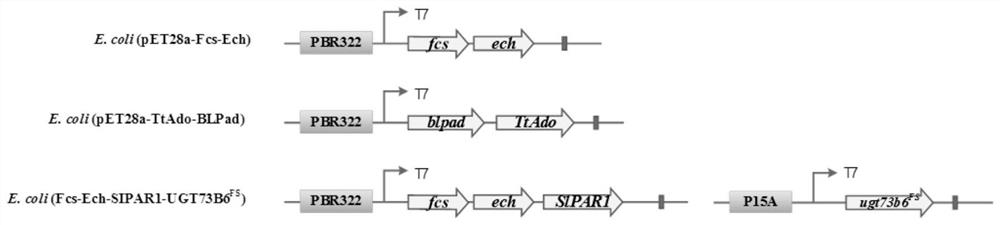
[0065] (1) Constructing a biocatalyst for the synthesis of gastrodin (the construction schematic diagram is as follows figure 1 shown)
[0066] Construction of plasmid pET28a-Fcs-Ech-SlPAR1 and plasmid pA7a-UGT73B6 FS , transform it into BL21(DE3) bacteria, construct biocatalyst E.coli(Fcs-Ech-SlPAR1-UGT73B6 FS ). The specific method of construction is as follows:
[0067] From the genome of Pseudomonas putida KT2440, Fcs and Ech were cloned by PCR, and co-constructed with SlPAR1 on the vector pET28a to obtain the plasmid pET28a-Fcs-Ech-SlPAR1; UGT73B6 Fs Constructed on vector pA7a to obtain plasmid pA7a-UGT73B6 FS ; Plasmid pET28a-Fcs-Ech-SlPAR1 and plasmid pA7a-UGT73B6 FS Transformed into the host BL21 (DE3) by heat shock or electric shock, the biocatalyst E.coli (Fcs-Ech-SlPAR1-UGT73B6 FS ).
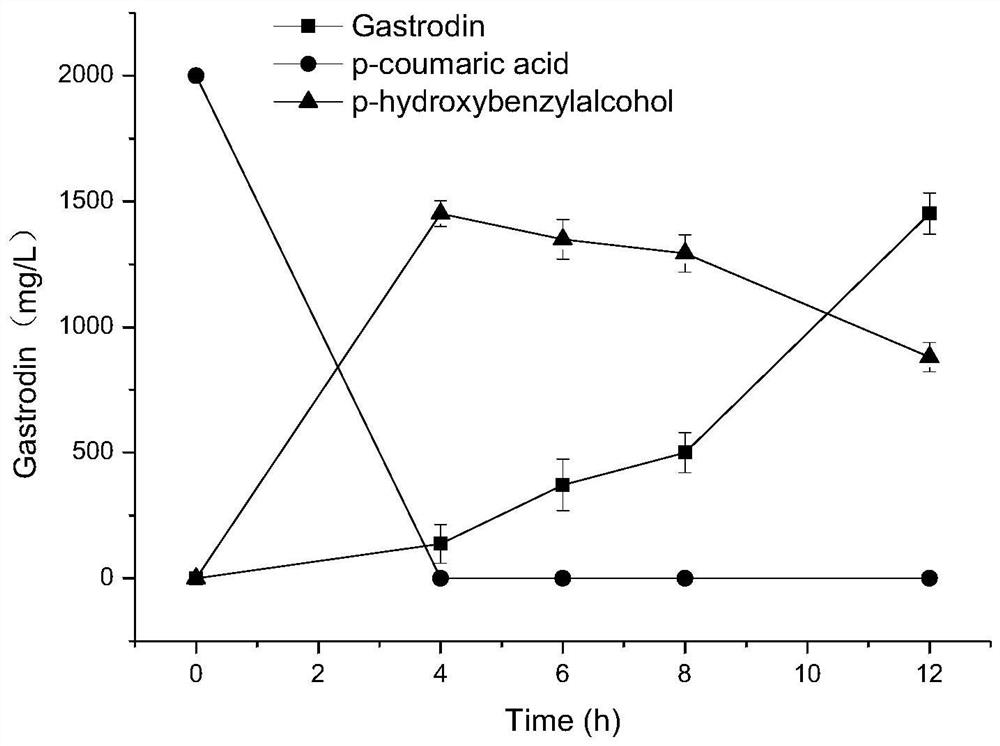
[0068] (2) biotransformation and synthesis of gastrodin (synthetic schematic diagram as Figure 12 shown)
[0069] The prepared biocatalyst E.coli (Fcs-Ech-SlPAR1-UGT73B6 FS...
Embodiment 2
[0071] (1) Construct biocatalyst to synthesize hydroquinone
[0072] Plasmids pET28a-Fcs-Ech-Vdh and plasmid pA7a-MNX1 were constructed and transformed into BL21(DE3) together to obtain biocatalyst E.coli (Fcs-Ech-Vdh-MNX1). The specific method is as follows: From the genome of Pseudomonas putida KT2440, Fcs, Ech, and Vdh were cloned by PCR to obtain pET28a-Fcs-Ech-Vdh, which was ligated into the vector pET28a by enzyme digestion, and pET28a-Fcs-Ech-Vdh was obtained, and pA7a was constructed by ligation of MNX1 into the vector pA7a by enzyme digestion. -MNX1; the plasmid pET28a-Fcs-Ech-Vdh and the plasmid pA7a-MNX1 were transformed into the host BL21(DE3) by heat shock or electric shock to prepare the biocatalyst E.coli (Fcs-Ech-Vdh-MNX1).
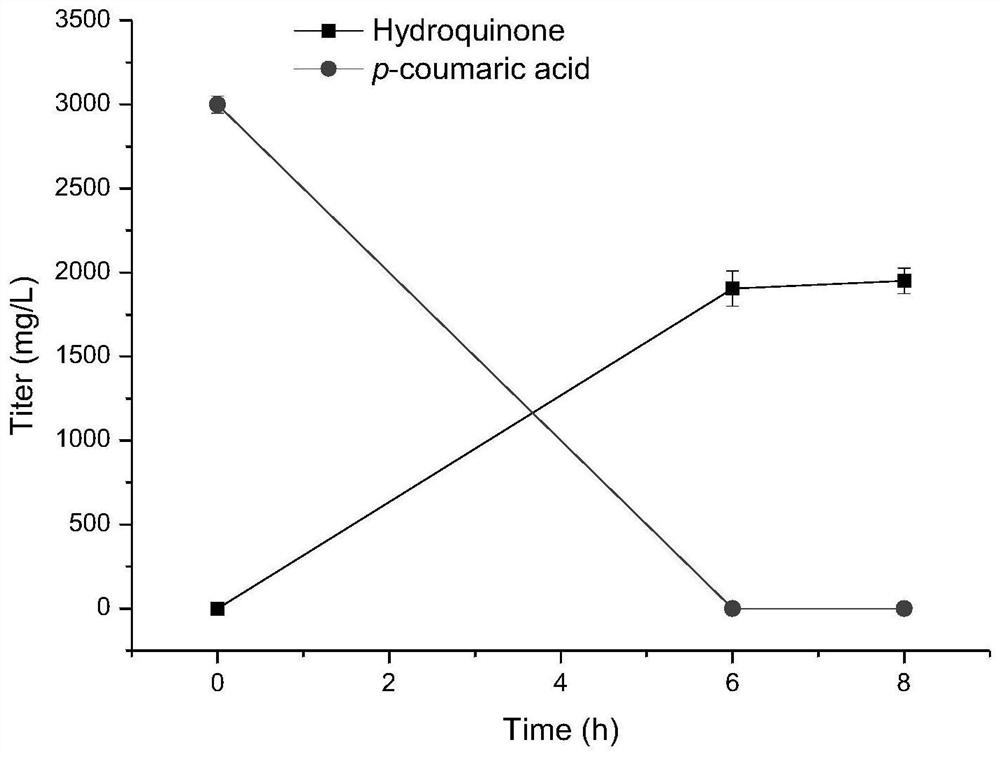
[0073] (2) biotransformation to synthesize hydroquinone (synthesis schematic diagram as Figure 13 shown)
[0074] Inoculate the prepared biocatalyst E.coli (Fcs-Ech-Vdh-MNX1) into 2mL LB for activation. After 10-12h at 37°C, transfer th...
Embodiment 3
[0076] (1) Constructing a biocatalyst for the synthesis of arbutin (the schematic diagram of the construction is as follows Figure 4 shown) corresponds to the E.coli (Fcs-Ech-Vdh-MNX1-AS) catalyst, among which SArbutin5 has the best effect.
[0077]Construction of plasmids pET28a-Fcs-Ech, pET28a-Fcs-Ech-Vdh, pA7a-Vdh-MNX1-AS, pA7a-MNX1-AS, pACYC-AS-MNX1, pA7a-AS-MNX1, pA7a-AS-7-MNX1 ( 7 represents the T7 promoter. Compared with pA7a-AS-MNX1, the T7 promoter is added before the MNX1 gene) to transform BL21(DE3) in pairs to obtain five biocatalysts SArbutin1, SArbutin2, SArbutin3, SArbutin4 and SArbutin5 . The specific method of construction is as follows:
[0078] From the genome of Pseudomonas putida KT2440, Fcs, Ech and Vdh were obtained by PCR cloning and ligated into the vector pET28a to construct pET28a-Fcs-Ech, pET28a-Fcs-Ech-Vdh; MNX1 and AS were ligated into the vector pA7a or pACYC was constructed to obtain pA7a-MNX1-AS, pA7a-AS-MNX1, pA7a-AS-7-MNX1; PCR cloned Vdh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com