Application of panaxadiol saponins fraction in preparing medicine for preventing dermatitis and scar
A technology of ginseng diol saponin and ginsenoside, which is applied in the field of medicine, can solve difficult problems such as medicinal value, and achieve the effect of stable drug effect and clear active ingredients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1 Preparation of Panaxadiol Saponin Fraction-A (PDSF-A) from Radix Ginseng
[0051] 1. Isolation and Purification of Panaxadiol Saponin Fraction-A (PDSF-A) :
[0052] Ginseng rhizome medicinal material (8000 grams, sample-A, five kinds of ginseng diol saponins Rb contained therein 1 , Rc, Rb 2 , Rb 3 and Rd content and their total content are shown in Table 5) after pulverizing into a coarse powder, use 50% ethanol aqueous solution to percolate and extract until the extract is colorless, combine the percolate to recover ethanol under reduced pressure and freeze-dry to obtain the extract (678.6 gram). The medicinal extract was dissolved in 3000 milliliters of 45% ethanol aqueous solution, and the sample liquid was separated by macroporous adsorption resin (Diaion HP-20, 6000 grams) column chromatography, and first eluted with 45% ethanol aqueous solution until the eluent had no obvious saponin reaction, and then eluted with 70% ethanol aqueous solution u...
Embodiment 2
[0075] Example 2 Preparation of Panaxadiol Saponin Component-B (PDSF-B) from American Ginseng Rhizome
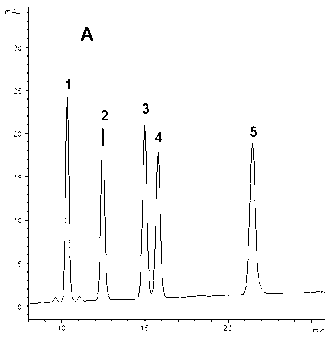
[0076] With American ginseng rhizome medicinal material (7000 grams, sample-B, five kinds of ginseng diol saponins Rb contained therein 1 , Rc, Rb 2 , Rb 3 and the content of Rd and their total content are shown in Table 5, and its HPLC collection is shown in Figure 1-3 ), adopt the same method of embodiment one to prepare the ginseng diol saponin component - B (PDSF-B, 101.3 g). HPLC analysis shows: PDSF-B also contains ginsenoside Rb 1 , Rc, Rb 2 , Rb 3 and Rd five kinds of ginsenosides, the total content of these five kinds of saponins is 91.60%, of which ginsenoside Rb 1 The highest content, followed by Rb 3 , Rd, Rb 2 and Rc; and Rb in Panaxadiol Saponin Fraction-B 1 / Rc / Rb 2 / Rb 3 / Rd content ratio and Rb in American ginseng rhizome (sample-B) 1 / Rc / Rb 2 / Rb 3 The content ratio of / Rd is basically the same (Table 5).
Embodiment 3
[0077] Example 3 Preparation of Panaxadiol Saponin Component-C ( PDSF -C)
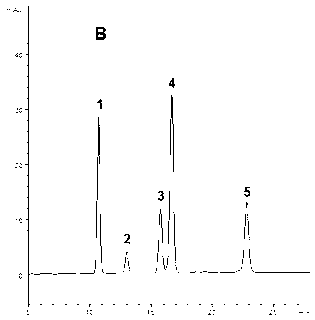
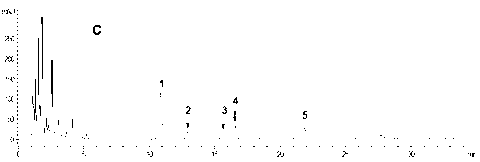
[0078] Purchased commodity ginseng rhizome total saponins (sample-C 1 , its total saponin content ≥ 80%) and American ginseng stem and leaf total saponin (sample-C 2 , its total saponin content ≥ 80%) show through HPLC analysis, five kinds of ginseng diol saponins contained in these two kinds of total ginsenoside products have their own characteristics respectively, namely: Sample-C 1 Both contain ginsenoside Rb 1 , Rc, Rb 2 , Rb 3 and Rd, but Rb 3 The content is very low (see attached diagram 2-1 and Table 5); Sample-C 2 Contains mainly Rb 3 and Rd, and Rb 1 , Rc and Rb 2 The content is very low (see attached Figure 2-2 and Table 5). This implementation comprehensively utilizes these two samples (sample-C 1 and sample-C 2 ) as this raw material to prepare the ginsengdiol saponin active component of the present invention, especially to obtain the total content of five kinds of ginseng...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com