Method for preparing p-coumalic acid by using spartina alterniflora
A technology of Spartina alterniflora and p-coumaric acid, which is applied in the separation/purification of carboxylic acid compounds, medical preparations containing active ingredients, and applications, etc., can solve problems such as affecting biodiversity, and achieve remarkable results and yields. High and low cost of planting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The research of embodiment 1 Spartina alterniflora active ingredient
[0030] Collect 100kg of the aboveground part of Spartina alterniflora, dry it in the sun, cut it into 2-3cm long, add 20 times of hot water to extract, and concentrate the extract to a dark brown liquid (about 10kg).
[0031] The technical solution disclosed in the patent application number 201510266409.2 can also be referred to to prepare the fine powder of Spartina alterniflora, which is dissolved in pure water and configured as a 1:10 sample mother liquor (aqueous solution, wherein the total saponin content is > 6.0%). Spartina powder used in the present invention was purchased from Jiangsu Hailifa Biotechnology Co., Ltd.
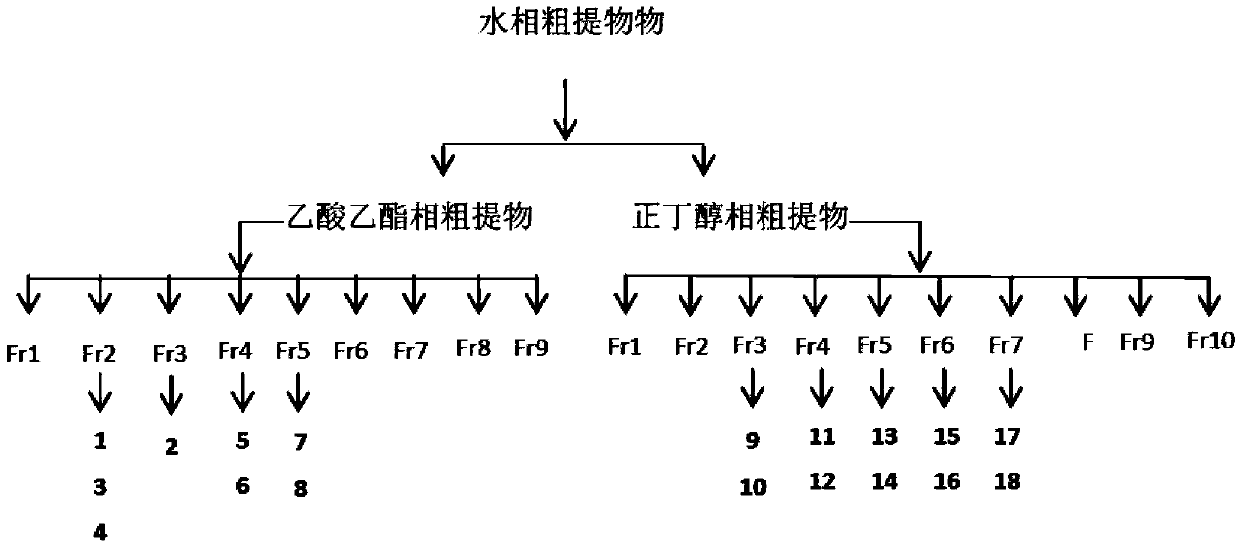
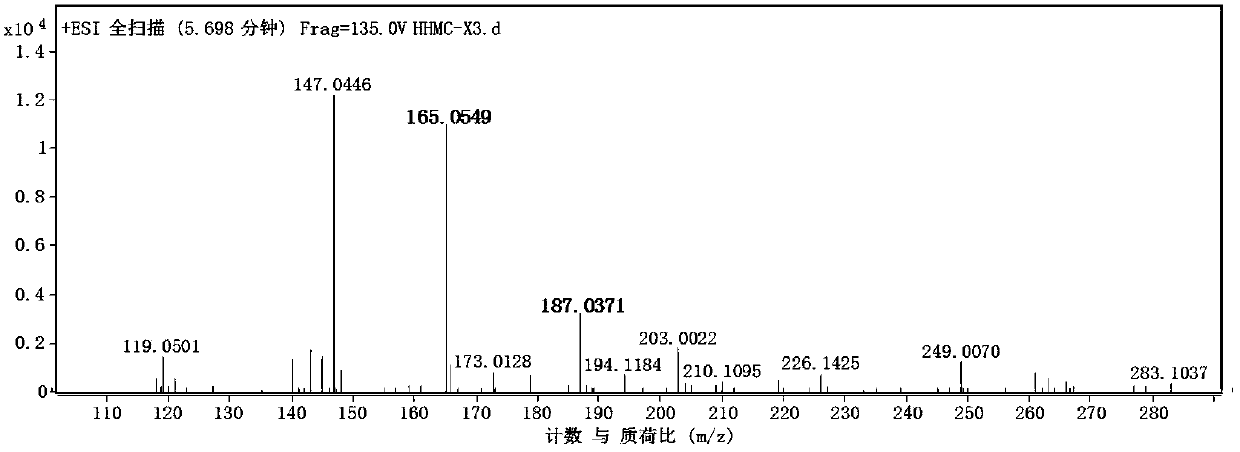
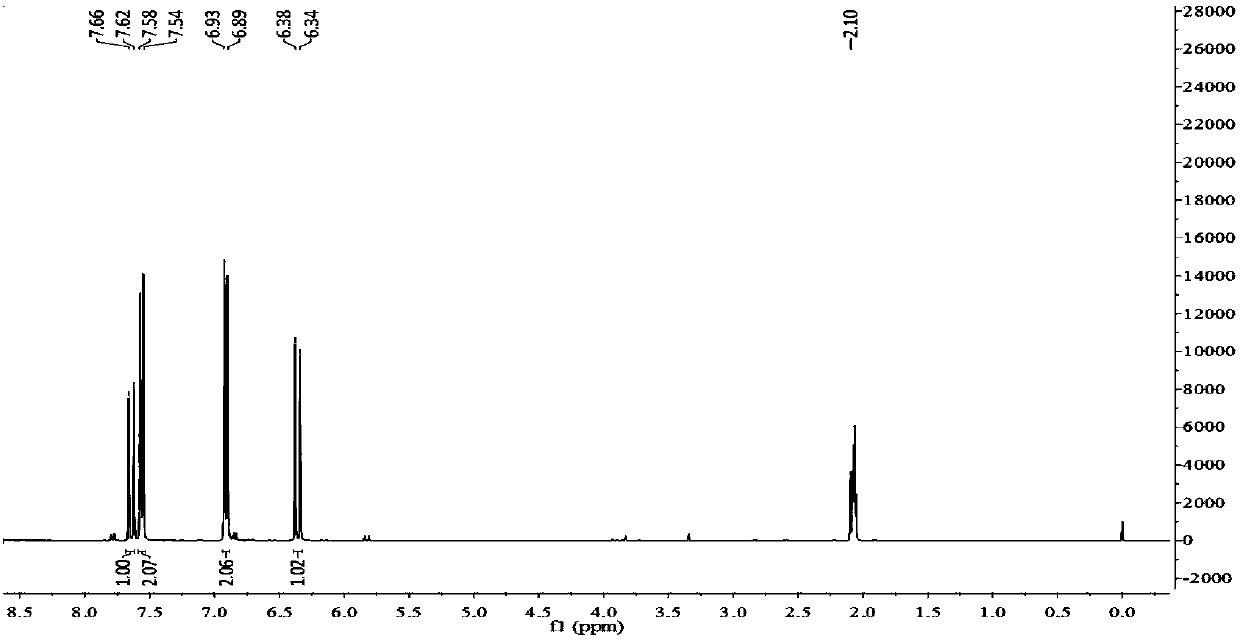
[0032] Take 2500g of concentrated solution of Spartina alterniflora, extract and extract 3 times with an equal volume of ethyl acetate, take the organic phase and concentrate it by rotary evaporation under reduced pressure to obtain 113.8g of crude paste of the ethyl acetate ph...
Embodiment 2
[0036] Example 2 Animal Test of Spartina alterniflora Extract and p-coumaric Acid for Lowering Blood Uric Acid
[0037] 1. Experimental materials
[0038] 1. Animals: 20-25 grams of male Kunming mice, 60
[0039] 2. Modeling agent: hypoxanthine
[0040] 3. Positive drug: benzbromarone
[0041] 4. Pseudopositive drugs: Spartina powder, desugared Spartina powder
[0042] 5. Negative agent: normal saline
[0043] 6. Test agent: p-coumaric acid isolated and identified from Spartina extract
[0044] 7. Solvent: sodium carboxymethylcellulose-CMC-Na
[0045] 8. Syringe: 1ml, 5ml
[0046] 9. Centrifuge tube: 1.5ml, 2ml, 5ml
[0047] 10. Capillaries for blood collection
[0048] 11. Feeder: flat head 9# needle
[0049] 2. Test equipment
[0050] Beckman LX20 automatic biochemical analyzer
[0051] 3. Experimental plan
[0052] 1. Reagent preparation:
[0053] (1) Preparation of 0.8% CMC-Na (sodium carboxymethyl cellulose): Weigh 2.4 grams of CMC-Na, first adjust it into a ...
Embodiment 3
[0113] Example 3 Comparison test of p-coumaric acid prepared by the method of the present invention and commercially available products for reducing blood uric acid
[0114] Referring to the method of Example 2, the blood uric acid-lowering effect of p-coumaric acid prepared by the method of the present invention and commercially available p-coumaric acid (purchased from Shanghai Yuanye Biotechnology Co., Ltd.) was investigated, and the results are shown in Table 10.
[0115] Both the p-coumaric acid 0.5mg / ml dosage group and the commercially available p-coumaric acid group 0.75mg / ml dosage group prepared by the present invention can reduce the serum uric acid level of mice with hyperuricemia, while the commercially available p-coumaric acid group The same dose of 0.5 mg / ml group could not reduce the serum uric acid level of mice with hyperuricemia (Table 10). This shows that the activity of the present invention on reducing the serum uric acid level of mice with hyperuricemia...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com