Method for preparing hyperbranched polymer by adopting consecutive click chemical reaction
A technology of hyperbranched polymer and click chemistry, which is applied in the field of synthesizing hyperbranched polymers containing thioether and alkynyl groups, can solve the problems that monomers cannot be purchased commercially, lengthy preparation time, low comprehensive yield, etc. Wide range of monomers, wide application prospects, high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
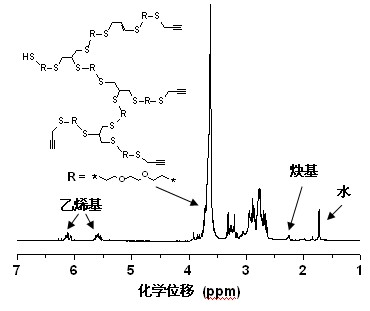
[0015] Step 1), under nitrogen protection, 3,6-dioxa-1,8-octanedithiol (3.64 g, 20 mmol), methanol (6.4 g, 200 mmol), KOH ( 1.12 g, 20 mmol), propargyl bromide (2.41 g, 20.4 mmol), at 15 o Reaction at C for 0.5 hour, filtered to remove the generated KBr salt precipitate, evaporated under reduced pressure to remove methanol;
[0016] Step 2), under nitrogen protection, dissolve the product obtained in step 1) in 40 mL of toluene, add 2 mol% of photosensitive free radical initiator benzoin dimethyl ether (102.5 mg), irradiate with ultraviolet light, and polymerize for 5 hours , through methanol precipitation, separation and drying to obtain a hyperbranched polymer (the number average molecular weight is 7800; the weight average molecular weight is 150000; the degree of branching is 0.44).
Embodiment 2
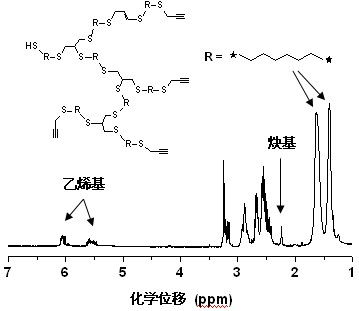
[0018] Step 1), under nitrogen protection, 3,6-dioxa-1,8-octanedithiol (3.64 g, 20 mmol), methanol (6.4 g, 200 mmol), KOH ( 1.01 g, 18 mmol), propargyl bromide (2.12 g, 18 mmol), at -20 o Reaction at C for 5 hours, filtered to remove the generated KBr salt precipitate, evaporated under reduced pressure to remove methanol;
[0019] Step 2), under nitrogen protection, dissolve the product obtained in step 1) in 40 mL of toluene, add 2 mol% of photosensitive free radical initiator benzoin dimethyl ether (102.5 mg), irradiate with ultraviolet light, and polymerize for 5 hours , the hyperbranched polymer was obtained by methanol precipitation, separation and drying.
Embodiment 3
[0021] Step 1), under nitrogen protection, 3,6-dioxa-1,8-octanedithiol (3.64 g, 20 mmol), methanol (0.32 g, 10 mmol), KOH ( 1.23 g, 22 mmol), propargyl bromide (2.60 g, 22 mmol), at 30 o Reaction at C for 0.5 hour, filtered to remove the generated KBr salt precipitate, evaporated under reduced pressure to remove methanol;
[0022] Step 2), under nitrogen protection, dissolve the product obtained in step 1) in 40 mL of toluene, add 2 mol% of photosensitive free radical initiator benzoin dimethyl ether (102.5 mg), irradiate with ultraviolet light, and polymerize for 5 hours , the hyperbranched polymer was obtained by methanol precipitation, separation and drying.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com