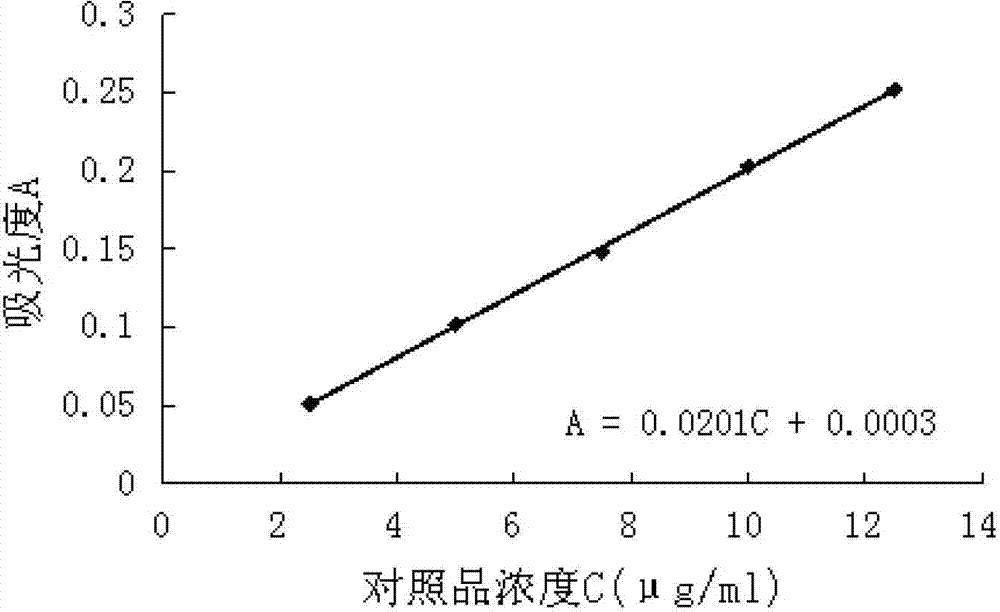
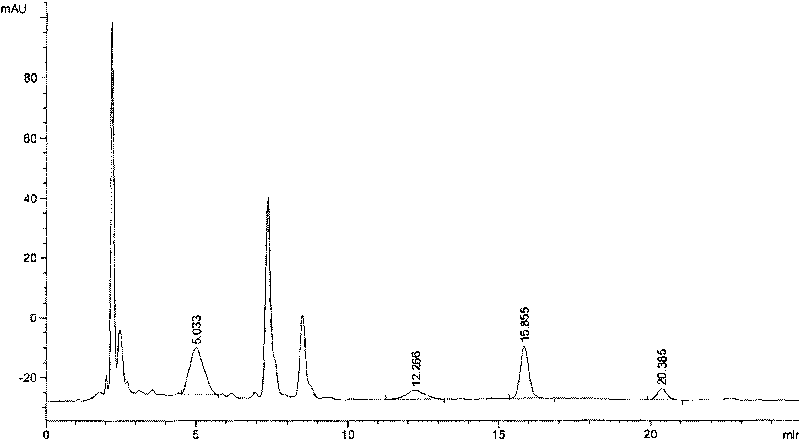
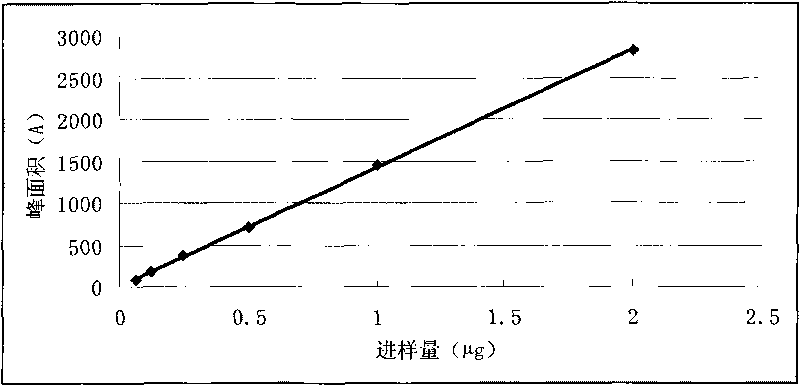
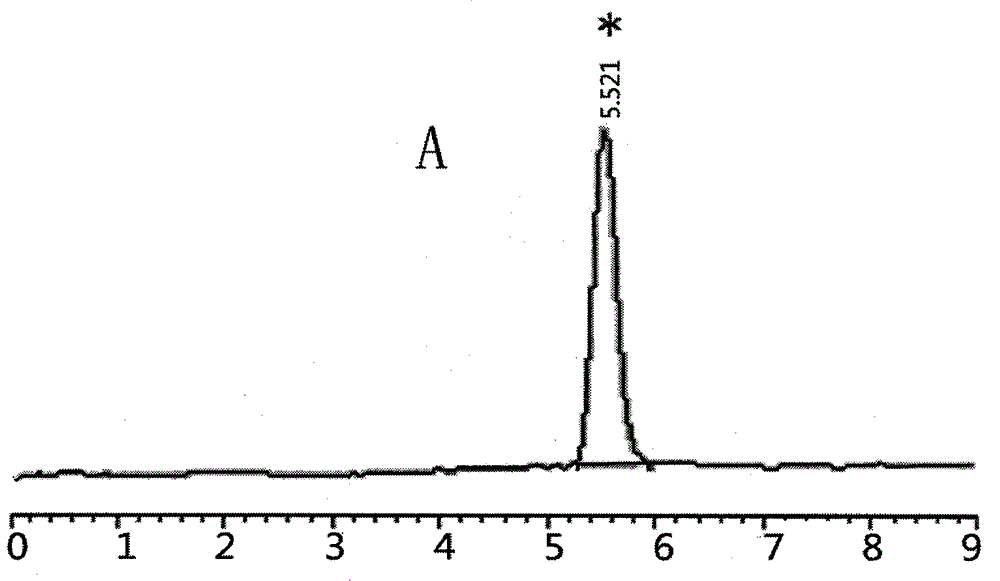
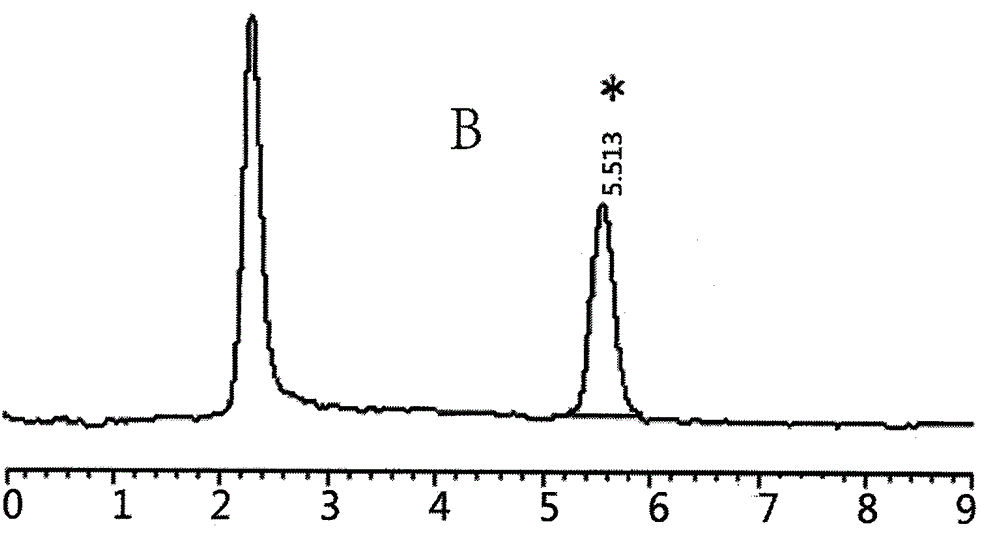
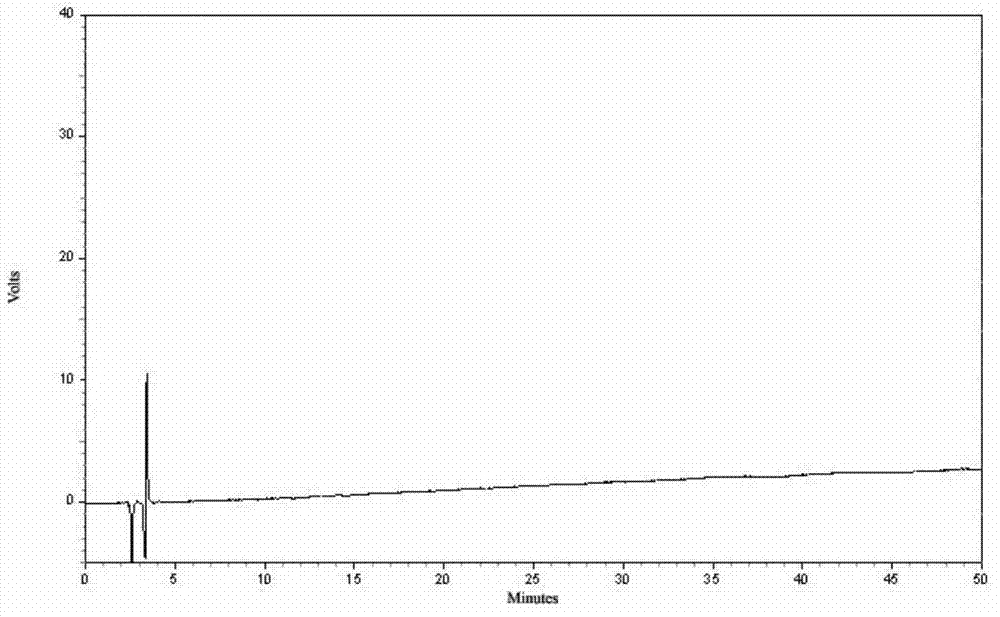
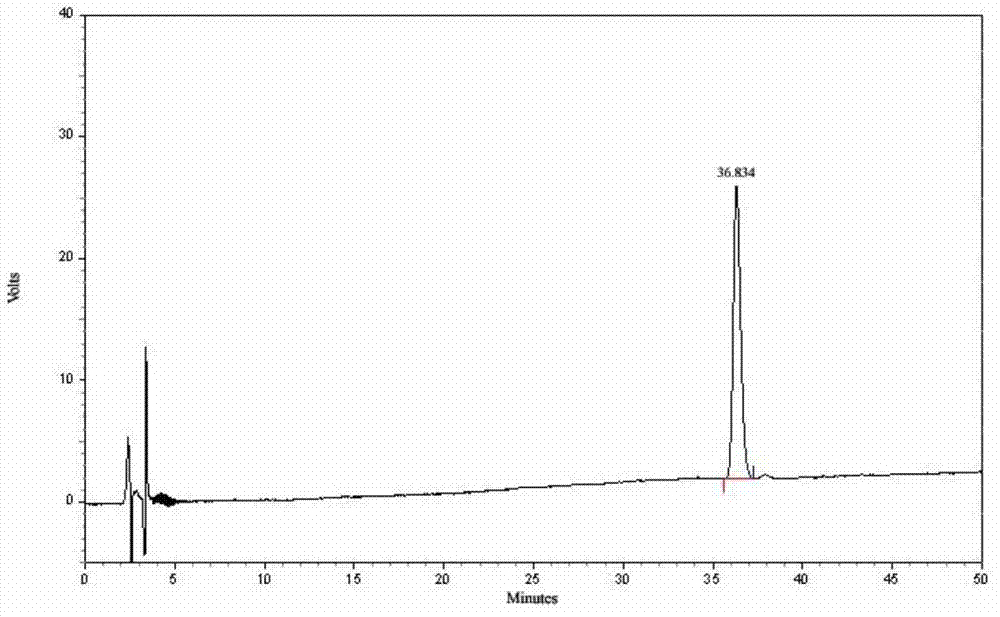
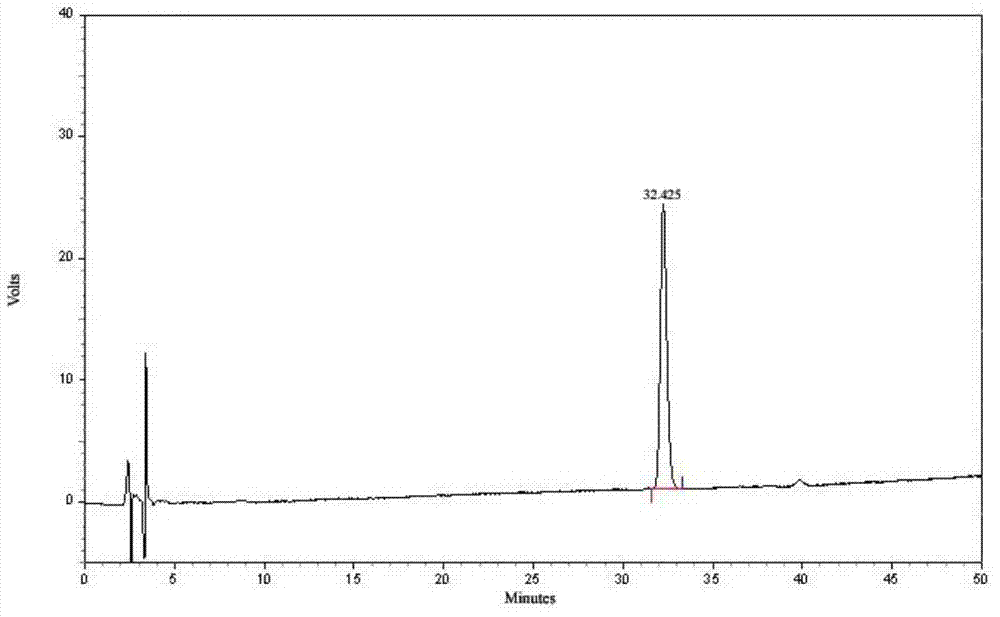
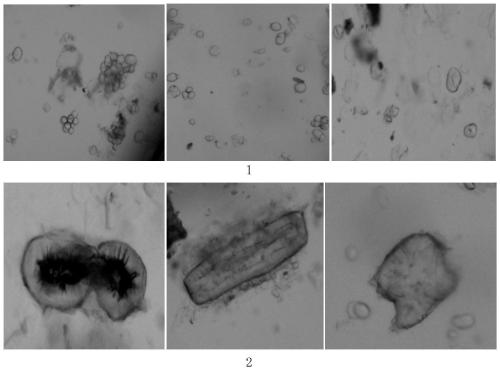
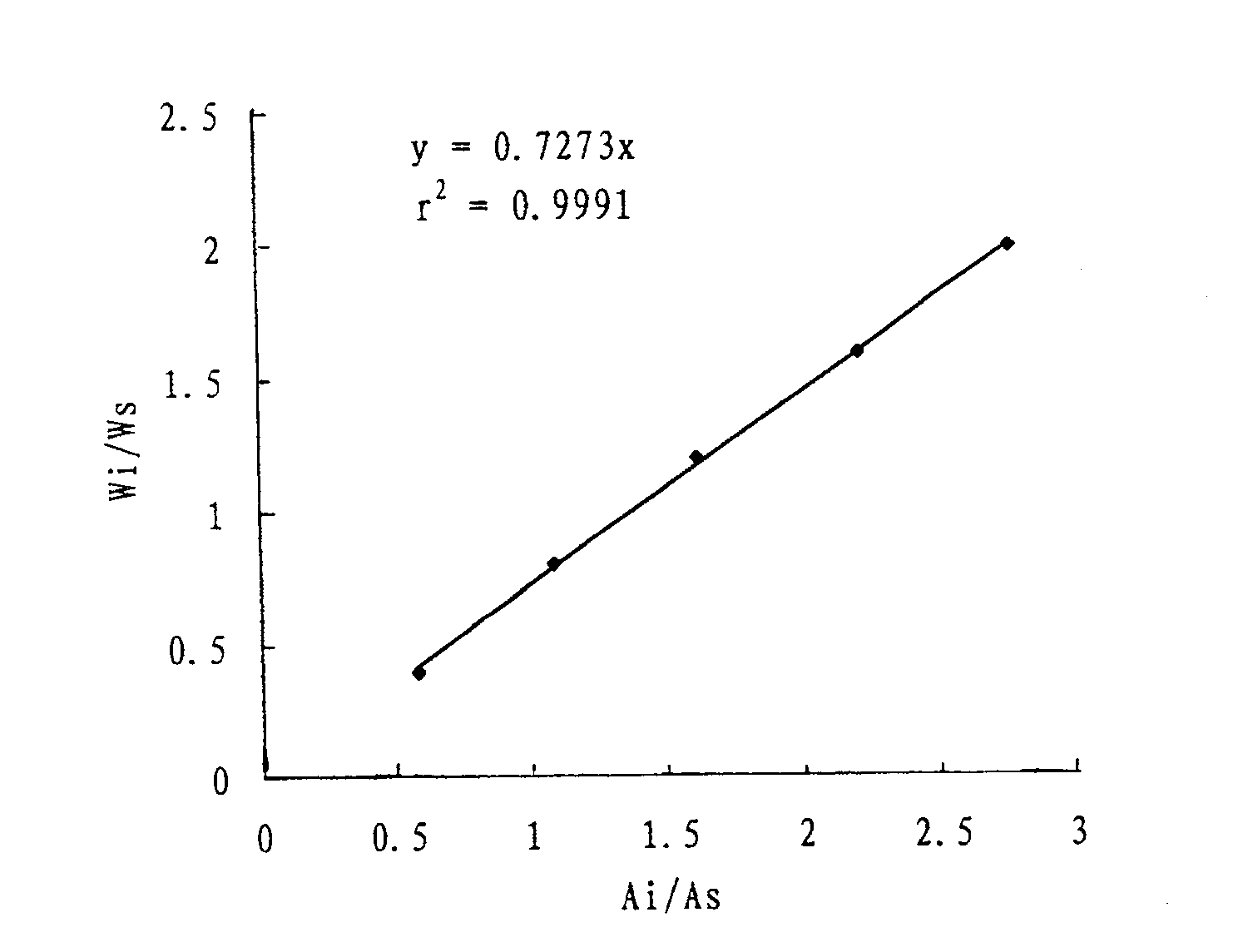
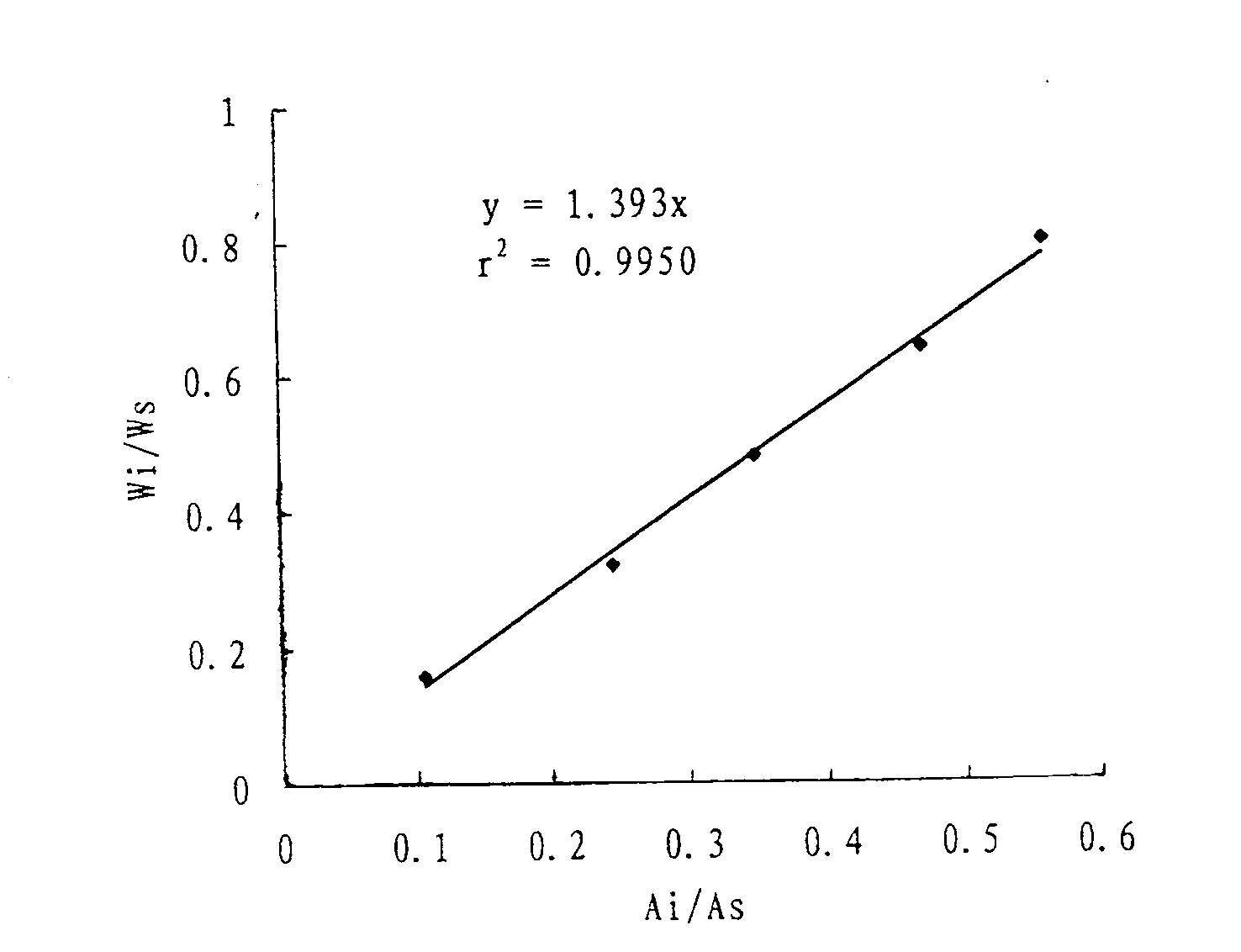
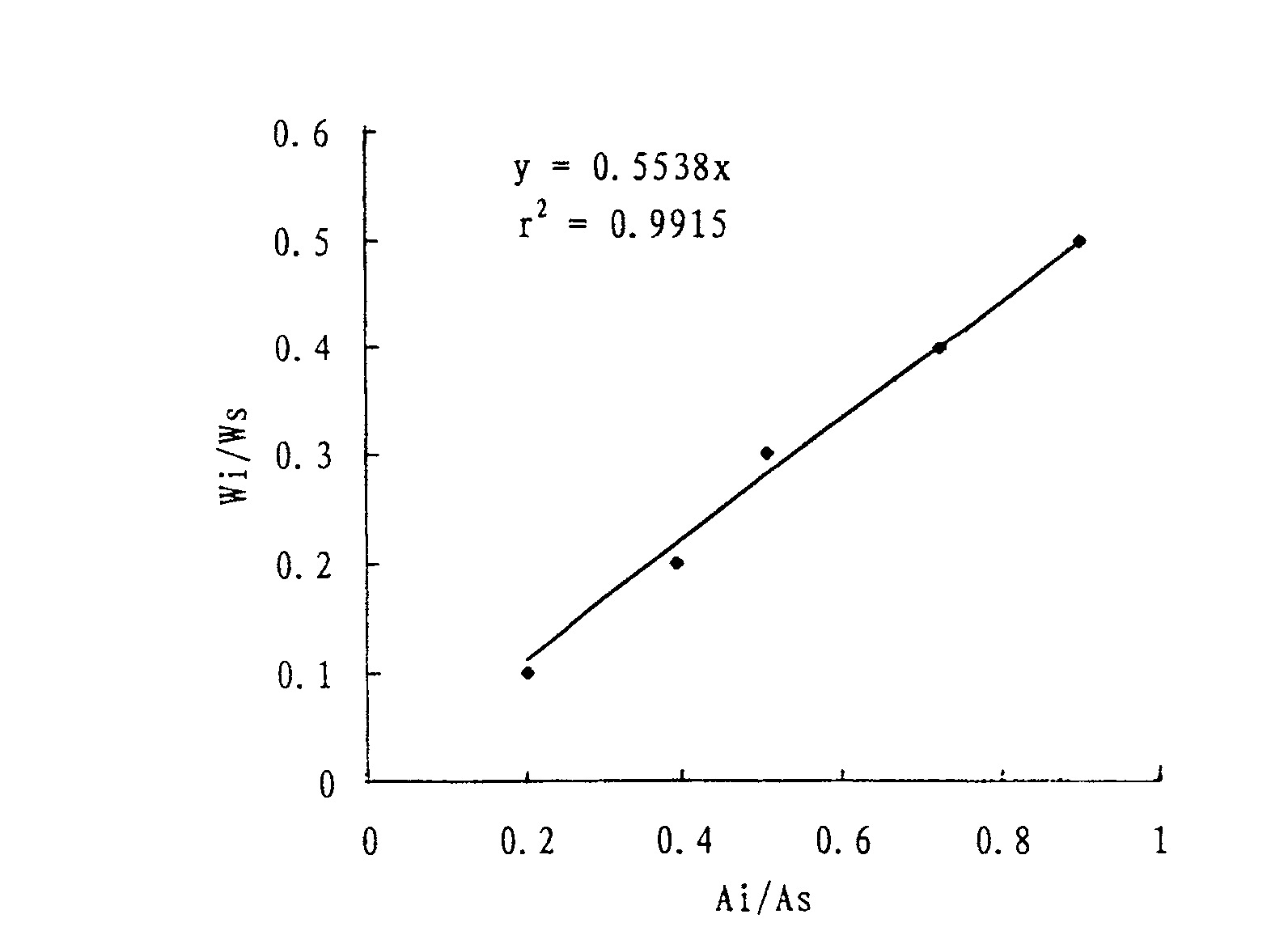
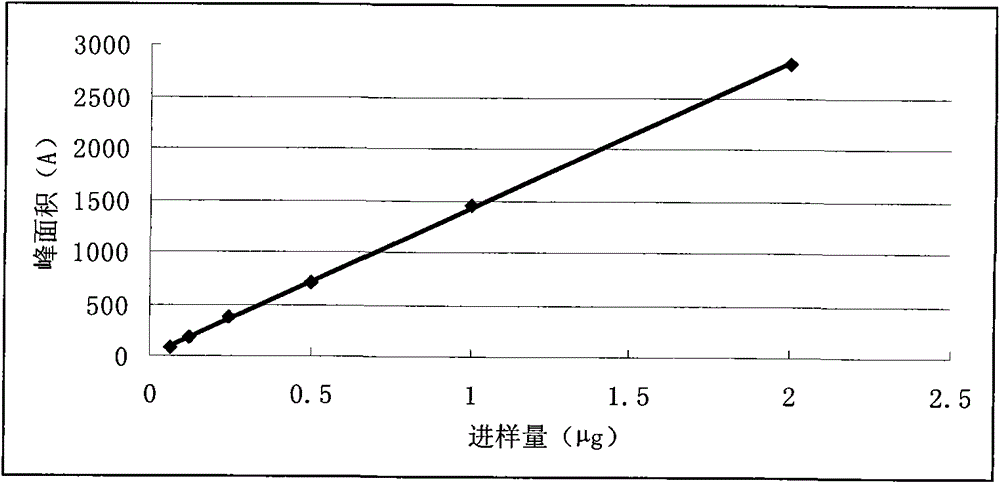
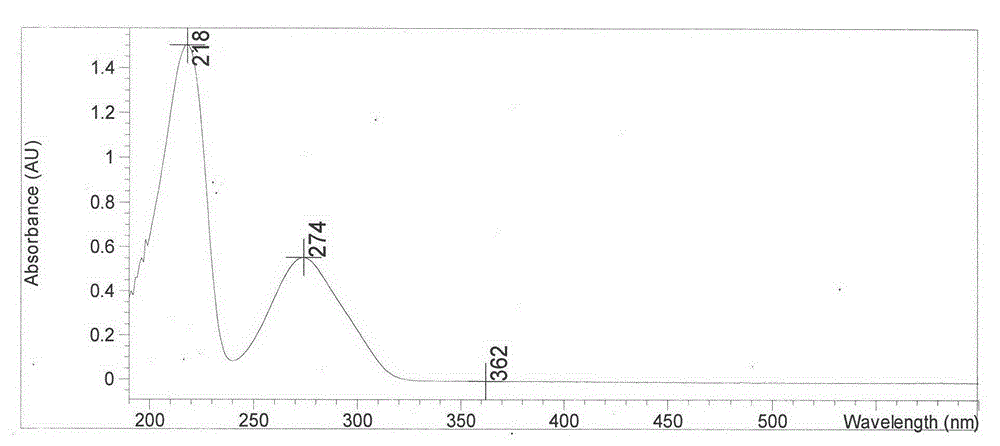
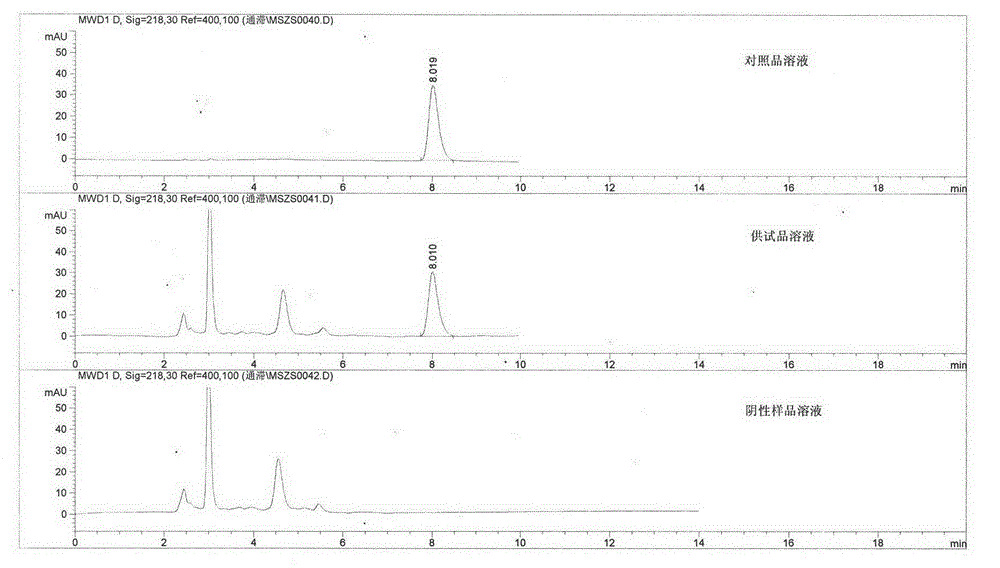
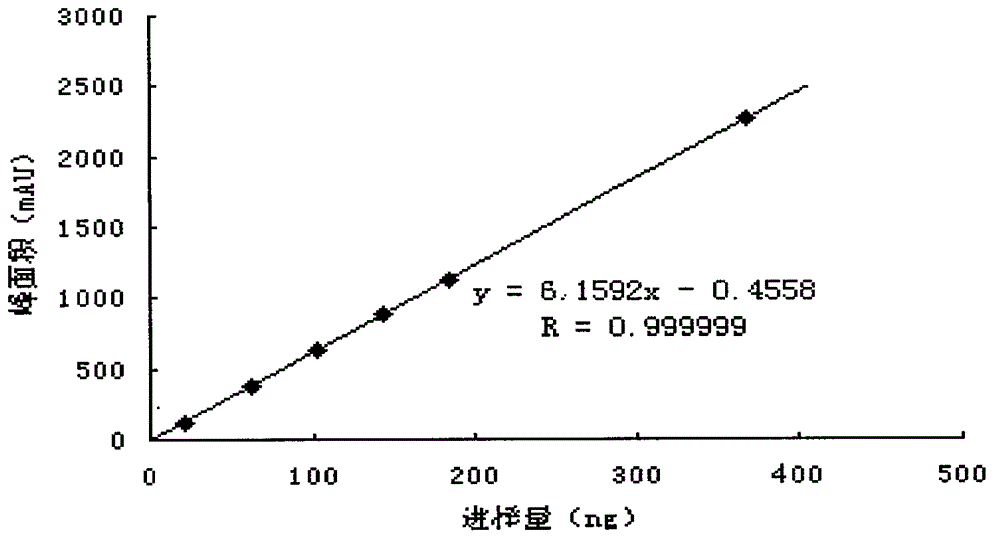
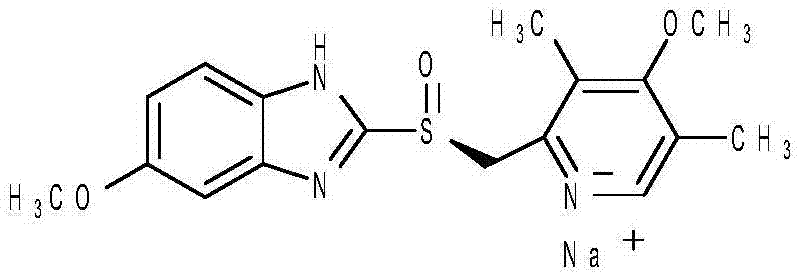
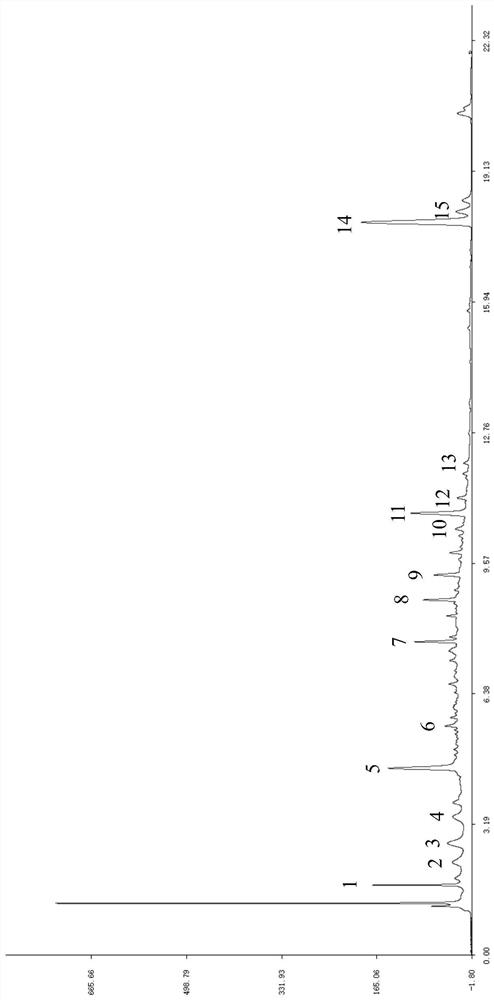
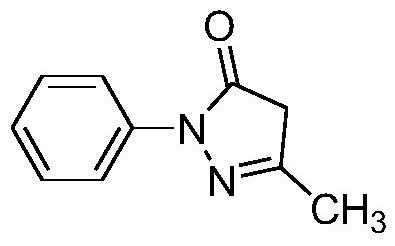
Patents
Literature
34results about How to "Reliable Quality Control Methods" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
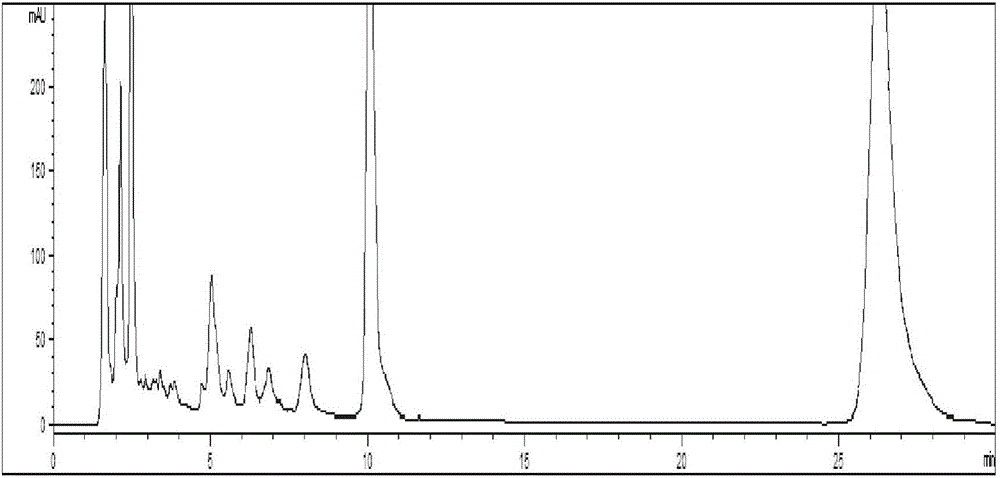
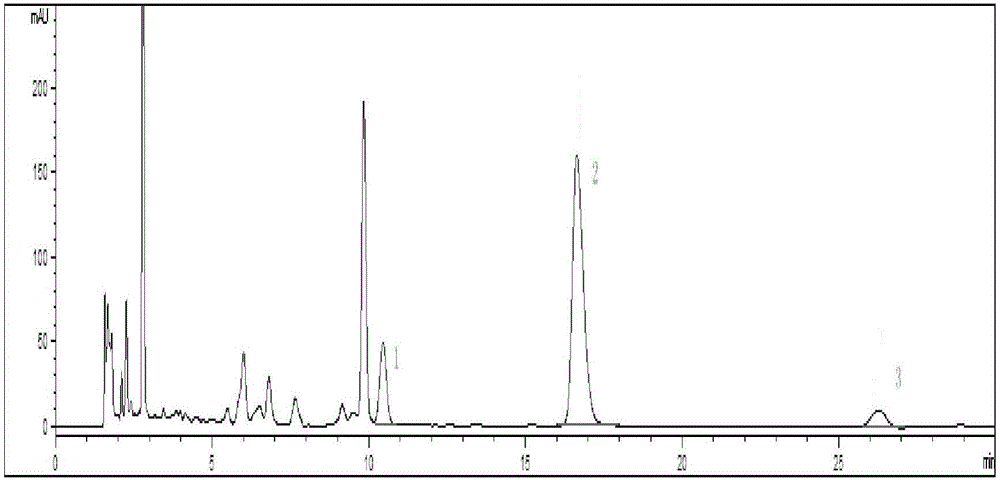
Method for detecting quality of alkaloids in Tibetan medicine herba aconiti tangutici and preparations thereof
ActiveCN102735789AQuality assuranceEnsure the safety of medicationComponent separationChemical analysis using titrationPhosphateMonopotassium phosphate
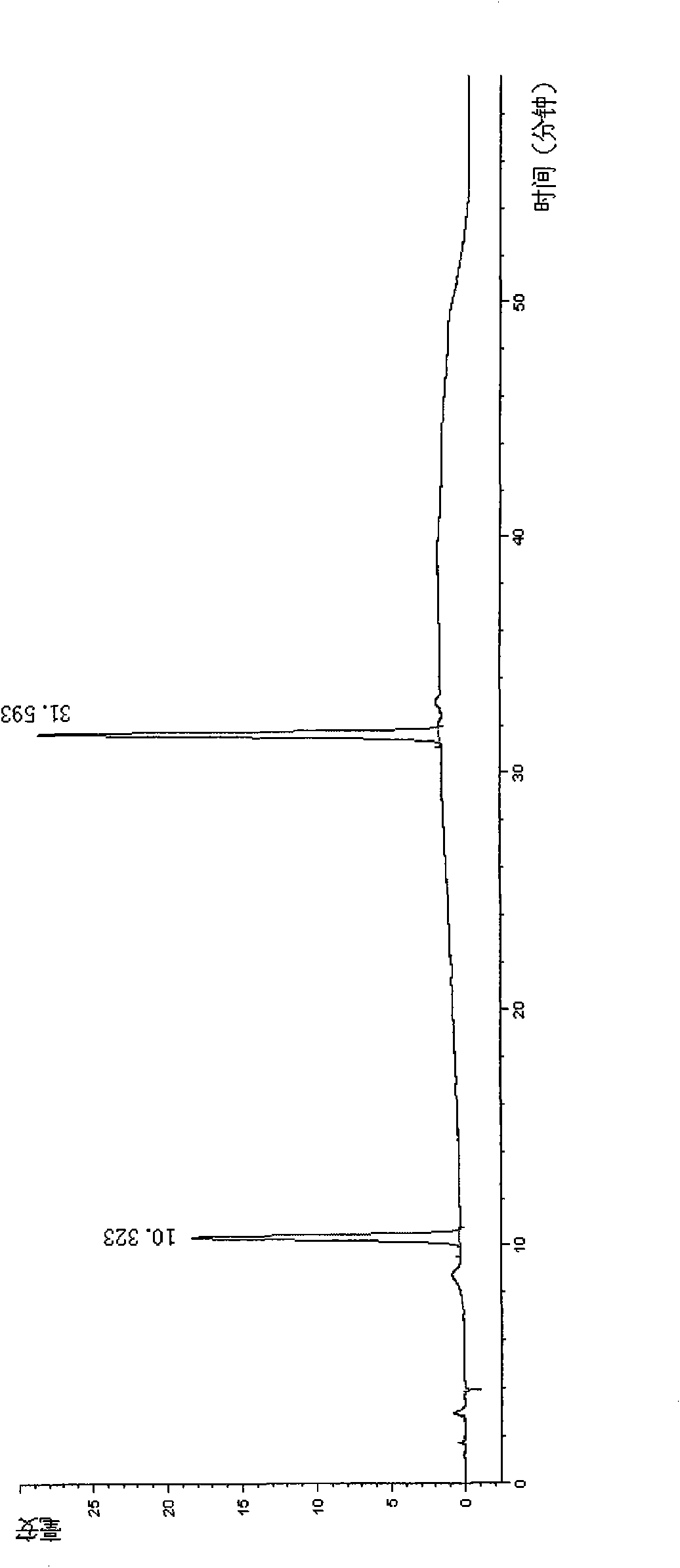
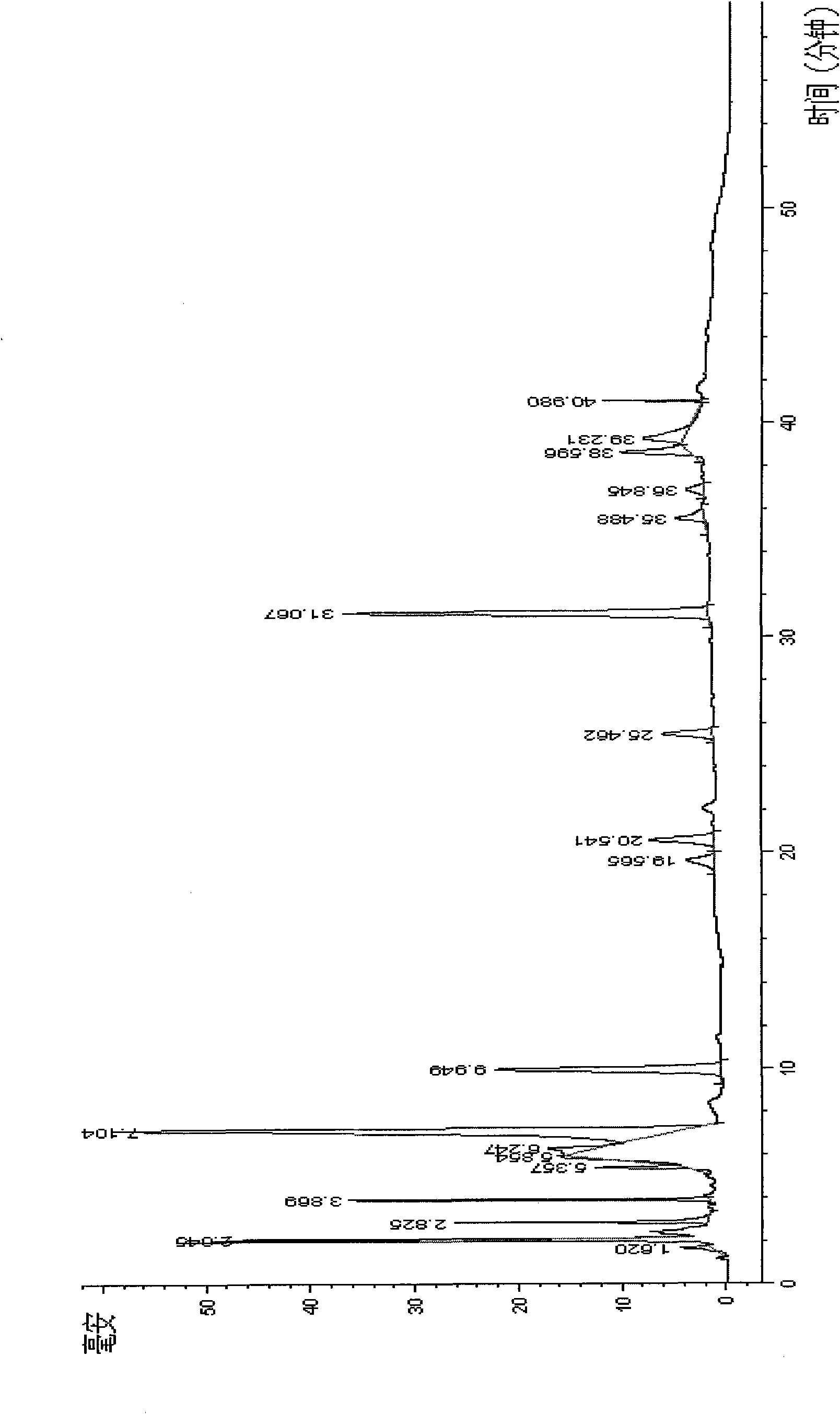
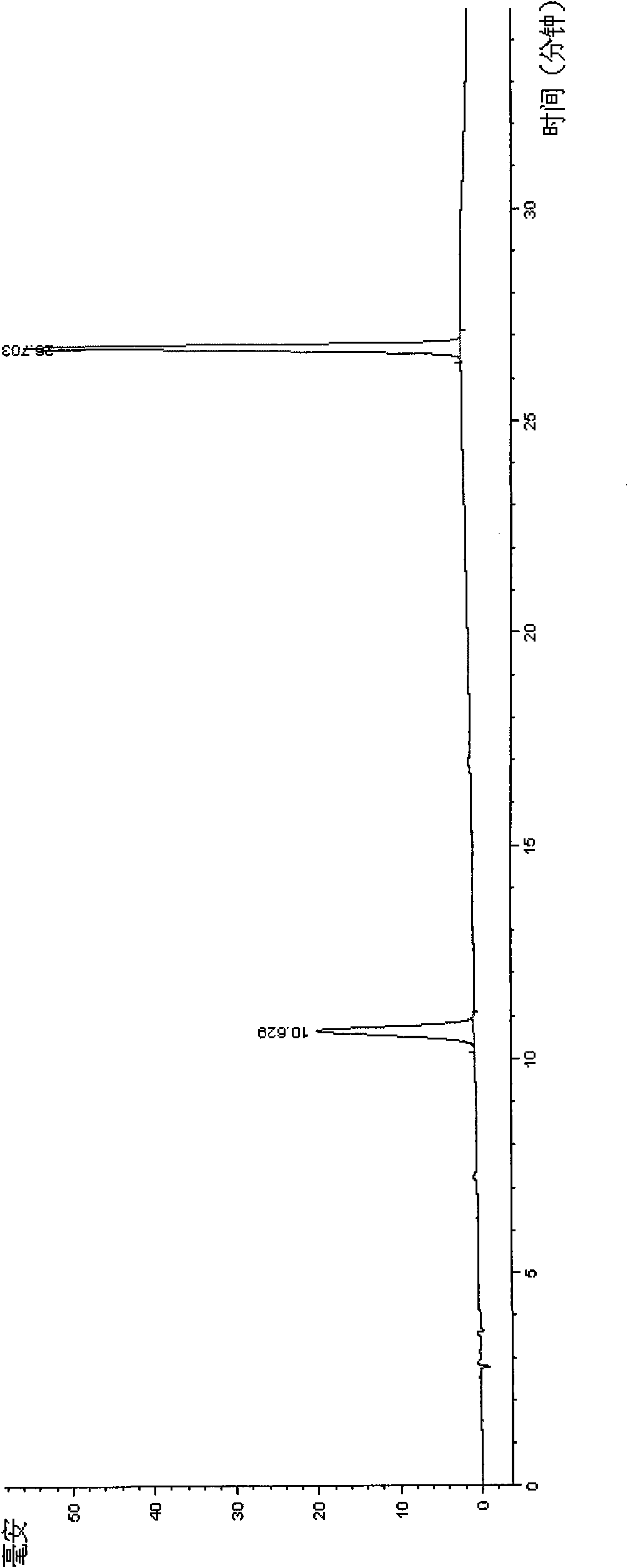
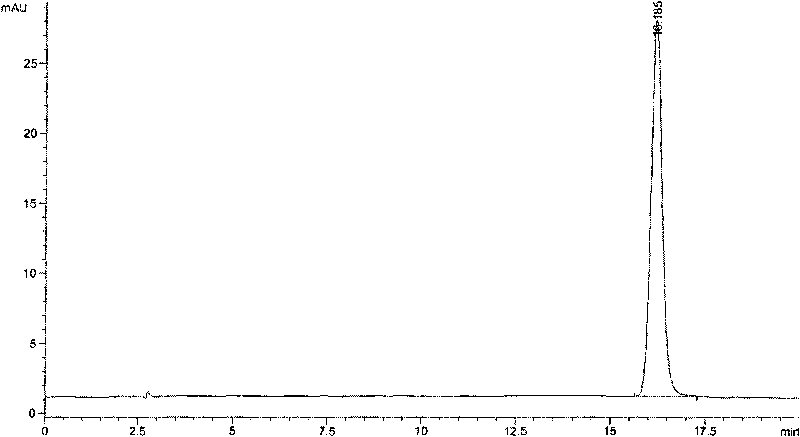
The invention discloses a method for detecting quality of alkaloids in a Tibetan medicine herba aconiti tangutici and preparations thereof. The method comprises the steps of identification, check and content determination, wherein thin layer chromatography is adopted for identification and is characterized by taking a herba aconiti tangutici control medicine as the control and normal hexane-ethyl acetate-methanol-ammonia water as a developer; high performance liquid chromatography is adopted for check and is characterized by taking a mixed solution of an aconitine control substance, a hypaconitine control substance and a new aconitine control substance as the control, acetonitrile-tetrahydrofuran as a mobile phase A and an ammonium acetate water solution with glacial acetic acid as a mobile phase B to carry out gradient elution; high performance liquid chromatography is adopted for hordenine content determination and is characterized by taking a hordenine control substance as the control and an acetonitrile-potassium dihydrogen phosphate water solution as a mobile phase; and back titration is adopted for total alkaloid content determination. The method is stable and reliable, has strong specificity, can ensure the safety, effectiveness and quality controllability of the alkaloids in the Tibetan medicine herba aconiti tangutici and the preparations thereof, and has higher scientificity and application values.
Owner:SHANDONG JINHE DRUG RES DEV
Quality detection method for tibetan medicine rhododendron anthopogonoide and tibetan medicine rhododendron anthopogonoide preparation
ActiveCN102809545AQuality assuranceQuality is safe and reliableComponent separationColor/spectral properties measurementsMedicinal herbsMedicine
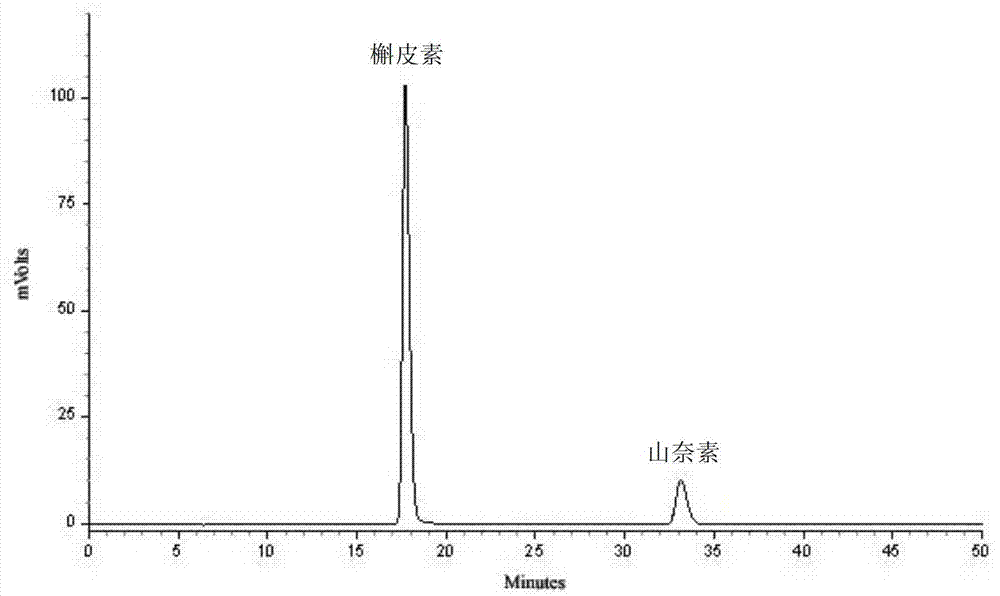
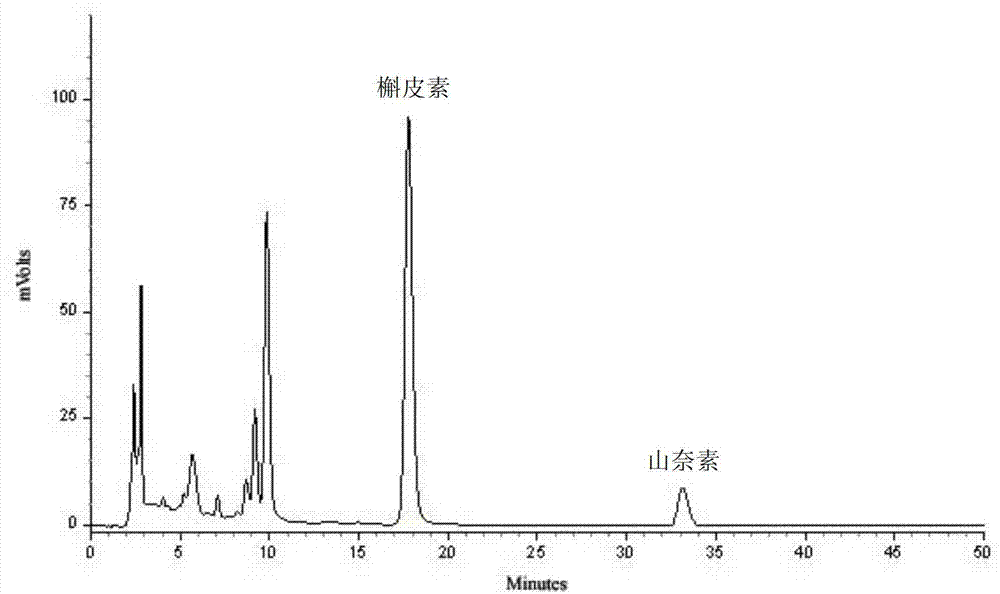
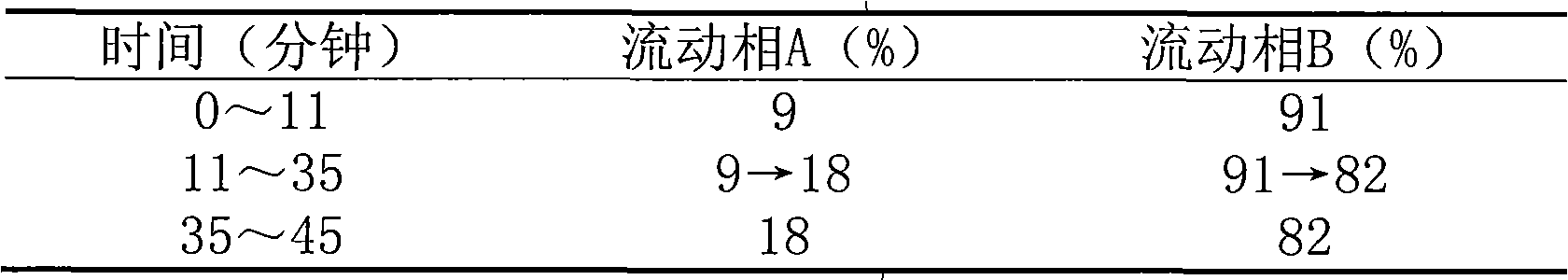
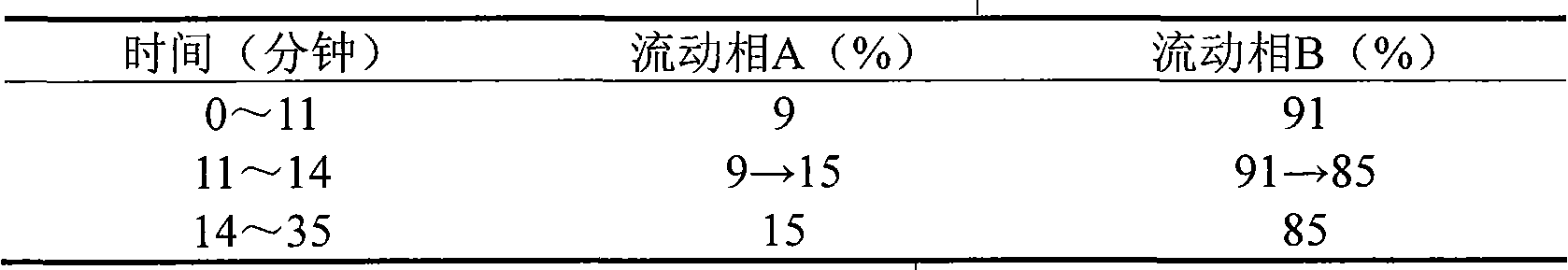
The invention provides a quality detection method for tibetan medicine rhododendron anthopogonoide and a tibetan medicine rhododendron anthopogonoide preparation. The method includes: determining the total flavonoid content in the rhododendron anthopogonoide and the rhododendron anthopogonoide preparation by ultraviolet-spectrophotometry; simultaneously determining the total quercetin content in the rhododendron anthopogonoide and the rhododendron anthopogonoide preparation in the same chromatograph by high-performance liquid chromatography; and establishing a finger-print chromatogram of flavonoids in the rhododendron anthopogonoide and the rhododendron anthopogonoide preparation by the high-performance liquid chromatography. Therefore, quality of the rhododendron anthopogonoide and the rhododendron anthopogonoide preparation can be guaranteed by detecting whether common peaks exist in the finger-print chromatogram or not, and further medication safety for patients is guaranteed. The quality detection method is stable in sample solution, high in precision and excellent in reproducibility, has certain specificity, is good in separating effect of each characteristic constituent in the finger-print chromatogram, can be used for quality detection of rhododendron anthopogonoide medical materials, and is beneficial to guarantee of safety, effectiveness, controllability in quality and good clinical effect for the tibetan medicine rhododendron anthopogonoide and the tibetan medicine rhododendron anthopogonoide preparation.
Owner:SHANDONG JINHE DRUG RES DEV
Lamiophlomis rotata extract, medicine composition containing same and quality control method
InactiveCN101612192AStable efficacyClear efficacyAntipyreticComponent separationMedicinal herbsMedicine
The invention provides a lamiophlomis rotata extract comprising gardenia glucoside methyl ester and 8-O-acetyl gardenia glucoside methyl ester, and the total weight of the lamiophlomis rotata extract is not lower than 5mg / g. The invention also provides a method for detecting the quality of a lamiophlomis rotata medicinal material and the extract thereof or a medicine composition containing the lamiophlomis rotata extract, which uses the gardenia glucoside methyl ester and the 8-O-acetyl gardenia glucoside methyl ester as index components to carry out quality detection. The extract of the invention has stable medicine effect and strong controllability. By using the gardenia glucoside methyl ester and the 8-O-acetyl gardenia glucoside methyl ester as the index components to control the quality of the extract, the medicine effect is definite, the controllability is strong, the quality control method of the invention is accurate, reliable, stable and controllable, and the quality of the lamiophlomis rotata medicine can be effectively monitored by using the method to measure the content of the gardenia glucoside methyl ester in the lamiophlomis rotata medicinal material or a preparation.
Owner:HENGKANG MEDICAL GROUP CO LTD
Preparation and quality detection methods of high-purity honeysuckle flower-baikal skullcap root soluble powder
InactiveCN102397331AImprove bioavailabilityGood curative effectAntibacterial agentsPowder deliverySodium bicarbonateSpray dried
The invention discloses preparation and quality detection methods of high-purity honeysuckle flower-baikal skullcap root soluble powder. The preparation method comprises the following steps of: 1, preparing an appropriate amount of baikal skullcap root, adding water for decocting for 2-4 times, combining decoctions, filtering, and concentrating the filtrate till the relative density is 1.10-1.35; 2, regulating the pH value to 1.0-2.0 with hydrochloric acid, preserving heat at the temperature 60-80 DEG C, standing, and filtering; 3, adding water into a precipitate, stirring uniformly, and regulating the pH value to 6.5-7.5 with 30-40 percent sodium hydroxide; 4, adding an equal amount of ethanol, stirring for dissolving, filtering, regulating the pH value of the filtrate to 1.0-2.0 with hydrochloric acid, preserving heat at the temperature 60-80 DEG C, standing, and filtering; 5, washing the precipitate with an appropriate amount of water and ethanol of different concentrations in sequence till the pH value is 6.5-7.5; 6, vaporizing the ethanol and drying under reduced pressure to obtain a baikal skullcap root extract; 7, preparing an appropriate amount of honeysuckle flower, adding water for decocting for 1-3 times, combining decoctions, filtering, and concentrating the filtrate under reduced pressure at the temperature 50-70 DEG C till the relative density is 1.10-1.25 to obtain clear paste; 8, adding ethanol till the ethanol content is 80-90 percent, standing for 24 hours, and filtering; 9, adding ethanol into filter residues till the ethanol content is 75-95 percent, standing for 18-30 hours, and filtering; 10, combining two filtrates, recovering ethanol, and concentrating under reduced pressure till the relative density is 1.15-1.30; 11, performing spray drying to obtain a honeysuckle flower extract; and 12, combining the baikal skullcap root extract with the honeysuckle flower extract, adding sodium bicarbonate to fixed amount, adding glucose to fixed amount, and mixing uniformly to obtain the honeysuckle flower-baikal skullcap root soluble powder.
Owner:BEIJING CENT BIOLOGY CO LTD
Preparation method and quality control method of mulberry leaf extract
InactiveCN105943621AEasy extractionEasy to keepComponent separationPlant ingredientsReverse osmosisFood material
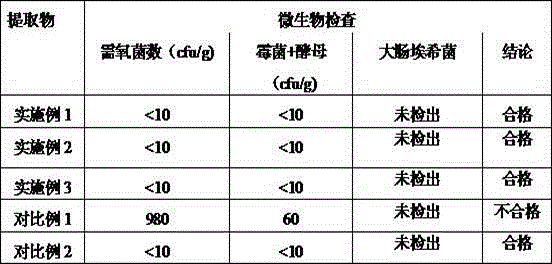
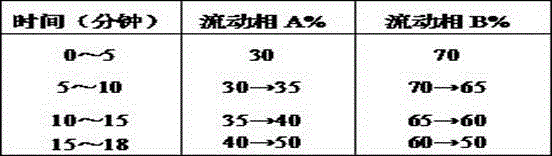
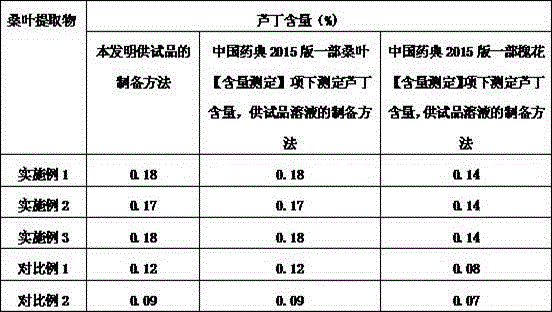
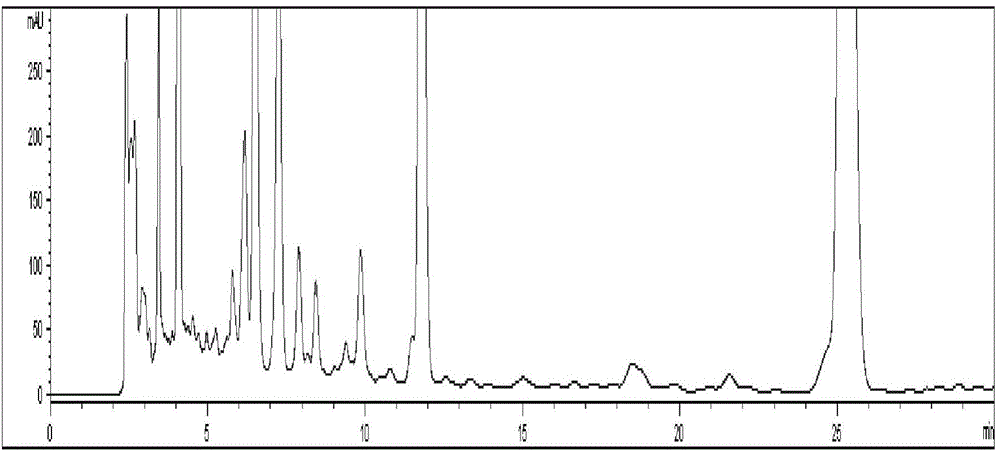
The invention discloses a preparation method and a preparation control method of a mulberry leaf extract. The preparation method comprises the steps of cutting mulberry leaves into shreds, adding water, decocting and extracting for two times, combining two decoction-filtering liquors, filtering through a sieve, enabling a primary filtrate to pass through a microfiltration membrane with the bore diameter of 0.01 to 0.02 micron, enabling a refined filtrate to pass through a reverse osmosis membrane with the bore diameter of 0.0001 to 0.001 micron, concentrating until a solid content is 15 to 18 percent, performing vacuum concentration on a concentrated initial solution under 60 DEG C, to form an extractum with the relative density of 1.10 to 1.13, and spray-drying the extractum to obtain the mulberry leaf extract. A quality control method is characterized by adopting high efficiency liquid chromatography to measure the content of rutin in the mulberry leaf extract. The preparation method can avoid rutin content loss caused by washing a mulberry leaf medicinal material and performing high temperature concentration, the rutin in the mulberry leaves is well extracted and reserved, microorganisms such as bacteria can be well removed, and a microorganism indicator is enabled to meet national health food material regulations; the quality control method is accurate, reliable and good in repeatability, and can be taken as a method for measuring the content of the mulberry leaf extract rutin.
Owner:GUANGDONG YIFANG PHARMA
Content detection method for compound houttuynia cordata mixture
ActiveCN104820029AReliable Quality Control MethodsGuaranteed clinical efficacyComponent separationAdditive ingredientSilica gel
The invention discloses a content detection method for a compound houttuynia cordata mixture. The content detection method mainly includes that water-saturated normal butanol is used for extraction, water-alcohol elution is performed after column feeding, Phenyl bonded silica gel is taken as a chromatographic column, and mobile phase is acetonitrile-0.5% acetic acid solution (volume ratio is 18.5:81.5). By the content detection method, the problem that quercitrin which serves as a feature ingredient of the compound houttuynia cordata mixture cannot be controlled effectively is solved, content of quercitrin, baicalin and forsythin in the compound houttuynia cordata mixture is detected synchronously, and drug safety, effectiveness and quality controllability are guaranteed.
Owner:浙江康恩贝中药有限公司
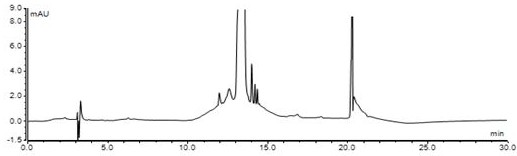
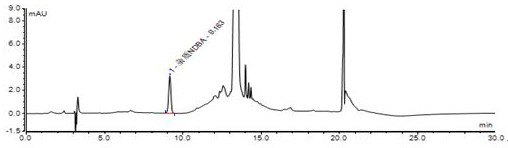
HPLC method for detecting genotoxic impurities in candesartan cilexetil
The invention relates to a method for detecting genotoxic impurities in candesartan cilexetil, and belongs to the technical field of medicine quality control. The detection method comprises the stepsof 1, preparing a reference substance solution; 2, preparing a test solution; 3, preparing a sample adding test solution; 4, taking Thermo Gold C18 as a chromatographic column, taking a methanol-watermixed solution with a volume ratio of 63:37 as a mobile phase A, and taking acetonitrile as a mobile phase B, wherein the detection wavelength is 240nm; introducing a sample and carrying out gradientelution according to a table 1, wherein the flow rate is 1.0 ml / min, the column temperature is 30 DEG C, and the sample size is 80[mu]l; and recording a chromatogram, and calculating the content of the genotoxic impurity NDBA. The invention provides an HPLC / UV detection method of genotoxic impurity NDBA in candesartan cilexetil.
Owner:迪嘉药业集团股份有限公司
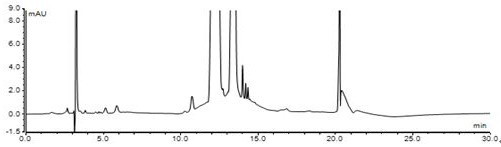
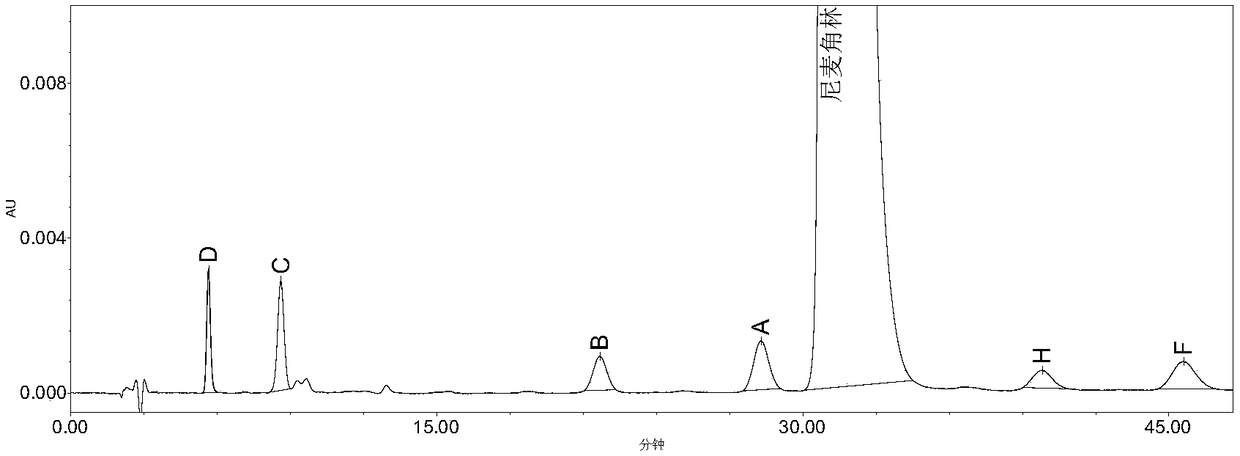
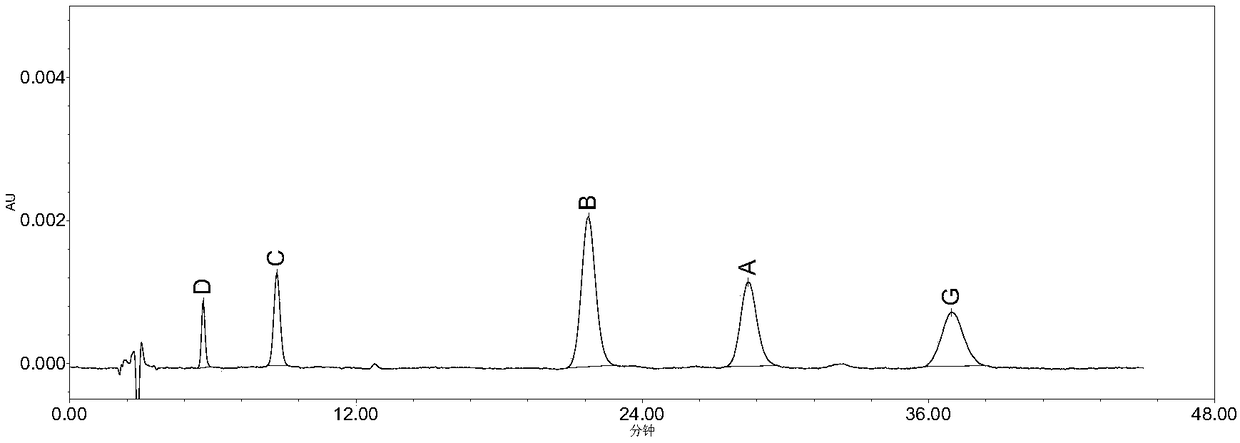
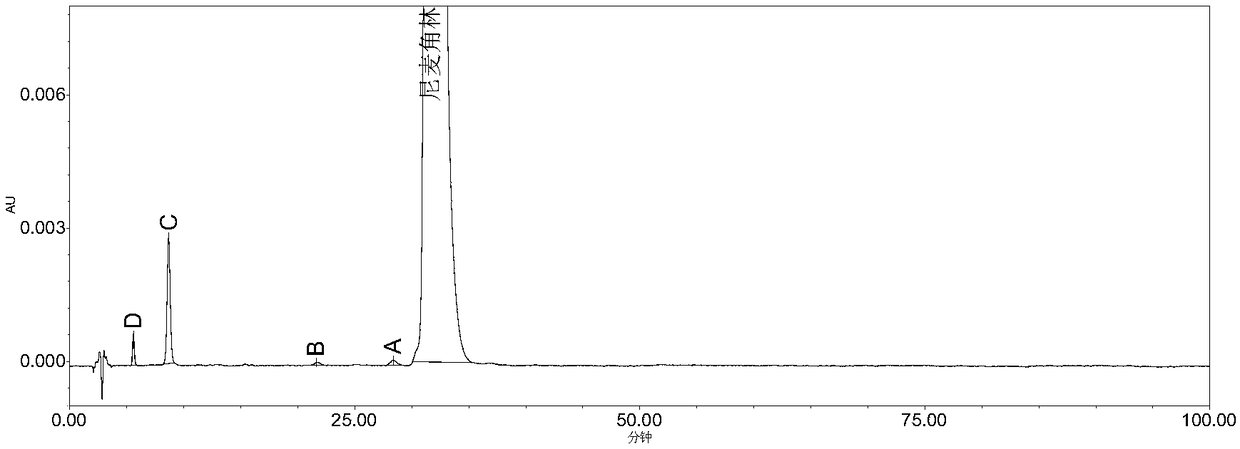
High performance liquid chromatography method for determining nicergoline related substances
ActiveCN108593818AReliable Quality Control MethodsStable quality control methodComponent separationIsocratic elutionColumn temperature
The invention belongs to the field of medicine analysis, and particularly relates to a high performance liquid chromatography method for determining nicergoline related substances. According to the high performance liquid chromatography method, a filler obtained by mixing strong cation exchange resin and a reversed phase C18 according to a volume ratio of 1 to 4 is used as a filling agent, acetonitrile is used as a mobile phase A, a phosphate buffer is used as a mobile phase B, the mobile phase A and the mobile phase B are mixed according to a ratio of 30 to 70 for isocratic elution, the detection wavelength is 288nm, and the column temperature is 30 DEG C. By the high performance liquid chromatography method, the content of an impurity D can be accurately determined and known impurities C, B, A, G, F and H and other unknown impurities in nicergoline can be simultaneously determined.
Owner:CHONGQING INST FOR FOOD & DRUG CONTROL
Lamiophlomis rotata extract, medicine composition containing same and quality control method thereof
ActiveCN101984983AStable efficacyClear efficacyAntipyreticComponent separationLamiophlomis rotataMedicine
Owner:HENGKANG MEDICAL GROUP CO LTD
Isatis root preparation and quality control method thereof
ActiveCN1528383AQuality improvementSimple and easy quality control methodOrganic active ingredientsComponent separationAlcoholAdenosine
The present inven relates to an isatis root preparation and its quality control method. First of all, said invention creates the following method and steps: using TCL to examine the isatis root preparation for that said isatis root medicinal material has the judgement method related to isatis leaf or not and isatis root preparation production process has the judgement standard for implementing alcohol precipition step or not; adopting HPLC quantitative analysis method of adenosine in isatis root medicinal material and isatis root preparation and providing quality control standard, and providing the adenosine content range in the isatis root medicinal materials from different sources and isatis root granules, said adenosine content range is 50-400 micrograms, so that said invention ensures normalization of production process and stable uniformity of quality.
Owner:GUANGZHOU BAIYUSN HUTCHISON WHAMPOA CHINESE MEDICINE
Common lamiophlomis extract, medicine composition containing same and quality control method
ActiveCN101732405AStable efficacyClear efficacyAntipyreticComponent separationMedicineQuality control
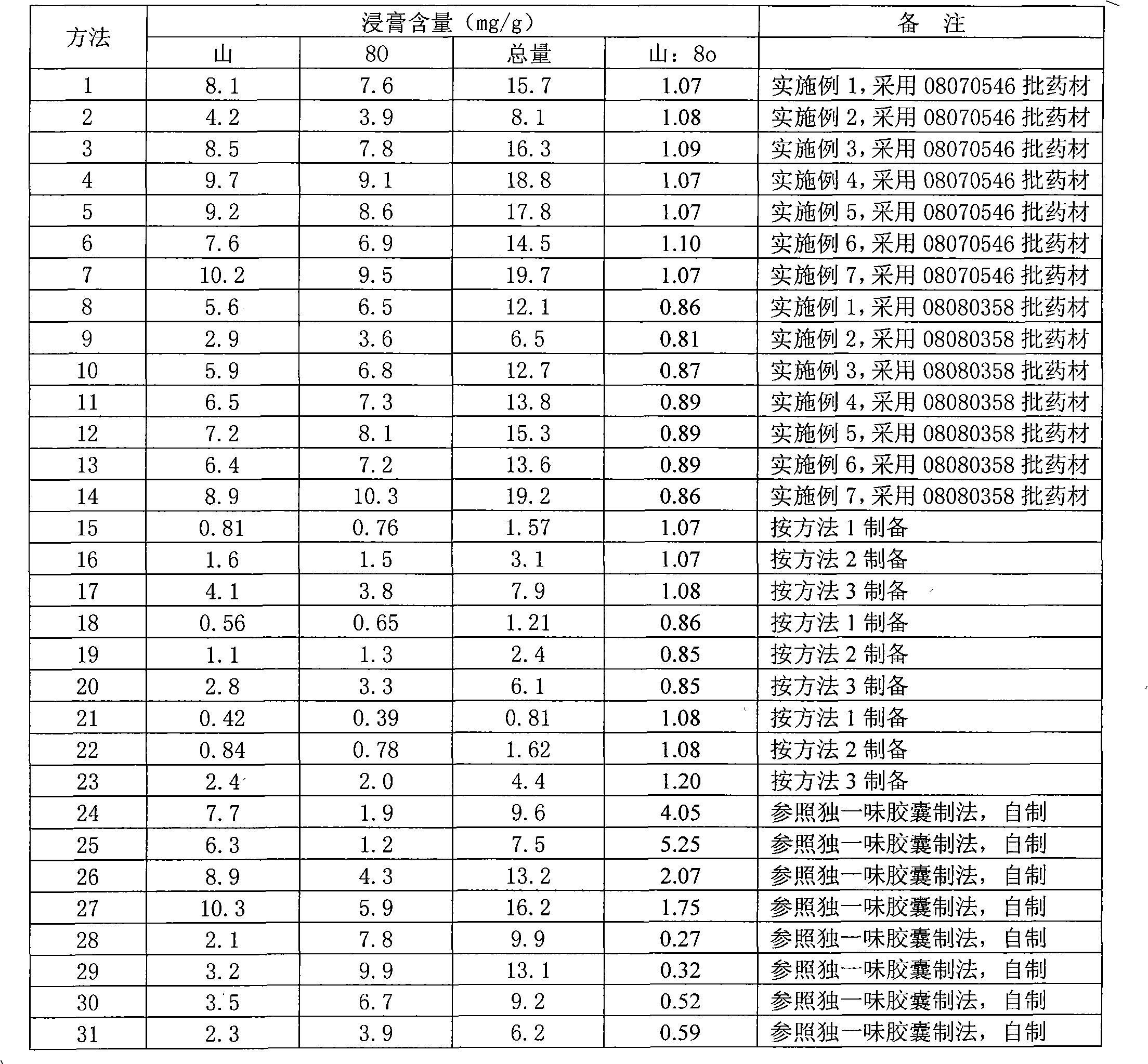
The invention provides a common lamiophlomis extract which contains 1.5-30mg / g of shanzhiside methylester. The invention also provides a medicine composition containing the common lamiophlomis extract and a method for controlling the quality of the common lamiophlomis extract or the medicine composition containing the common lamiophlomis extract. The invention has stable medicine effect of the extract, definite medicine effect and strong controllability and controls the quality of the extract by taking the shanzhiside methylester as an index component. The quality control method is accurate, reliable, stable and controllable, is used for measuring the content of the shanzhiside methylester in a common lamiophlomis medicinal material or a preparation and can effectively monitor the qualityof a common lamiophlomis medicine.
Owner:康县独一味生物制药有限公司
Detection method for medicinal material Ardisia mamillata Hance or extract thereof
InactiveCN105116059AReliable Quality Control MethodsGood linear relationshipComponent separationActive componentMedicine
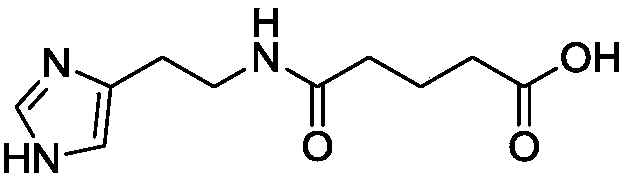
The invention provides a quantitative analysis method for a medicinal material Ardisia mamillata Hance or extract thereof. The method employs high performance liquid chromatography-evaporation light scattering detection process (HPLC-ELSD) to determine the content of the active component ardisiacrispin B in the medicinal material Ardisia mamillata Hance or extract thereof, so the inherent quality of the medicinal material Ardisia mamillata Hance or extract thereof can be objectively and scientifically evaluated. The precision, stability, repeatability, sample recovery, tolerance and the like of the method all meet quantitative analysis requirements. The method is used for determining the contents of the medicinal material Ardisia mamillata Hance produced from different areas for the first time and provides a novel quality control and detection means for medicinal development of the component ardisiacrispin B.
Owner:SOUTHWEST UNIVERSITY FOR NATIONALITIES
Quality control method for leech and preparation containing leech
ActiveCN101703524AReliable evaluationReliable evaluation of leechesComponent separationPreparing sample for investigationQuantitative determinationQuality control
The invention relates to a quality control method for a leech and a preparation containing the leech. The method takes succinic acid or sodium succinate as an index component, and carries out quality control on the leech and the preparation containing the leech through the qualitative and / or quantitative determination of the index component. The quality of the leech and the preparation containing the leech can be accurately controlled by the quality control method which can more reliably and more accurately evaluate the leech and the preparation containing the leech so as to improve the quality control level of medicaments and ensure the quality uniformity and the curative effect stability of the preparation.
Owner:LUNAN PHARMA GROUP CORPORATION
Method for detecting quality of alkaloids in Tibetan medicine herba aconiti tangutici and preparations thereof
ActiveCN102735789BQuality assuranceEnsure the safety of medicationChemical analysis using titrationComponent separationPhosphateMonopotassium phosphate
Owner:SHANDONG JINHE DRUG RES DEV
Quality control method for medicinal material of dotted ardisia
PendingCN111308032AStrong specificityGood reproducibilityInvestigation of vegetal materialComponent separationMedicinal herbsThin layer chromatographic
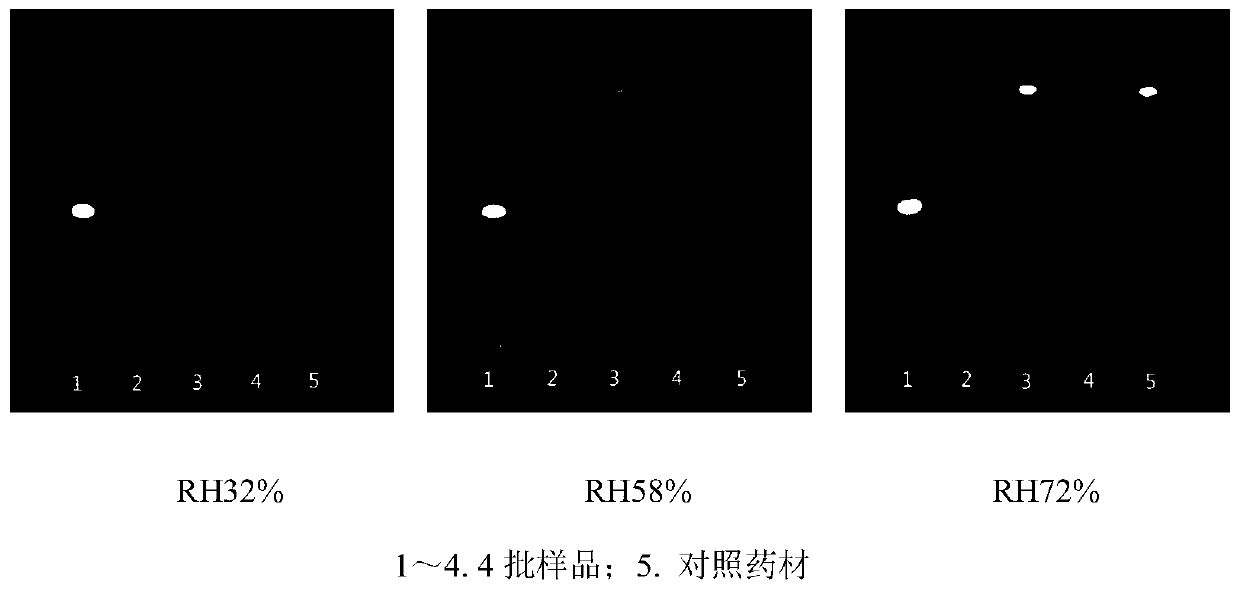
The invention discloses a quality control method for a medicinal material of dotted ardisia, and relates to the technical field of traditional Chinese medicinal materials. The method comprises the following steps: (1) microscopically identifying medicinal material powder; (2) determining the water content, total ash content and extract content ranges of the medicinal materials: the water content is not more than 15.0%, the total ash content is not more than 9.0%, and the extract content is not less than 12.0% by using dilute ethanol as a solvent according to a hot dipping method under the condition of an alcohol-soluble extract determination method (the general rule 2201 of the four parts of the Chinese Pharmacopoeia 2015 edition); (3) thin-layer chromatography identification, in which inthe chromatogram of the test sample, fluorescence spots with the same color should be shown at the position corresponding to the chromatogram of the dotted ardisia reference medicinal material solution. The scientific, complete, reliable and effective quality control method for the dotted ardisia medicinal material is established, and the method is high in specificity and good in reproducibility;meanwhile, the quality standard of the dotted ardisia medicinal material is established by adopting the method, and the internal quality and the medication quality of the medicinal material can be effectively evaluated and controlled.
Owner:GUIZHOU SHENGSHI LONGFANG PHARMA
Thin-layer chromatography identification of Solamum melongena and quality control method thereof
InactiveCN110297062AImprove clarityEasy to separateComponent separationPreparing sample for investigationThin layer chromatogramHigh definition
The invention belongs to the technical field of the medicinal material detection, especially a thin-layer chromatography identification of Solamum melongena and a quality control method thereof. The Solamum melongena can be identified through a Solamum melongena thin-layer chromatography identification method, a more reliable quality control method can be determined. Through the Solamum melongenathin-layer chromatography identification method provided by the invention, the obtained atlas has high definition, good separation degree, obvious spot and good reproducibility.
Owner:GUIZHOU MEDICAL UNIV
A kind of impurity detection method of ingaverine and preparation thereof
ActiveCN106257276BQuality improvementEffective detection and separationComponent separationSilanesGradient elution
The invention provides ingavrin and a method for detecting impurity of an ingavrin preparation. The method is a high-performance liquid chromatography, takes octadecylsilane chemically bonded silica or octa-silane chemically bonded silica as a chromatographic column of a filler, takes a mixed solvent of an organic phase and a water phase as a mobile phase for gradient elution, and ten impurities in ingavrin and its preparation can be determined. The method has the advantages of low process and low cost, by employing a gradient elution method, ten impurities in ingavrin and its preparation can be effectively determined and separated, the detection method is scientific, reasonable and objective, so that the ingavrin quality can be better controlled.
Owner:JIANGSU CHIA TAI FENGHAI PHARMA
A HPLC method for detecting genotoxic impurities in candesartan cilexetil
The invention relates to a method for detecting genotoxic impurities of candesartan cilexetil, belonging to the technical field of drug quality control. Detection method of the present invention, comprises the following steps: step 1 prepares reference substance solution; Step 2 prepares need testing solution; Step 3 adds the preparation of need testing solution; Step 4. takes Thermo Gold C18 as chromatographic column, with volume The methanol-water mixture with a ratio of 63:37 was used as mobile phase A, and acetonitrile was used as mobile phase B; the detection wavelength was 240nm; the sample was injected and gradient eluted according to Table 1. The flow rate was 1.0 ml / min, the column temperature was 30°C, and the injection volume was 80 µl. Record the chromatogram and calculate the content of genotoxic impurity NDBA. The invention provides an HPLC / UV detection method for candesartan cilexetil genotoxic impurity NDBA.
Owner:迪嘉药业集团股份有限公司
Quality control method for Keyin pills
ActiveCN101632774BStable quality control methodsFast quality control methodComponent separationDermatological disorderMedicineCurative effect
Owner:BEIJING ASIA EAST BIO PHARMA CO LTD
Capillary electrophoresis uantitative analytical method for impurities content in refined p-phthalic acid
InactiveCN100516862CSimple and fast operationQuantitatively accurateMaterial analysis by electric/magnetic meansSpecial data processing applicationsBenzoic acidCapillary electrophoresis
The related CE inner-scaling analysis method for the impurity in fine TPA comprises: selecting one of PPA, PMXPA and PTBBA as the inner scale, and quantitative analyzing the impurity content in the fine TPA. This invention is simple and fast for real-time monitor and analysis.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
Common lamiophlomis extract, medicine composition containing same and quality control method
ActiveCN101732405BStable efficacyClear efficacyComponent separationAntipyreticBiotechnologyMedicinal herbs
The invention provides a common lamiophlomis extract which contains 1.5-30mg / g of shanzhiside methylester. The invention also provides a medicine composition containing the common lamiophlomis extract and a method for controlling the quality of the common lamiophlomis extract or the medicine composition containing the common lamiophlomis extract. The invention has stable medicine effect of the extract, definite medicine effect and strong controllability and controls the quality of the extract by taking the shanzhiside methylester as an index component. The quality control method is accurate, reliable, stable and controllable, is used for measuring the content of the shanzhiside methylester in a common lamiophlomis medicinal material or a preparation and can effectively monitor the quality of a common lamiophlomis medicine.
Owner:康县独一味生物制药有限公司
Tongzhisurunjiang tablet for activating retardation and softening and preparation and quality control method thereof
ActiveCN102784275BHigh tablet hardnessImprove brittlenessComponent separationAntipyreticSide effectSciatica
The invention discloses a tablet for activating the retardation and softening, which can smoothen retardation and relieve swelling and pain, and can treat articular bone pain, rheumatism, rheumatoid arthritis and sciatica. According to the tablet, a core of the tablet comprises seven traditional Chinese medicines which are respectively folium sennae, colchicum autumnale and medicine terminalia fruit and the like, and microcrystalline cellulose, aerosil, light magnesium carbonate, an adhesive and magnesium stearate. The invention also discloses a preparation method for the tablet, and a quality control method for the Tongzhisurunjiang tablet. The tablet activating the retardation and softening disclosed by the invention has the characteristics of high tabletting strength, high friability and is easy to form, and has characteristics of resistance to oil leakage, and high stability; compared with troche, the tablet is more convenient to take and carry, is low in cost, and less in side effect. The preparation technology disclosed by the invention is simple, and the quality control method is reliable; and the quality of a product can be controlled well.
Owner:WUHAN JIANMIN ZHONGWEI PHARMA CO LTD
Quality control method for leech and preparation containing leech
ActiveCN101703524BReliable evaluationReliable Quality Control MethodsComponent separationPreparing sample for investigationQuantitative determinationQuality control
Owner:LUNAN PHARMA GROUP CORPORATION
Esomeprazole sodium and method for detecting impurity content in esomeprazole sodium for injection
The invention provides esomeprazole sodium and a method for detecting content of the esomeprazole sodium for injection. A chromatographic column, using octadecyl silane bonded silica gel or octo-alkyl silane bonded silica gel as a filling agent, implements the gradient elution with mixed solvent of an organic phase and a water phase as a mobile phase to measure the esomeprazole sodium and the content of eight impurities thereof. The method is simple in process and low in cost, and adopts the gradient elution method to measure the esomeprazole sodium and the content of eight important impurities thereof; the detection method is scientific, reasonable and objective, so that the quality of the esomeprazole sodium can be preferably controlled.
Owner:JIANGSU CHIA TAI FENGHAI PHARMA
Quality control method of radix cudramiae medicinal materials
PendingCN111122806AControl internal qualityAssess and control intrinsic qualityInvestigation of vegetal materialComponent separationBiotechnologyMedicinal herbs
The invention discloses a quality control method of a radix cudramiae medicinal material, and relates to the technical field of traditional Chinese medicinal materials. The method comprises the following steps: (1) microscopically identifying medicinal material powder; (2) determining the water content, total ash content and extract content ranges of the medicinal materials: the water content is not more than 13.0%, the total ash content is not more than 6.0%, the extract is determined by a hot dipping method under the condition of an alcohol-soluble extract determination method (the general rule 2201 of the four parts of the Chinese Pharmacopoeia 2015 edition), and dilute ethanol is used as a solvent and is not less than 10.0%; and (3) thin-layer chromatography identification; wherein inthe chromatogram of the test sample, fluorescent spots with the same color should be shown at the position corresponding to the chromatogram of the kaempferol reference substance. The scientific, complete, reliable and effective quality control method for the radix cudramiae medicinal material is established, and the method is high in specificity and good in reproducibility; meanwhile, the qualitystandard of the radix cudramiae medicinal material is established by adopting the method, and the internal quality and the medication quality of the medicinal material can be effectively evaluated and controlled.
Owner:GUIZHOU SHENGSHI LONGFANG PHARMA
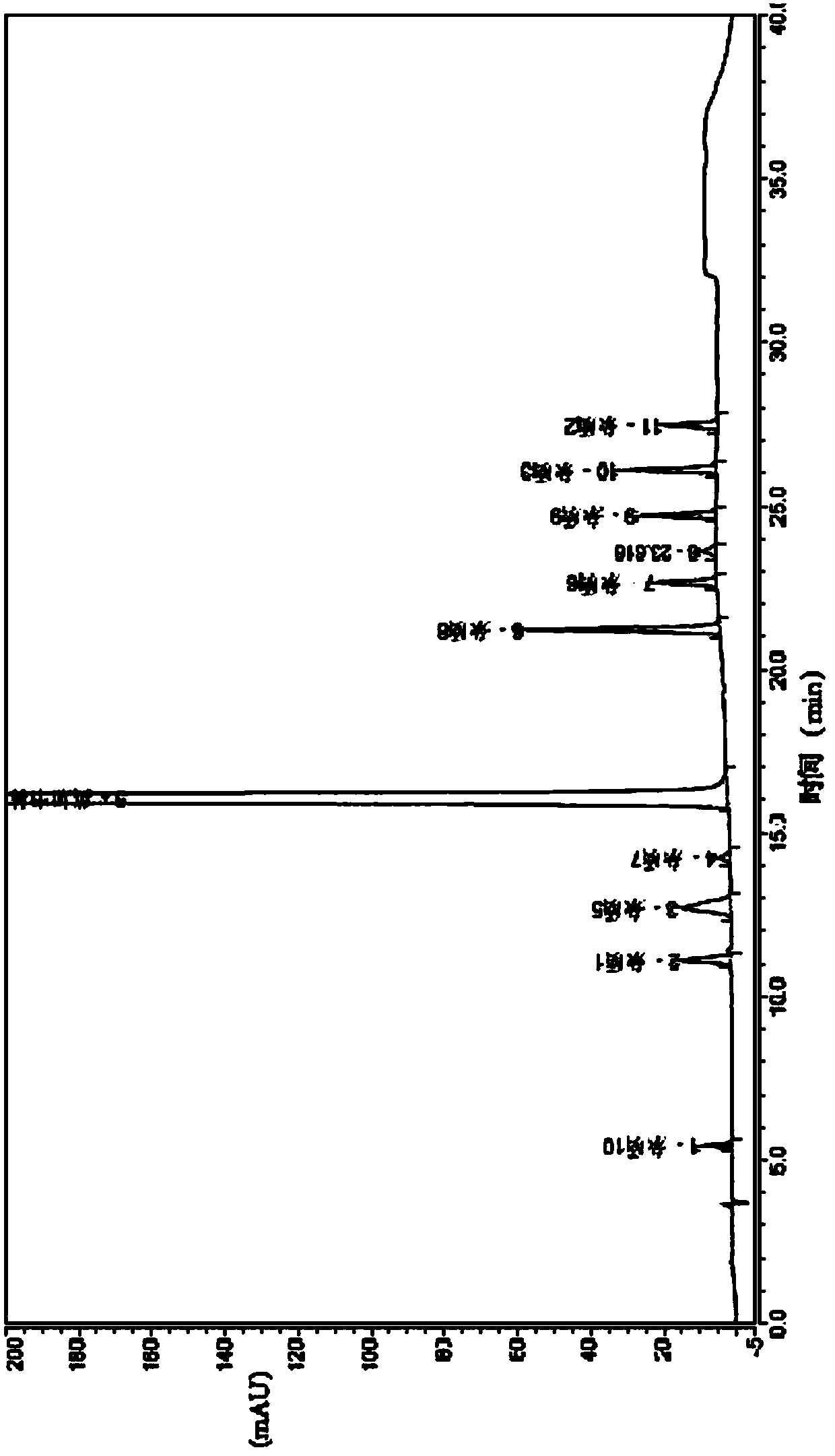
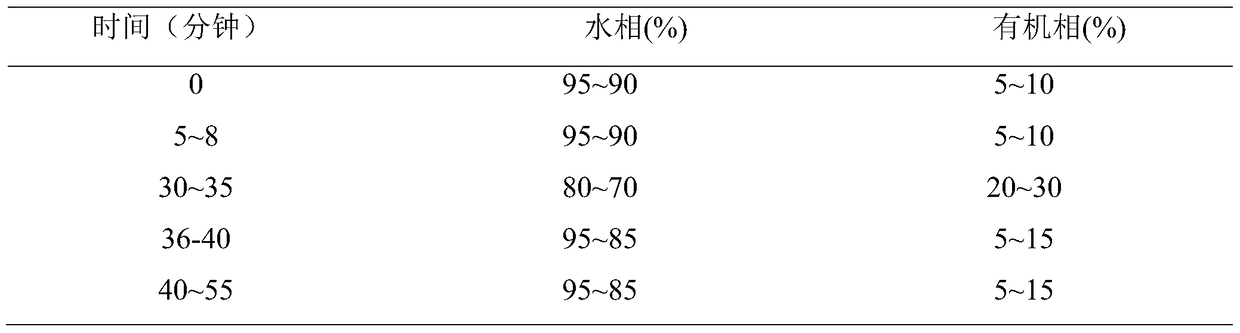
A method for establishing fingerprints of Zhinao capsules and a method for quality evaluation
ActiveCN113720954BEvaluation authenticityGoodness of evaluationComponent separationFiltration membraneFluid phase
A method for establishing fingerprints of Zhinao capsules and a quality evaluation method, belonging to the technical field of product control and management of compound traditional Chinese medicine preparations, taking an appropriate amount of the contents of Zhinao capsules, adding methanol for ultrasonic extraction, and filtering through a filter membrane; accurately weighing a reference substance and adding methanol to prepare the product. A certain concentration of reference substance solution; choose acetonitrile-water as the mobile phase, and use gradient elution; choose DAD as the detector, and choose BEH C as the chromatographic column 18 ; Measure and obtain UPLC spectra of 10 batches of samples, import the obtained UPLC spectra into the similarity evaluation software, calculate the similarity of the samples, and determine the standard fingerprints; if the similarity is greater than 0.90, it meets the quality requirements of Zhinao Capsules, so as to complete the intelligence to be tested Quality assessment of brain capsule samples. The invention utilizes ultra-high performance liquid chromatography to construct the fingerprints of Zhinao capsules, analyzes multiple batches of Zhinao capsules to determine their standard fingerprints, and establishes a stable and reliable quality control method for Zhinao capsules.
Owner:FIRST AFFILIATED HOSPITAL OF ANHUI UNIV OF CHINESE MEDICINE
Quality control method of bauhinia championii medicinal materials
PendingCN111122804AScientific Quality Control MethodsComplete Quality Control MethodInvestigation of vegetal materialComponent separationBauhinia championiiMedicinal herbs
The invention discloses a quality control method of bauhinia championii medicinal materials, and relates to the technical field of traditional Chinese medicinal materials. The method comprises the following steps: (1) microscopically identifying medicinal material powder; (2) determining the water content, total ash content and extract content ranges of the medicinal materials: the water content is not more than 15.0%, the total ash content is not more than 9.0%, and the extract content is not less than 15.0% by using dilute ethanol as a solvent according to a hot dipping method under the condition of an alcohol-soluble extract determination method (the general rule 2201 of the four parts of the Chinese Pharmacopoeia 2015 edition); and (3) thin-layer chromatography identification; whereinin the chromatogram of the test sample, fluorescent spots with the same color should be shown at the position corresponding to the chromatogram of the gallic acid reference substance solution. The scientific, complete, reliable and effective quality control method for bauhinia championii medicinal materials is established, and the method is high in specificity and good in reproducibility; meanwhile, the quality standard of the bauhinia championii medicinal material is established by adopting the method, and the internal quality and the medication quality of the medicinal material can be effectively evaluated and controlled.
Owner:GUIZHOU SHENGSHI LONGFANG PHARMA
Fingerprint establishment method and quality evaluation method of Zhinao capsule
ActiveCN113720954AEvaluation authenticityGoodness of evaluationComponent separationHplc dadGradient elution
The invention relates to a fingerprint establishment method and a quality evaluation method of the Zhinao capsule, and belongs to the technical field of compound traditional Chinese medicine preparation quality control management. A proper amount of Zhinao capsule content is taken, methanol is added for ultrasonic extraction, and a filter membrane is used for filtration; a reference substance is weighed precisely and adding methanol to prepare a reference substance solution with a certain concentration; acetonitrile-water is selected as a mobile phase, and gradient elution is adopted; DAD is selected as a detector, and BEH C18 is selected as a chromatographic column; 10 batches of sample UPLC maps are obtained through measurement, the obtained UPLC maps are imported into similarity evaluation software, the sample similarity is calculated, and a standard fingerprint map is determined; if the similarity is greater than 0.90, the quality requirement of the Zhinao capsule is met, so that the quality evaluation of the to-be-tested Zhinao capsule sample is completed. According to the invention, the fingerprint spectrum of the Zhinao capsule is constructed by using the ultra-high performance liquid chromatography, the standard fingerprint spectrum of the Zhinao capsule is determined by analyzing multiple batches of the Zhinao capsules, and a stable and reliable quality control method of the Zhinao capsule is established.
Owner:FIRST AFFILIATED HOSPITAL OF ANHUI UNIV OF CHINESE MEDICINE
A method for detecting related substances in edaravone sodium chloride injection
ActiveCN108072710BRich varietyHigh sensitivityComponent separationSodium Chloride InjectionSilica gel
The invention mainly provides a method for detecting related substances in an edaravone sodium chloride injection through a high-performance liquid chromatography. The method provided by the inventiontakes octadecyl silane bonded silica gel or octyl silane bonded silica gel as a filling agent and 0.2 percent to 0.4 percent of glacial acetic acid triethylamine-methanol as a mobile phase; the detection wavelength is 240 to 260nm, the column temperature is 20 to 40 DEG C and the flow speed is 0.5 to 1.0ml / min; gradient elution is carried out and edaravone and the related substances in the edaravone sodium chloride injection can be simultaneously detected. The method is simple to operate and high in sensitivity and the quality of products can be controlled relatively well.
Owner:JIANGSU CHIA TAI FENGHAI PHARMA
Quality control method of isatis root preparation
InactiveCN1248696CQuality improvementSimple and easy quality control methodOrganic active ingredientsComponent separationAlcoholAdenosine
The present inven relates to an isatis root preparation and its quality control method. First of all, said invention creates the following method and steps: using TCL to examine the isatis root preparation for that said isatis root medicinal material has the judgement method related to isatis leaf or not and isatis root preparation production process has the judgement standard for implementing alcohol precipition step or not; adopting HPLC quantitative analysis method of adenosine in isatis root medicinal material and isatis root preparation and providing quality control standard, and providing the adenosine content range in the isatis root medicinal materials from different sources and isatis root granules, said adenosine content range is 50-400 micrograms, so that said invention ensures normalization of production process and stable uniformity of quality.
Owner:GUANGZHOU BAIYUSN HUTCHISON WHAMPOA CHINESE MEDICINE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com