Method for preparing trans-ferulaic acid, p-cumaric acid and pentosan
A technology of trans-ferulic acid and pentosan, applied in the preparation of acyl halide, organic chemistry, etc., can solve the problem of many impurities, and achieve the effects of high purity of phenolic acid, reduction of waste liquid discharge, and reduction of usage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
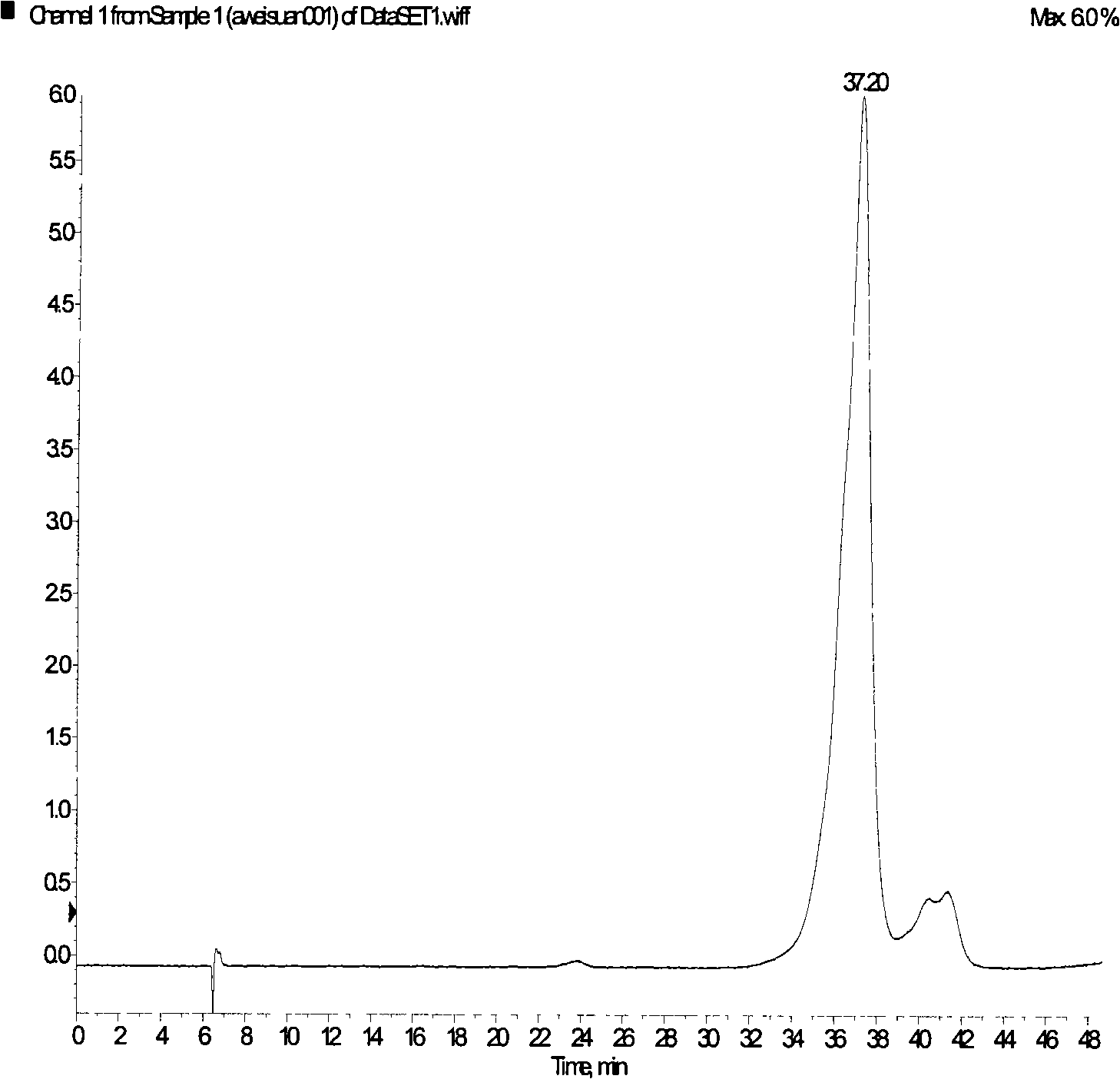
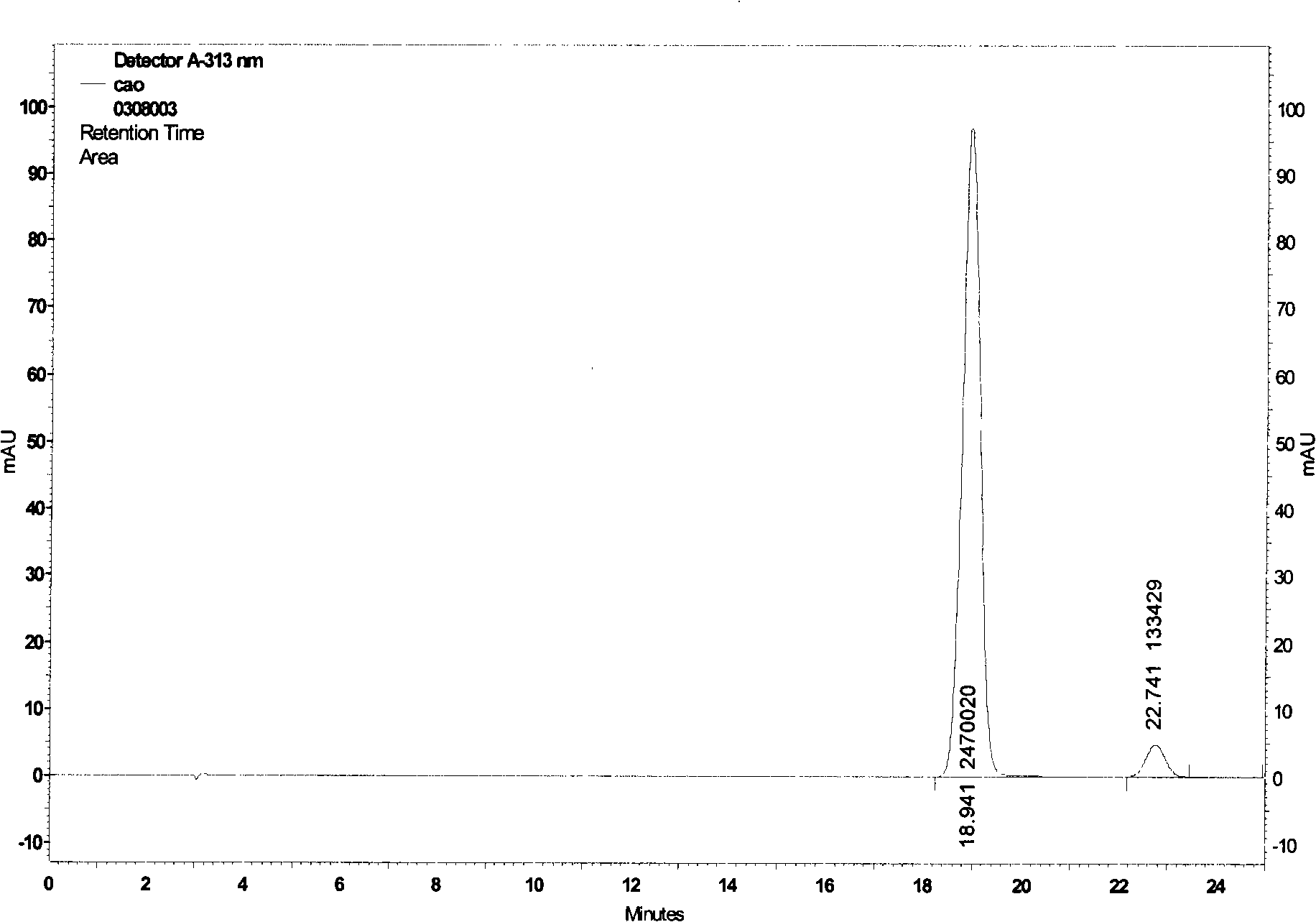
Image
Examples
Embodiment 1
[0035] The present invention will be described in further detail below in conjunction with the embodiments, but the implementation of the present invention is not limited thereto. Embodiment 1: utilize bagasse to prepare trans-ferulic acid, p-coumaric acid and pentosan
[0036] (1) Pretreatment of raw materials: bagasse is dried and pulverized;
[0037] (2) Dilute alkali treatment releases p-coumaric acid:
[0038] The pretreated raw materials are soaked in a 0.5% sodium hydroxide solution with a mass concentration of 0.5% according to a solid-to-liquid ratio of 1g: 10ml, stirred continuously, treated at 15°C for 24 hours, filtered, and the filter residue is used for later use;
[0039] The obtained filtrate is concentrated to 1 / 20 of the volume of the original low-concentration alkaline hydrolysis solution with an ultrafiltration device with a molecular weight cut-off of 5000, and the concentrated solution is used to prepare pentosan, and the permeate is used for subsequent ...
Embodiment 2
[0051] The concentrated solution described in step (2) or (4) is neutralized to pH5 with a mass concentration of 1% hydrochloric acid or sulfuric acid, centrifuged, and ethanol is added to the supernatant until the final ethanol volume concentration is 50%, filtered or centrifuged, and the precipitate is Pentosan. Example 2: Utilization of corncob trans-ferulic acid, p-coumaric acid and pentosan
[0052] (1) Pretreatment of raw materials: corncobs are dried and pulverized;
[0053] (2) Dilute alkali treatment releases p-coumaric acid:
[0054] The pretreated raw material is soaked in sodium hydroxide with a mass concentration of 0.8% according to the solid-to-liquid ratio of 1g: 15ml, stirred continuously, treated at 60°C for 4 hours, filtered, and the filter residue is set aside;
[0055] The obtained filtrate is concentrated to 1 / 40 of the volume of the original low-concentration alkaline hydrolysis solution with an ultrafiltration device with a molecular weight cut-off of...
Embodiment 3
[0068] Example 3: Preparation of trans-ferulic acid, p-coumaric acid and pentosan by straw
[0069] (1) Pretreatment of raw materials: drying and crushing of straw;
[0070] (2) Dilute alkali treatment releases p-coumaric acid:
[0071] Soak the pretreated raw material in a 0.6% mass concentration potassium hydroxide solution according to a solid-to-liquid ratio of 1g: 12ml, stir continuously, and process at 30°C for 14 hours, filter, and filter residue for subsequent use;
[0072] The obtained filtrate is concentrated to 1 / 30 of the volume of the original low-concentration alkaline hydrolysis solution with an ultrafiltration device with a molecular weight cut-off of 20,000. The concentrated solution is used to prepare pentosan, and the permeate is used for subsequent use;
[0073] (3) Obtaining p-coumaric acid:
[0074] Resin and step (2) permeated liquid were stirred and adsorbed in a stirring tank for 5 hours according to the volume concentration 1: 130, the resin was sep...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com