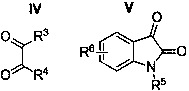
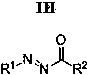A kind of 2,2,5-trisubstituted 1,3,4 oxadiazole derivative and its synthesis method
A synthetic method, oxadiazole technology, applied in the field of organic synthesis, can solve the problems of harsh reaction conditions, lack of synthetic methods, poor functional group tolerance, etc., and achieve the effect of mild reaction conditions, good tolerance and wide application range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Synthesis of 2,2,5-trisubstituted 1,3,4-oxadiazole derivatives, R in general structural formula I 1 = Ph, R 2 = Ph, R 3 = Ph, R 4 =OEt.
[0036]
[0037] In a 25mL schlenk bottle with a magnetic stirring bar, add 1.5mL dichloromethane, N-acyldiazaalkene (R 1 = Ph, R 2 =Ph) (84mg, 0.40mmol), and a-ketoester (R 3 = Ph, R 4 =OEt) (36mg, 0.2mmol), the resulting reaction mixture was placed at -78°C and stirred for 15 minutes, then 55μL (0.3mmol) of hexamethylphosphorous triamide diluted with 0.5mL of dichloromethane was added to a concentration of 0.6 mol / L, added dropwise to the above reaction mixture within 10 minutes, after the dropwise addition, the reaction was slowly warmed up to room temperature and stirred for 8 hours, after the reaction was completed, the solvent was removed by rotary evaporation, and the crude product was chromatographed on a 200-300 mesh silica gel column The target compound of oxadiazole was obtained by purification, and the eluent was ...
Embodiment 2
[0041] Synthesis of 2,2,5-trisubstituted 1,3,4-oxadiazole derivatives, R in general structural formula I 1 = Ph, R 2 = 4-MeC 6 h 4 , R 3 = Ph, R 4 =OEt.
[0042]
[0043] The synthetic steps are basically the same as in Example 1, and the difference is listed as follows:
[0044] The N-acyldiazaolefin R used 1 = Ph, R 2 = 4-MeC 6 h 4 , the dosage was 90mg (0.4mmol), the reaction time at room temperature was 11 hours, and 60mg of the pure product in the form of light yellow oil was obtained, and the yield was 78%.
[0045] The data detection is as follows:
[0046] 1 H NMR (400MHz, C 6 D. 6 )δ7.94 (d, J=8.2Hz, 2H, ArH), 7.83 (dd, J=8.1, 1.5Hz, 2H, ArH), 7.49 (dd, J=8.8, 1.0Hz, 2H, ArH), 7.14 –7.03(m,5H,ArH),6.86(d,J=8.0Hz,2H,ArH),6.77(t,J=7.3Hz,1H,ArH),3.84(dq,J=10.7,7.1Hz,1H ,OCH 2 ), 3.72 (dq, J=10.7, 7.1Hz, 1H, OCH 2 ),1.95(s,3H,CH 3 ),0.60(t,J=7.1Hz,3H,OCH 2 CH 3 ); HRMS-ESI([M+H] + ) Calcd for C 24 h 23 N 2 o 3 387.1703, found 387.1705.
Embodiment 3
[0048] Synthesis of 2,2,5-trisubstituted 1,3,4-oxadiazole derivatives, R in general structural formula I 1 = Ph, R 2 =4-ClC 6 h 4 , R 3 = Ph, R 4 =OEt.
[0049]
[0050] The synthetic steps are basically the same as in Example 1, and the difference is listed as follows:
[0051] The N-acyldiazaolefin R used 1 = Ph, R 2 =4-ClC 6 h 4 , the dosage is 98 mg (0.4 mmol), the reaction solvent is tetrahydrofuran, the reaction mixture is stirred at -50 ° C for 10 minutes, and the reaction time at room temperature is 11 hours to obtain 49 mg of light yellow oily pure product with a yield of 60%.
[0052] The data detection is as follows:
[0053] 1 H NMR (400MHz, C 6 D. 6 )δ7.78(dd, J=8.1,1.5Hz,2H,ArH),7.64(d,J=8.8Hz,2H,ArH),7.44(dd,J=8.8,1.0Hz,2H,ArH),7.14 –7.04(m,5H,ArH),6.95(d,J=8.8Hz,2H,ArH),6.78(dt,J=8.4,1.0Hz,1H,ArH),3.83(dq,J=10.8,7.1Hz ,1H,OCH 2 ), 3.71 (dq, J=10.8, 7.1Hz, 1H, OCH 2 ),0.59(t,J=7.1Hz,3H,OCH 2 CH 3 ); HRMS-ESI([M+H] + ) Calcd for C 23 h 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com