Novel p-coumaric acid sulfonate derivative, preparation method and application thereof
A kind of technology of coumaric acid sulfonate and p-coumaric acid, applied in the field of novel p-coumaric acid sulfonate derivative and preparation thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
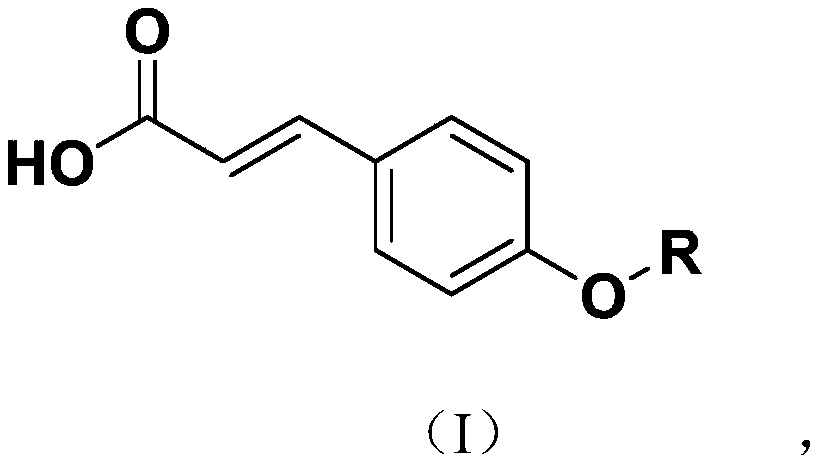
[0024] A class of novel p-coumaric acid sulfonate derivatives of the present invention, the structural formula of described novel p-coumaric acid sulfonate derivatives is as shown in formula (I):
[0025]
[0026] Wherein, R is selected from:
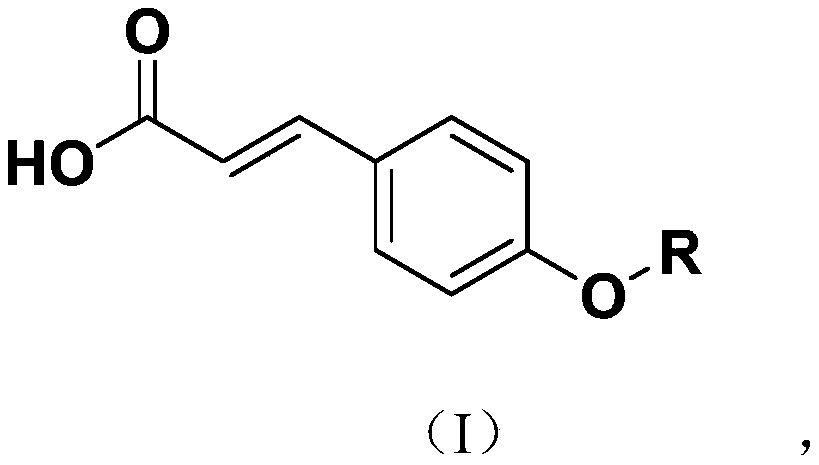
[0027] The synthetic process of a class of novel p-coumaric acid sulfonate derivatives of the present invention, it has following general formula:
[0028]
[0029] Wherein, R is selected from:
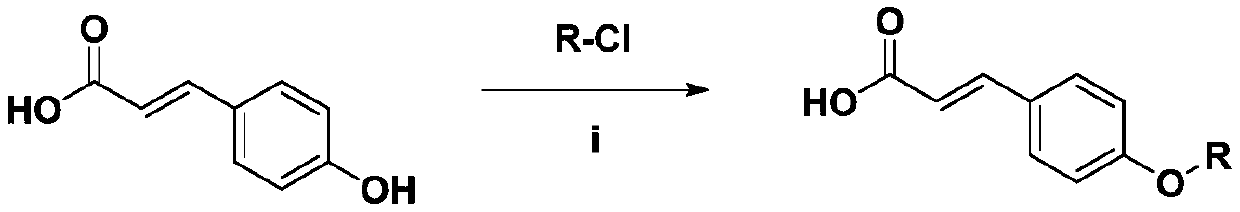
[0030] The preparation method of described a class of novel p-coumaric acid sulfonate derivatives of the present invention, comprises the steps:
[0031] Dissolve p-coumaric acid in dichloromethane, add various sulfonyl chlorides, stir for 20 min under ice-bath conditions, add 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride and 1-Hydroxybenzotriazole, move to room temperature and stir for 12 hours, follow the reaction by thin layer chromatography, filter the precipitate after the reaction, extract the filtrate with an ext...
Embodiment 2
[0039] The difference between embodiment 2 and embodiment 1 is:
[0040] The preparation method of described a class of novel p-coumaric acid sulfonate derivatives of the present invention, comprises the steps:
[0041] Dissolve p-coumaric acid in dichloromethane, add various sulfonyl chlorides, stir for 40 min under ice-bath conditions, add 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride and 1-Hydroxybenzotriazole, move to room temperature and stir for 4 hours, follow the reaction by thin-layer chromatography, filter the precipitate after the reaction, extract the filtrate with an extractant, wash with saturated sodium chloride solution, and distill under reduced pressure Remove the organic solvent, and use the mixed solvent of acetone and ethanol to recrystallize to obtain a new type of p-coumaric acid sulfonate derivatives.
[0042] The extractant is dichloromethane, and the number of washings of the saturated sodium chloride solution is 4 times.
Embodiment 3
[0044] The difference between embodiment 3 and embodiment 1 is:
[0045] The preparation method of described a class of novel p-coumaric acid sulfonate derivatives of the present invention, comprises the steps:
[0046] Dissolve p-coumaric acid in dichloromethane, add various sulfonyl chlorides, stir for 30 min under ice-bath conditions, add 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride and 1-Hydroxybenzotriazole, move to room temperature and stir for 8 hours, follow the reaction by thin layer chromatography, filter the precipitate after the reaction, extract the filtrate with extractant, wash with saturated sodium chloride solution, and distill under reduced pressure Remove the organic solvent, and use the mixed solvent of acetone and ethanol to recrystallize to obtain a new type of p-coumaric acid sulfonate derivatives.
[0047] The extractant is dichloromethane, and the number of washings of the saturated sodium chloride solution is 6 times.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com