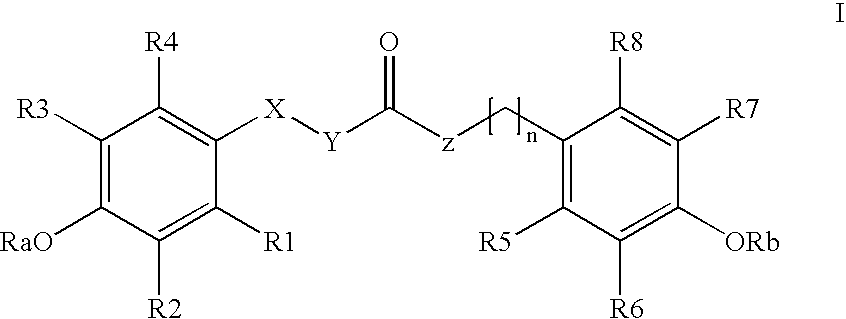
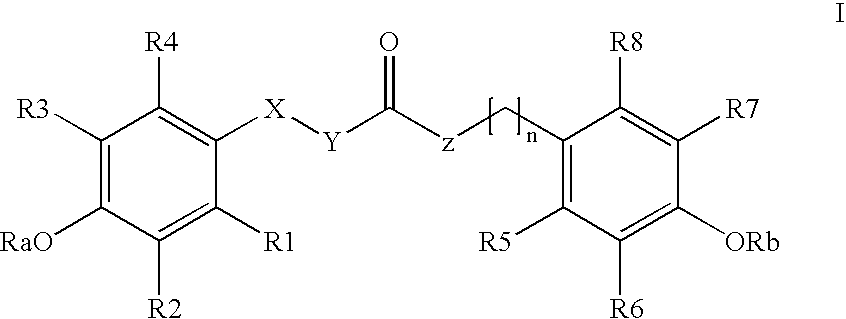
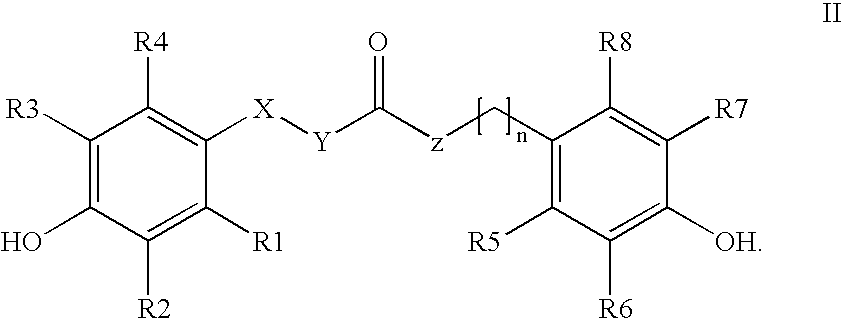
Para-coumaric acid or para-hydroxycinnamic acid derivatives and their use in cosmetic or dermatological compositions
a technology of para-coumaric acid and para-hydroxycinnamic acid, which is applied in the direction of hair cosmetics, drug compositions, anti-noxious agents, etc., can solve the problems of irreversible damage to the genetic material of keratinocytes, formation of free radicals that are even more toxic, and not all melanins are protectiv
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
N-trans-feruloyldopamine(SO—I-146)
[0046]
[0047] A solution of ferulic acid (300 mg; 1.54 mmol) and of triethylamine (1.5 eq; 2.31 mmol) in DMF (3.5 mL) is cooled to 3 or 4° C. using an ice bath. An amine, 3-hydroxytyramine (dopamine) (1 eq; 1.54 mmol) is added to the medium, followed by addition of a solution of BOP (benzotriazol-1-yloxy)tris(dimethylamino)phosphonium hexafluorophos-phate; (1 eq; 1.54 mmol) in dichloromethane (3.5 mL); the mixture is stirred for about thirty minutes in the ice bath and then for 20 hours at room temperature. Stirring is then stopped and the dichloromethane is evaporated off under vacuum. 30 mL of water are added to the remaining solution and the mixture obtained is extracted with ethyl acetate (3×75 mL). The organic phase is successively washed with 100 mL of 1N HCl solution, 100 mL of water and 100 mL of 1M sodium bicarbonate (NaHCO3) solution. It is then dried over sodium sulfate and evaporated to dryness. The product obtained is in the form of a w...
example 2
N-trans-feruloyl-3,4-dimethoxydopamine
[0048] The protocol derived from Example 1 is applied with ferulic acid and 2-(3,4-dimethoxyphenyl)ethylamine instead of ferulic acid and dopamine; the compound obtained is N-trans-feruloyl-3,4-dimethoxydopamine.
example 3
N-trans-feruloyltyramine
[0049] The protocol derived from Example 1 is applied with ferulic acid and tyramine instead of ferulic acid and dopamine; the compound obtained is N-trans-feruloyltyramine.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| w/w | aaaaa | aaaaa |
| w/w | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com