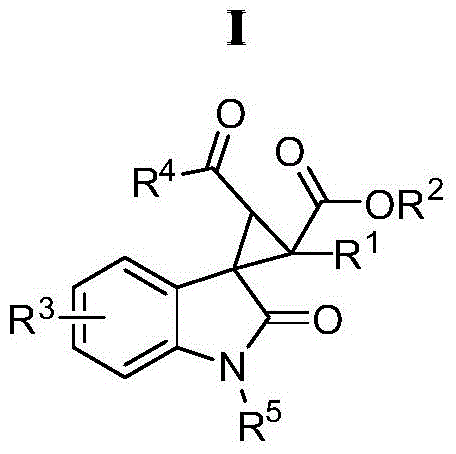
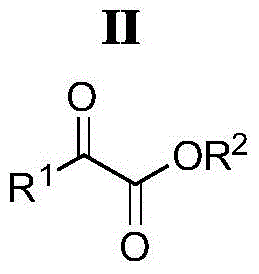
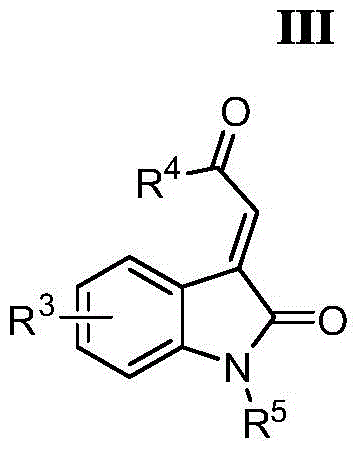
A kind of oxindole spirocyclopropane derivative and its synthetic method
A technology of oxindole spirocyclopropane and its synthesis method, which is applied in the field of oxindole spirocyclopropane derivatives and their synthesis, can solve the problems of limited types of substituents, and achieve good stability, rich types, and easy-to-obtain raw materials Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Synthesis of oxindole spirocyclopropane derivatives, R in the general structural formula 1 = Ph, R 2 =OEt, R 3 = H, R 4 = Ph, R 5 =Bn.
[0036]
[0037] In a 25mL schlenk bottle with a magnetic stirring bar, add 1.5mL dichloromethane, α-ketoester (R 1 = Ph, R 2 =OEt) 36mg (0.20mmol) and α, β-unsaturated oxindole compound (R 3 = H, R 4 = Ph, R 5 =Bn) 71mg (0.21mmol), the resulting reaction mixture was placed at -78°C and stirred for 10 minutes, then 38μL (0.21mmol) of hexamethylphosphorous triamide diluted with 0.5mL of dichloromethane, the concentration was 0.42mol / L, added dropwise to the above reaction mixture within 5 minutes. After the dropwise addition, the reaction was slowly warmed up to room temperature and continued to stir for 22 hours. After the reaction was completed, the solvent was removed by rotary evaporation. The target compound of oxindole spirocyclopropane was obtained by analysis and purification, the eluent was petroleum ether (boiling ...
Embodiment 2
[0040] Synthesis of oxindole spirocyclopropane derivatives, R in the general structural formula 1 = Ph, R 2 =OEt, R 3 =5-Me,R 4 = Ph, R 5 =Bn.
[0041]
[0042] The synthetic steps are basically the same as in Example 1, and the difference is listed as follows:
[0043] The α,β-unsaturated oxindole compound R used 3 =5-Me,R 4 = Ph, R 5 =Bn, the dosage was 75 mg (0.21 mmol), the reaction time at room temperature was 24 hours, and 102 mg of pure light yellow solid was obtained with a yield of 99%.
[0044] 1 HNMR (400MHz, CDCl 3 )δ8.30(d, J=7.4Hz, 2H, ArH), 7.63(t, J=7.4Hz, 1H, ArH), 7.53(t, J=7.4Hz, 2H, ArH), 7.33-7.18(m ,8H,ArH),7.09(d,J=7.4Hz,2H,ArH),6.94(d,J=7.9Hz,1H,ArH),6.68(d,J=7.9Hz,1H,ArH),6.42( s,1H,ArH),5.12(d,J=15.8Hz,1H,1 / 2Ph CH 2 ), 4.91 (d, J=15.8Hz, 1H, 1 / 2Ph CH 2 ),4.50(s,1H,CH),4.26-4.12(m,2H,OCH 2 ),2.09(s,3H,CH 3 ), 1.23(t, J=7.1Hz, 3H, OCH 2 CH 3 ); HRMS-ESI([M+H] + )CalcdforC 34 h 30 NO 4 516.2169, found 516.2180.
Embodiment 3
[0046] Synthesis of oxindole spirocyclopropane derivatives, R in the general structural formula 1 = Ph, R 2 =OEt,R 3 =5-MeO, R 4 = Ph, R 5 =Bn.
[0047]
[0048] The synthetic steps are basically the same as in Example 1, and the difference is listed as follows:
[0049] The α,β-unsaturated oxindole compound R used 3 =5-MeO, R 4 = Ph, R 5 =Bn, the dosage is 77mg (0.21mmol), the reaction mixture was stirred at -70°C for 15 minutes, the phosphorus reagent was added dropwise within 15 minutes, and the reaction time at room temperature was 21 hours to obtain 102mg of white solid pure product, the yield was 96% .
[0050] 1 HNMR (400MHz, CDCl 3 )δ8.29(d, J=8.4Hz, 2H, ArH), 7.64(t, J=7.3Hz, 1H, ArH), 7.54(t, J=7.7Hz, 2H, ArH), 7.28-7.18(m ,8H,ArH),7.10(d,J=7.1Hz,2H,ArH),6.72-6.65(m,2H,ArH),6.26(brs,1H,ArH),5.11(d,J=15.8Hz,1H ,1 / 2Ph CH 2 ), 4.90 (d, J=15.8Hz, 1H, 1 / 2Ph CH 2 ),4.52(s,1H,CH),4.30-4.13(m,2H,OCH 2 ),3.46(s,3H,OCH 3 ), 1.24(t, J=7.1Hz, 3H, OCH 2 CH ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com