Lamiophlomis rotata extract, medicine composition containing same and quality control method thereof
A unique flavor and extract technology, applied in the field of medicine, can solve the problems of reporting index components without quality control, and achieve the effect of clear drug effect, strong controllability and stable drug effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
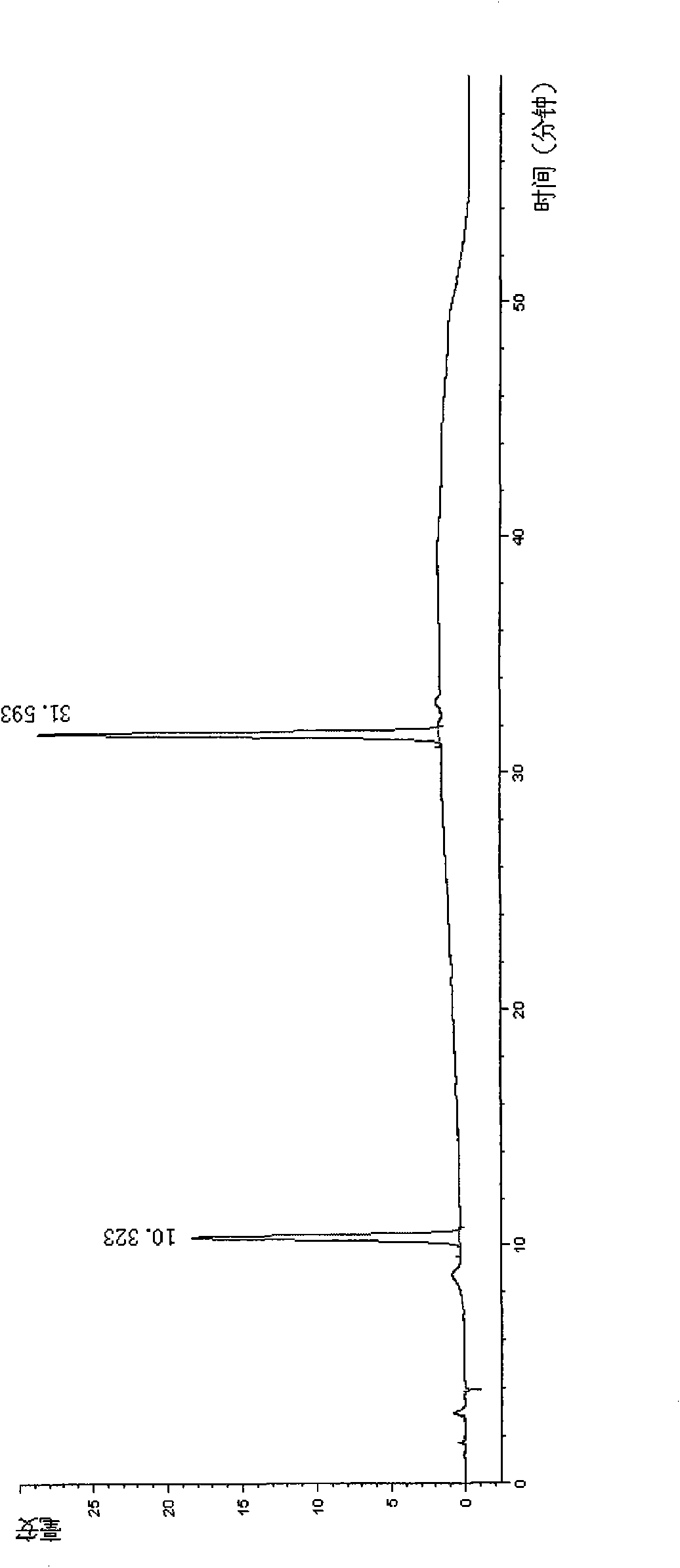
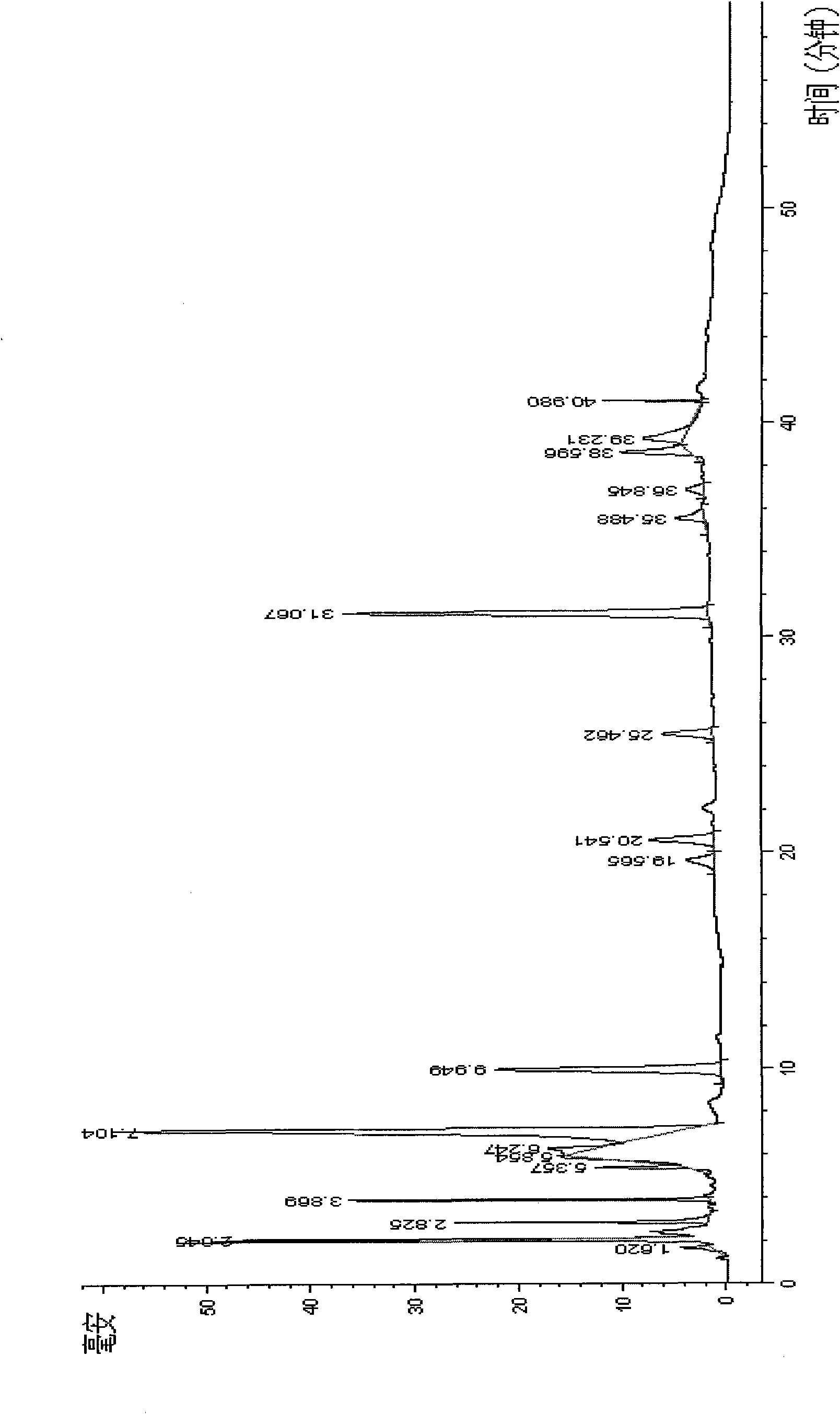
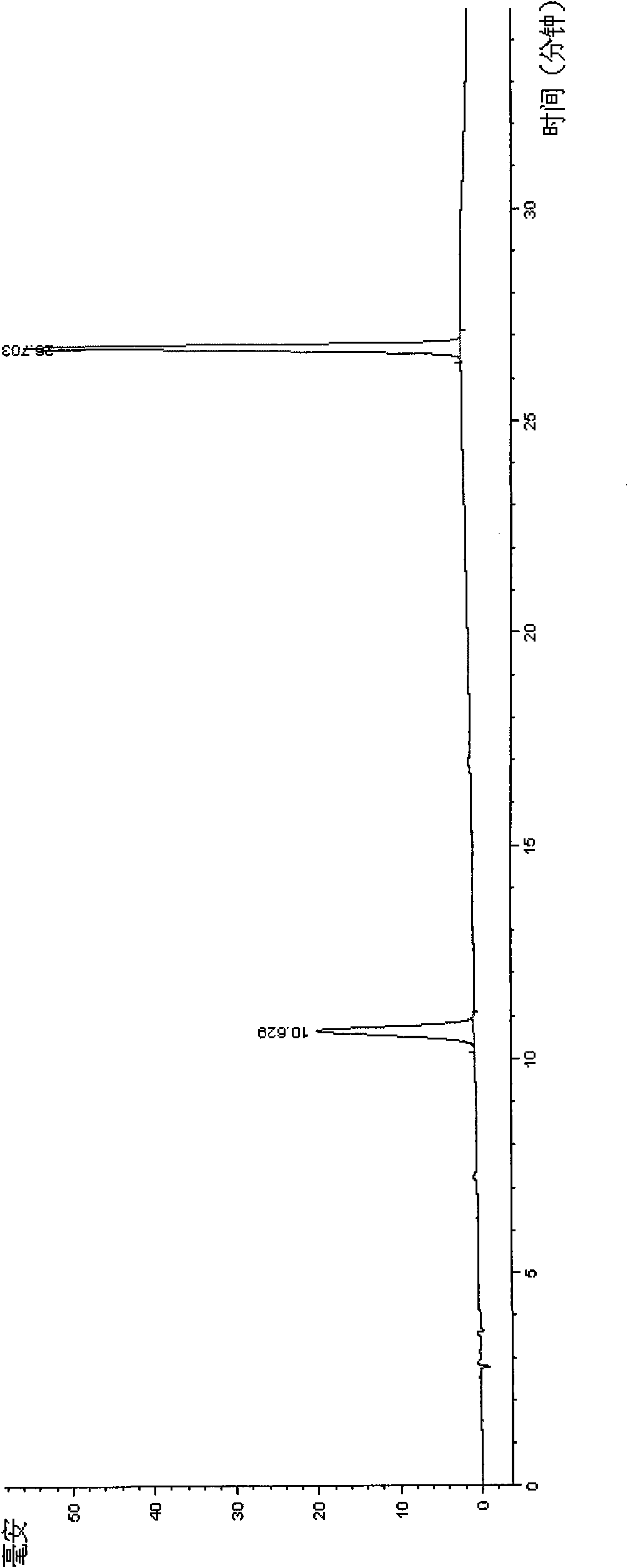
Image
Examples
Embodiment 1
[0029] The preparation of embodiment 1 pharmaceutical material extract of the present invention
[0030] Take 1000g of unique herb, crush it, add water and decoct 3 times, the first time is 2 hours, the second and third times are 1 hour respectively, and the amount of water added each time is 8 times, the decoction is combined, filtered, and the filtrate is concentrated to relative density 1.30 (85 ℃) clear paste, dry below 80 ℃, pulverize, and the paste rate is 15-25%.
Embodiment 2
[0031] The preparation of embodiment 2 pharmaceutical material extracts of the present invention
[0032] Take 1000g of unique medicinal material, crush it, add 20 times of water and soak for 24 hours, the water temperature is 85°C, filter, the filtrate is concentrated into a clear paste with a relative density of 1.30 (85°C), dried, crushed, and the paste yield is 12-18%
Embodiment 3
[0033] The preparation of embodiment 3 pharmaceutical material extracts of the present invention
[0034] Take 1000g of unique medicinal material, grind it, add water and pressurize extraction in a pressure extraction tank for 3 times, the first time is 2 hours, the second and third time are 1 hour respectively, the amount of water added each time is 8 times, the temperature is 120 ° C, combined The extract is filtered, concentrated, dried and pulverized to obtain a paste rate of 18-25%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| separation | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com