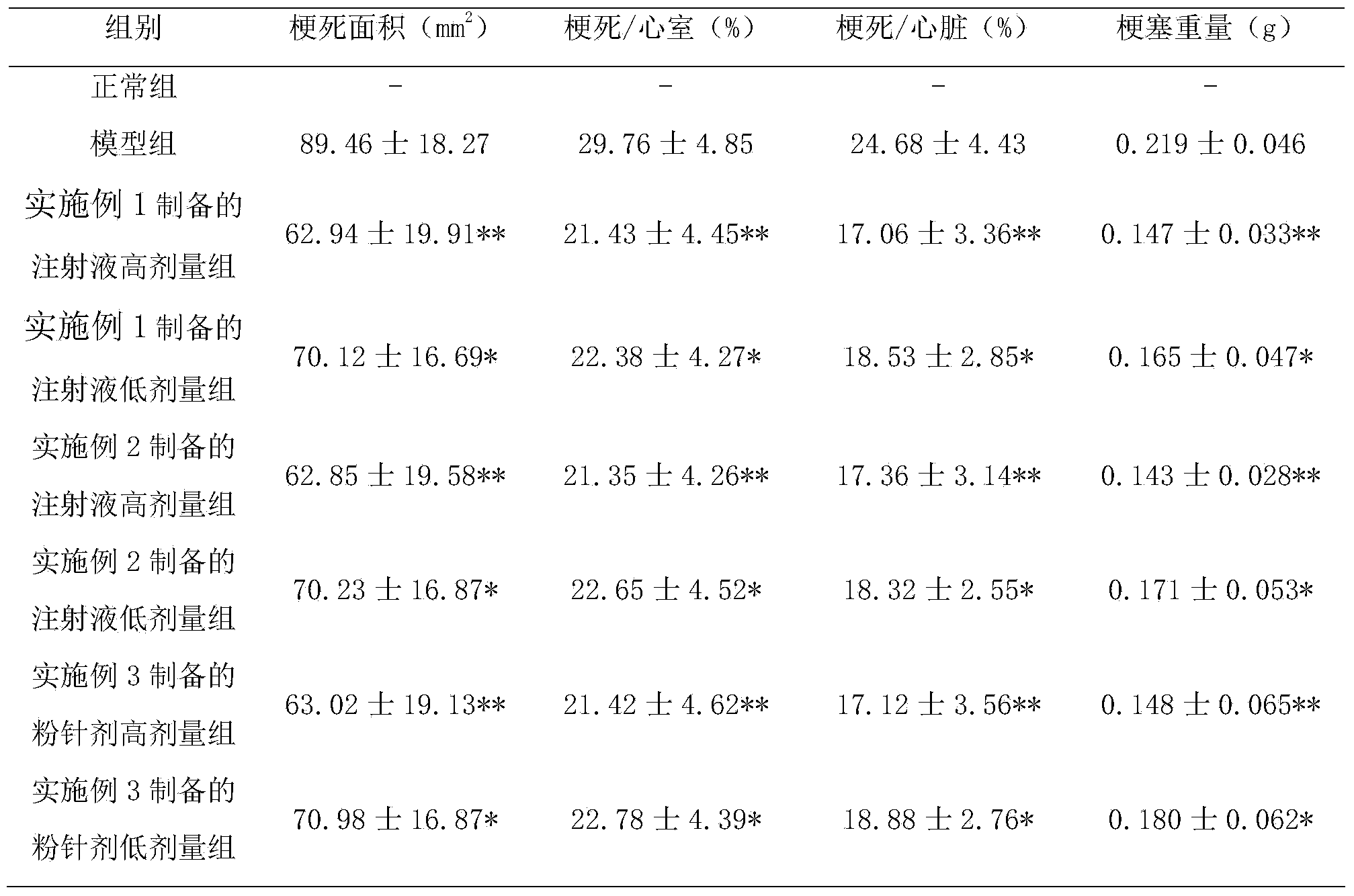
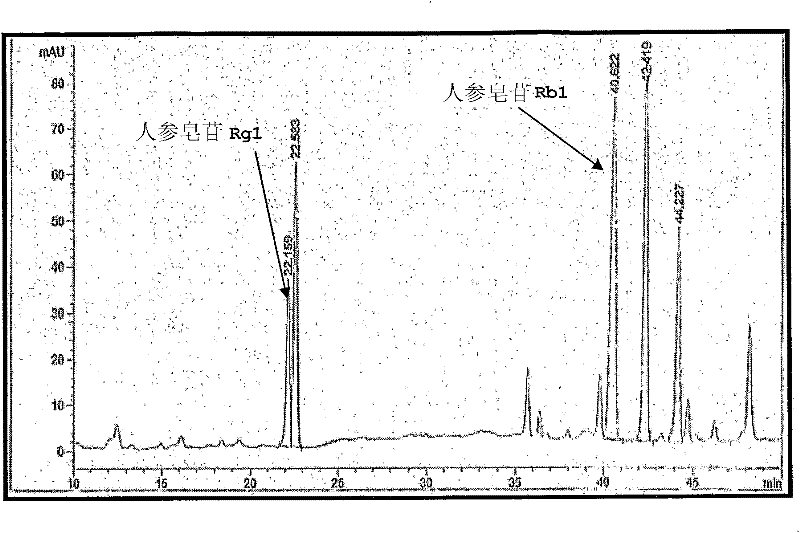
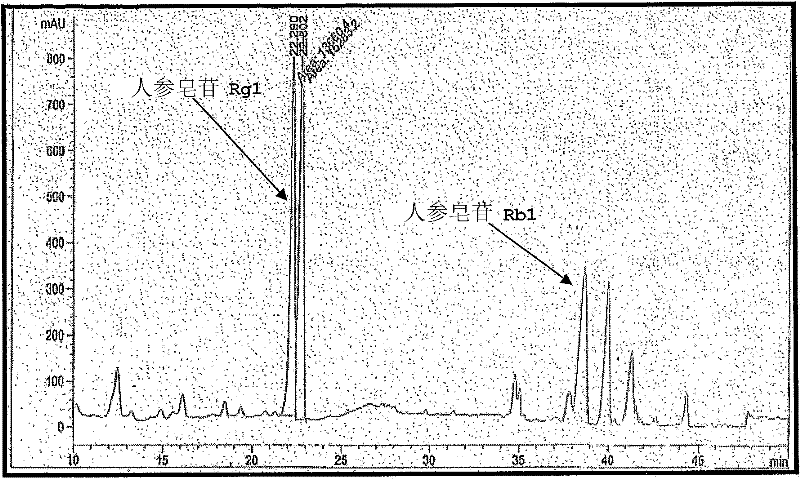
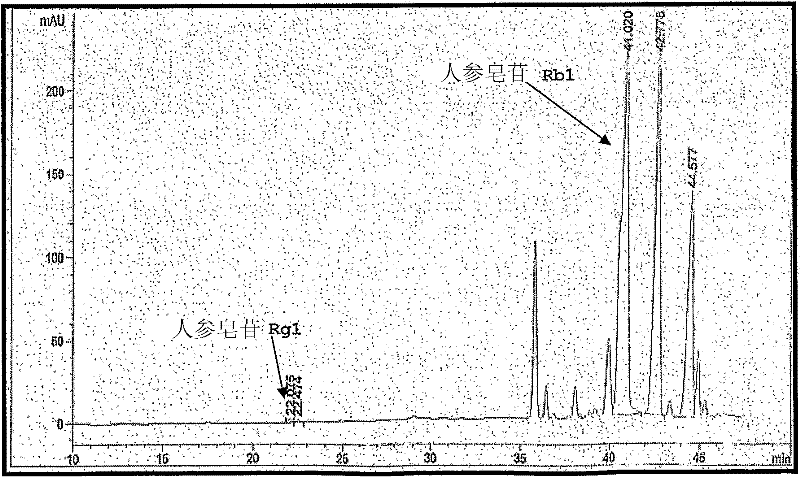
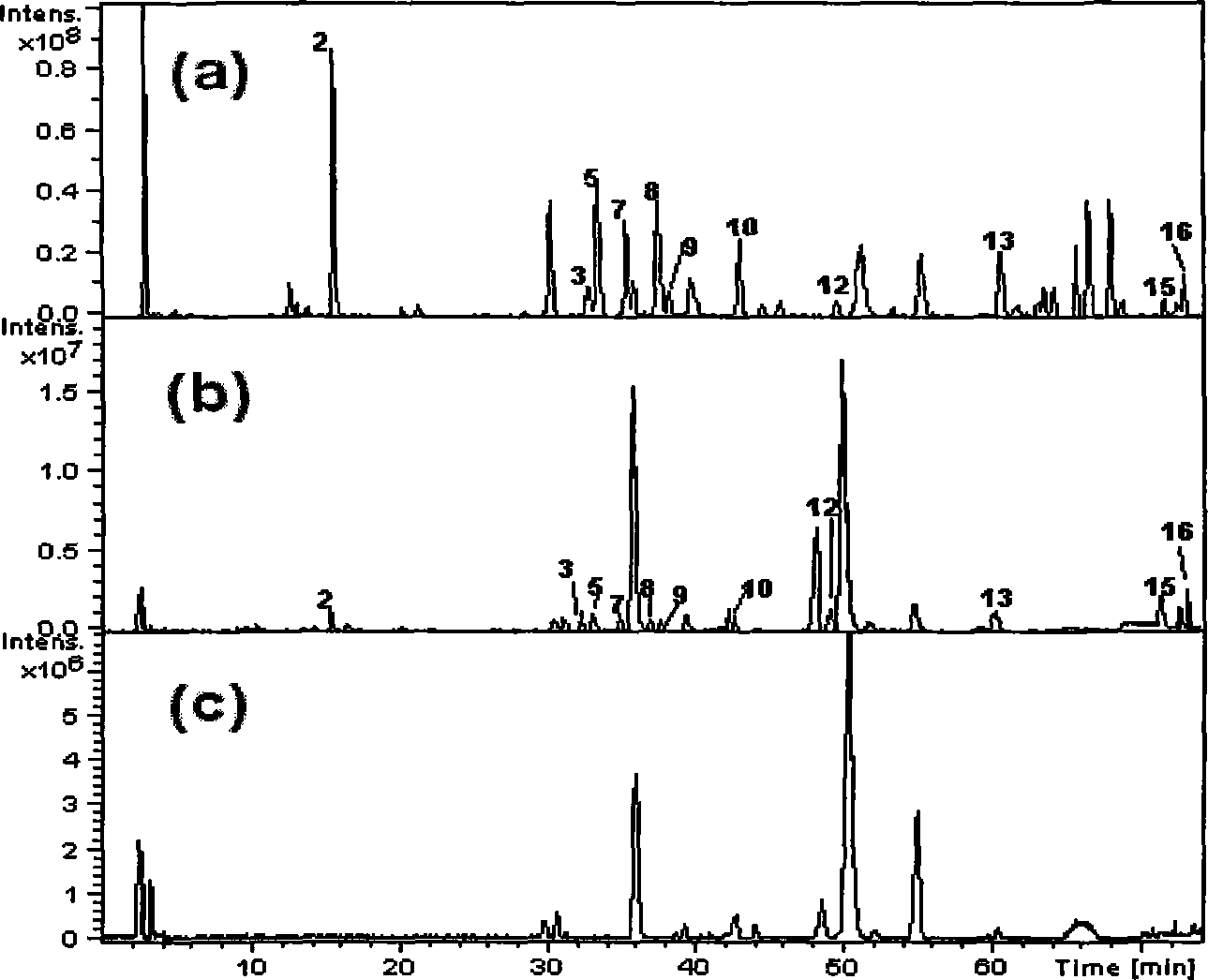
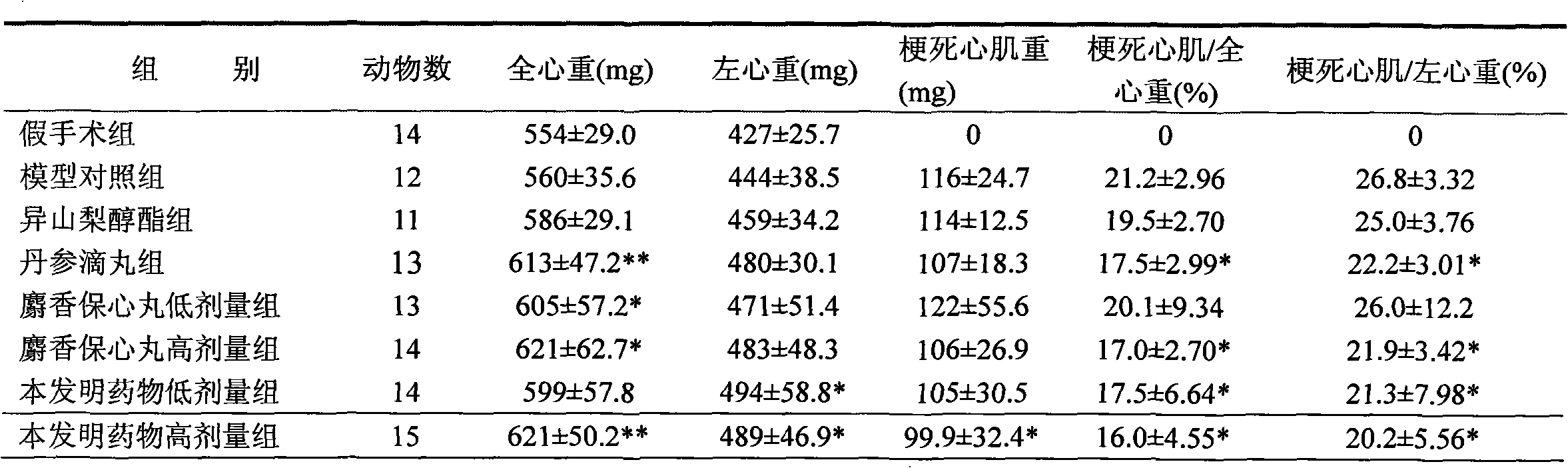
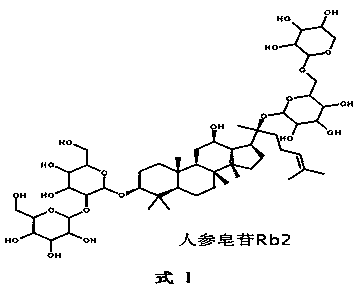
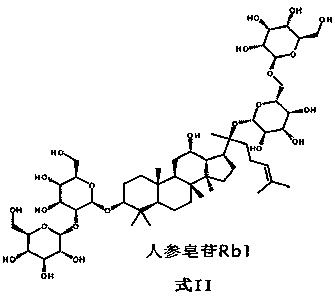
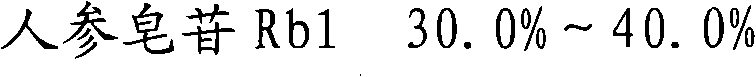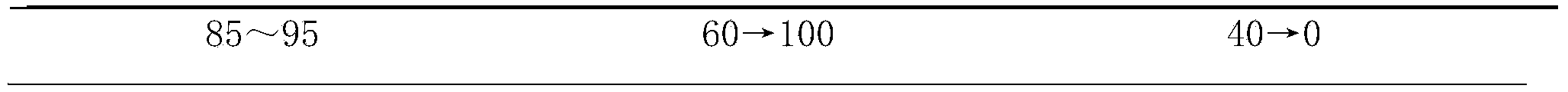Patents
Literature
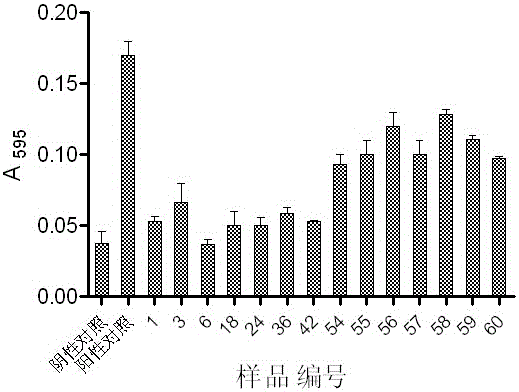
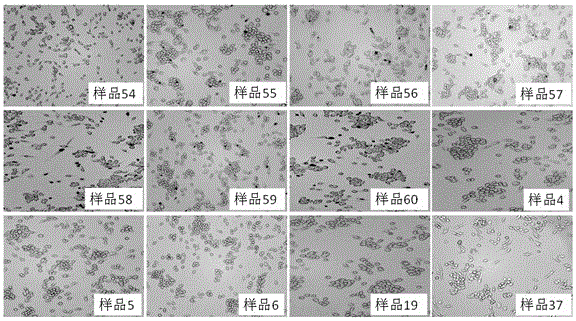
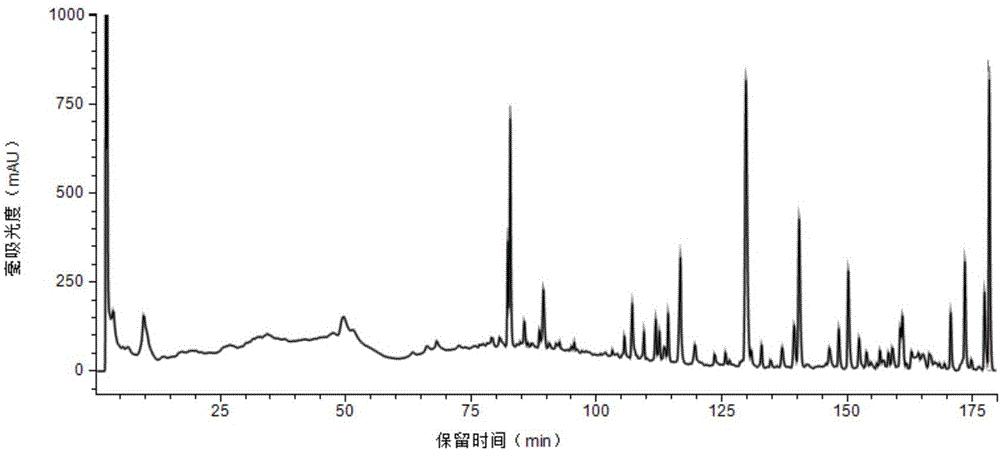
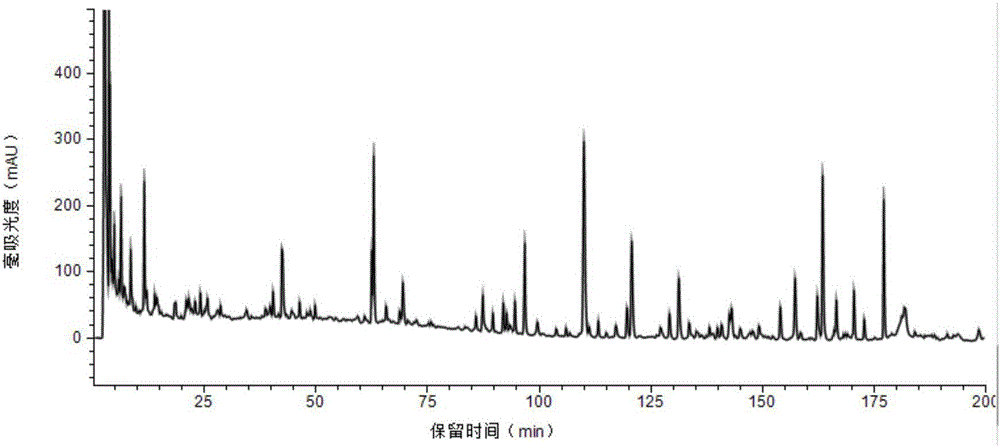
53 results about "Ginsenoside Rc" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
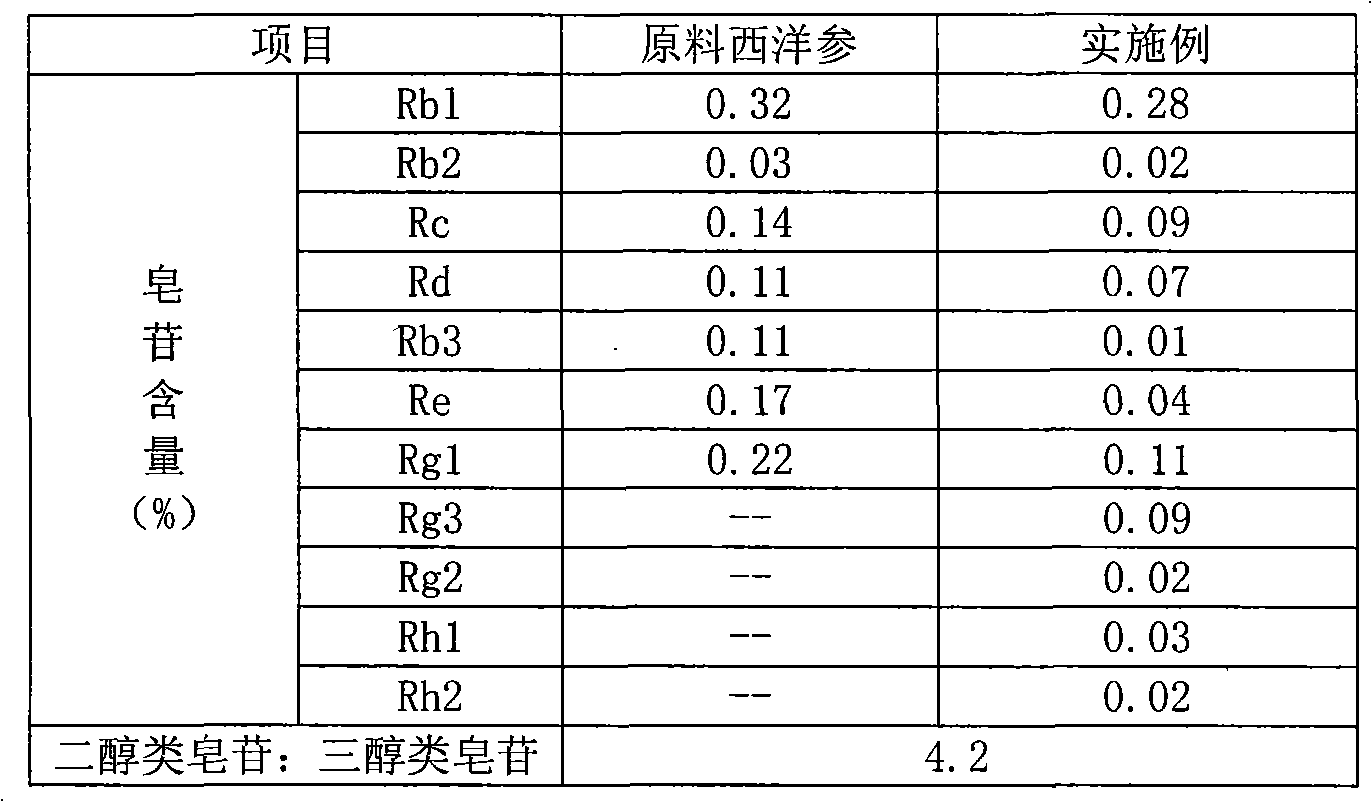
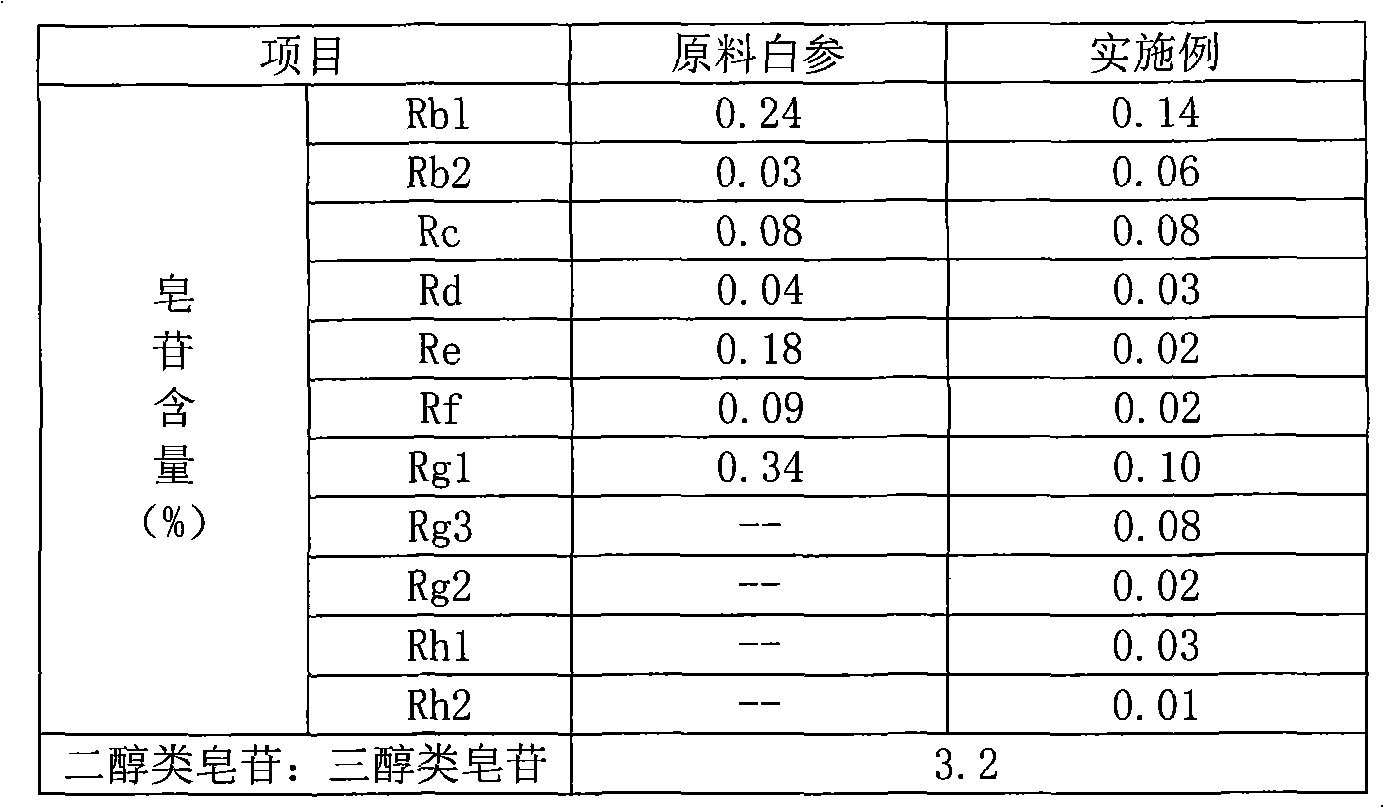
Ginsenoside Rc is a constituent of Panax ginseng (ginseng) from Human Metabolome Database (HMDB) Cellular Locations. The metabolome in Cellular Locations. Cytoplasm Extracellular Membrane. from Human Metabolome Database (HMDB) Literature.
Personal care compositions and methods for the beautification of mammalian skin and hair
Personal care composition comprising from about 0.05% to about 5% of at least one aquaporin-stimulating compound selected from the group consisting of xanthine, caffeine; 2-amino-6-methyl-mercaptopurine; 1-methyl xanthine; 2-aminopurine; theophylline; theobromine; adenine; adenosine; kinetin; p-chlorophenoxyacetic acid; 2,4-dichlorophenoxyacetic acid; indole-3-butyric acid; indole-3-acetic acid methyl ester; beta-naphthoxyacetic acid; 2,3,5-triiodobenzoic acid; adenine hemisulfate; n-benzyl-9-(2-tetrahydropyranyl)adenine; 1,3-diphenylurea; 1-phenyl-3-(1,2,3-thiadiazol-5-yl)urea; zeatin; indole-3-acetic acid; 6-benzylaminopurine; alpha-napthaleneacetic acid; 6-2-furoylaminopurine; green tea extract; white tea extract; menthol; tea tree oil; ginsenoside-RB1; ginsenoside-RB3; ginsenoside-RC; ginsenoside-RD; ginsenoside-RE; ginsenoside-RG1; ginseng root extract; ginseng flower extract; pomegranate extract, extracts from Ajuga turkestanica; extracts from viola tricolor and combinations thereof; an additional ingredient selected from the group consisting of niacinamide, glycerin and mixtures thereof, and a dermatologically-acceptable carrier.
Owner:THE PROCTER & GAMBLE COMPANY
Radix notoginseng extract and preparation thereof
ActiveCN101732378AHigh purity of ingredientsIncrease concentrationCardiovascular disorderPlant ingredientsGinsenoside RcPanax notoginseng extract
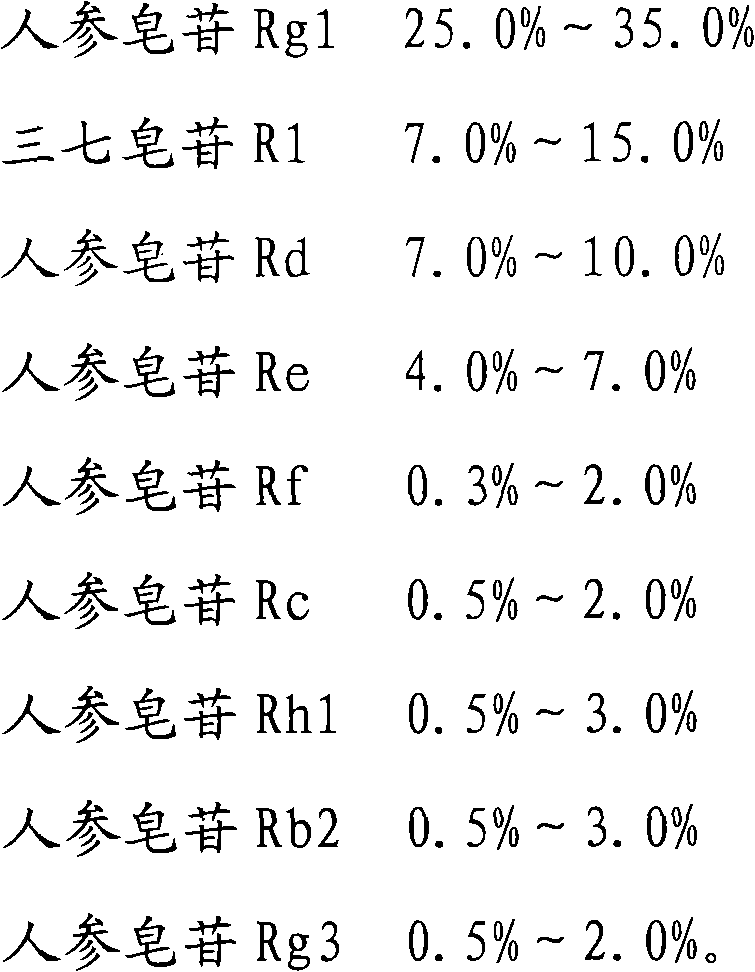
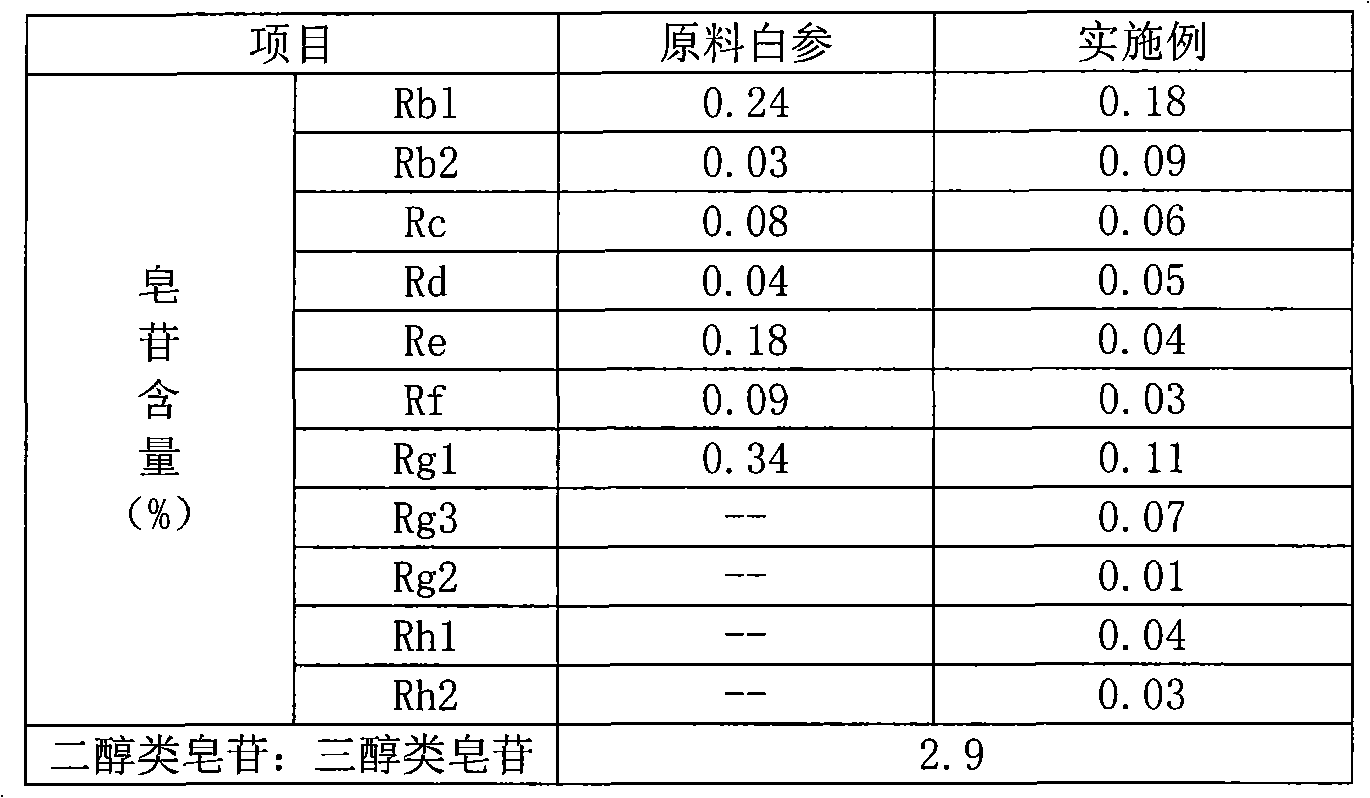
The invention provides a radix notoginseng extract which contains 5-10% of notoginsenoside R1, 25-36% of ginsenoside Rg1, 2.5-5% of ginsenoside Re, 30-39% of ginsenoside Rb1, 5-10% of ginsenoside Rd and at least 2% of ginsenoside Rf, ginsenoside Rh1, ginsenoside Rc, ginsenoside Rb2 and ginsenoside Rg3, wherein the ginsenoside R1, the ginsenoside Rg1, the ginsenoside Re, the ginsenoside Rb1 and the ginsenoside Rd account for 75-95% of the total weight. The invention also provides a preparation method of the radix notoginseng extract. The radix notoginseng extract prepared by the method has little impurities, the purity of the component of total saponin is higher, and especially, the components of the ginsenoside Rf, the ginsenoside Rh1, the ginsenoside Rc, the ginsenoside Rb2, the ginsenoside Rg3 and the like with very low content are purified. The medicinal preparation prepared by the radix notoginseng extract has better curative effect and higher safety.
Owner:HARBIN ZHENBAO PHARMA
Extracting purified ginsenoside from leaves of Panax quinquefolium and ginseng at the same time and the preparing method thereof
InactiveCN101032535ASimple processLow costOrganic active ingredientsSteroidsSide effectGinsenoside Rc
The present invention relates to Chinese medicine and its extracting and processing technology, and is especially the effective part extracted from American ginseng leaf and ginseng leaf and its preparation process. The extracted effective part contains six kinds of ginsenoside substances, including ginsenoside Re, ginsenoside Rb1, ginsenoside Rb2, ginsenoside Rb3, ginsenoside Rc and ginsenoside Rd. Its preparation process includes water extracting American ginseng leaf and ginseng leaf in the weight ratio of 1 to 0.3, macroporous resin adsorption of the water extract liquid, water eluting to eliminate impurity, further eluting with two kinds of elutents, collecting the eluted liquid, and refining the total solid matter to reach purity up to 63.45 %. The extracted effective part has less side effects, and may be used widely in compound medicine preparation and functional food and for separating ginsenoside substance.
Owner:吉林人参研究院
Medicinal composition and preparation method thereof
ActiveCN102028700AIncrease the maximum tolerated doseRaise the median lethal doseOrganic active ingredientsPeptide/protein ingredientsHemolysisAcute toxicity testing
The invention provides a medicinal composition, which comprises the following components: ginsenoside Rb1, ginsenoside Rg1, notoginsenoside R1, ginsenoside Rd, ginsenoside Re, ginsenoside Rf, ginsenoside Rc, ginsenoside Rh1, ginsenoside Rb2 and ginsenoside Rg3. The invention also provides a preparation method for the medicinal composition. The medicinal composition has definite components; compared with the total notoginsenoside in the prior art, the medicinal composition has stable quality and good controllability; and results of experiments of acute toxicity, undue toxicity, hemolysis and the like show that the medicinal composition has higher safety and wide clinical application prospect.
Owner:KPC PHARM INC
Method for extracting and separating total saponins of panax ginseng from american ginseng
ActiveCN102772462AHigh purityHigh yieldNervous disorderAntinoxious agentsAMERICAN GINSENG ROOTGinsenoside Rc
The invention discloses a method for extracting and separating total saponins of panax ginseng from american ginseng. The method employs a series of high-efficiency extraction, separation and purification technical measures of alcohol-backflow extracting, water precipitating, purifying by a macroporous adsorption resin, decolorizing with an ion exchange resin, etc. The obtained total saponins of panax ginseng are white powder and comprise ginsenoside Rg1, ginsenoside Re, ginsenoside Rb1, ginsenoside Rc and ginsenoside Rd, wherein the total purity of ginsenoside Rg1, ginsenoside Re and ginsenoside Rb1 is more than 74.0%. The production process is simple and practical, has no pollution, and is suitable for large-scale production.
Owner:HEBEI YILING MEDICINE INST
Production method of flavor panax ginseng
The invention relates to a preparation method of flavor ginseng; after ginseng is added with nature fruit juice or organic acid for reaction, the content of the precious ginsenoside in products is enhanced, the efficacies of ginseng is improved, while the specific flavor of ginseng is maintained. The main quality characteristics is that: the flavor ginseng includes at least one of the following ingredients: ginsenoside Rg3, ginsenoside Rh1 and ginsenoside 20R-Rh2; wherein, the ratio of the total amount of precious ginsenoside group, ginsenoside Rb1, ginsenoside Rb2, ginsenoside Rd and ginsenoside Rc to the total amount of ginsenoside Re, ginsenoside Rg1 and ginsenoside Rf is more than 2.5; the preparation method can convert part of the component causing internal heat, namely, panaxatriol ginsenoside, into precious ginsenoside.
Owner:JILIN HONGJIU BIO TECH
Method of preparing ginseng saponine monomer from ginseng leaf
A process for preparing ginsenoside monomer from ginseng leaf includes such steps as preparing general ginsenoside from ginseng leaf, chromatography with alumina column to obtain several groups of ginsenosides, and chromatography by column to obtain different ginsenoside monomers.
Owner:HAINAN ASIA PHARM CO LTD
Application of panaxadiol saponins fraction in preparing medicine for preventing dermatitis and scar
ActiveCN102743402AHigh purityStable efficacyOrganic active ingredientsCosmetic preparationsMedicinal herbsGlucocorticoid
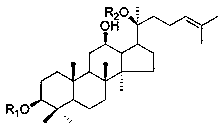
The invention provides application of a panaxadiol saponins fraction in preparing a medicine for preventing dermatitis and scar and a health-care cosmetic. The panaxadiol saponins fraction comprises the following main constituents: ginsenoside Rb1, ginsenoside Rb2, ginsenoside Rb3, ginsenoside Rc and ginsenoside Rd. The medicine or the cosmetic is prepared from active constituents in the single panaxadiol saponins fraction or / and other medicines together and a pharmaceutically acceptable or cosmetic acceptable carrier. The medicine or the cosmetic is prepared by utilizing raw materials: ginseng rhizome medicinal materials, American ginseng rhizome medicinal materials, ginseng stem leaf medicinal materials, American ginseng leaf medicinal materials and total extractives or total saponins of the ginseng rhizome medicinal materials, the American ginseng rhizome medicinal materials, the ginseng stem leaf medicinal materials and American ginseng leaf medicinal materials through a chromatographic separation and purification method combining macroporous resin column chromatography and octadecylsilane chemically bonded silica column chromatography. The scar can be prevented from forming while the tissue regeneration and repair are promoted by the medicine or the cosmetic. Compared with glucocorticoids, cellular immunity is integrally regulated, the drug action is stable after the medicine is suspended, and the drug action advantage is obvious. The medicine or the health-care cosmetic is highly safe. The structural formula of panaxadiol saponins is shown in the specification.
Owner:ZHEJIANG UNIV
Use of ginsenosides Rh2, Ck and Rg3 in increasing myocardial contractility
InactiveCN1879640AReduced or disappeared ralesReduce edemaOrganic active ingredientsPill deliveryVeinGinsenoside Rc
The invention relates to the application of panaxoside Rh2, Ck and Rg3 to increase the heart contraction force, wherein the medicine compound comprises active panaxoside Rh2 and / or Ck and / or Rg3 and medicine carrier, to be made into injection, oral agent and film agent, to cure acute and low heart cardiac failure or reduced heart contraction force.
Owner:彭康康
Extracting method of ginsenoside
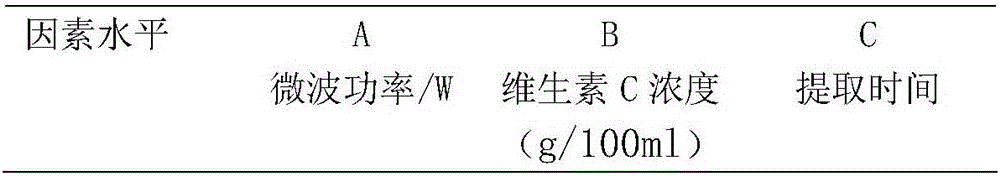
ActiveCN105663195ASimple and fast operationReduce manufacturing costOrganic active ingredientsNervous disorderAlcoholVitamin C
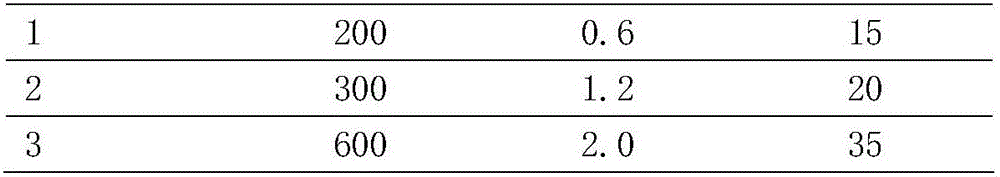
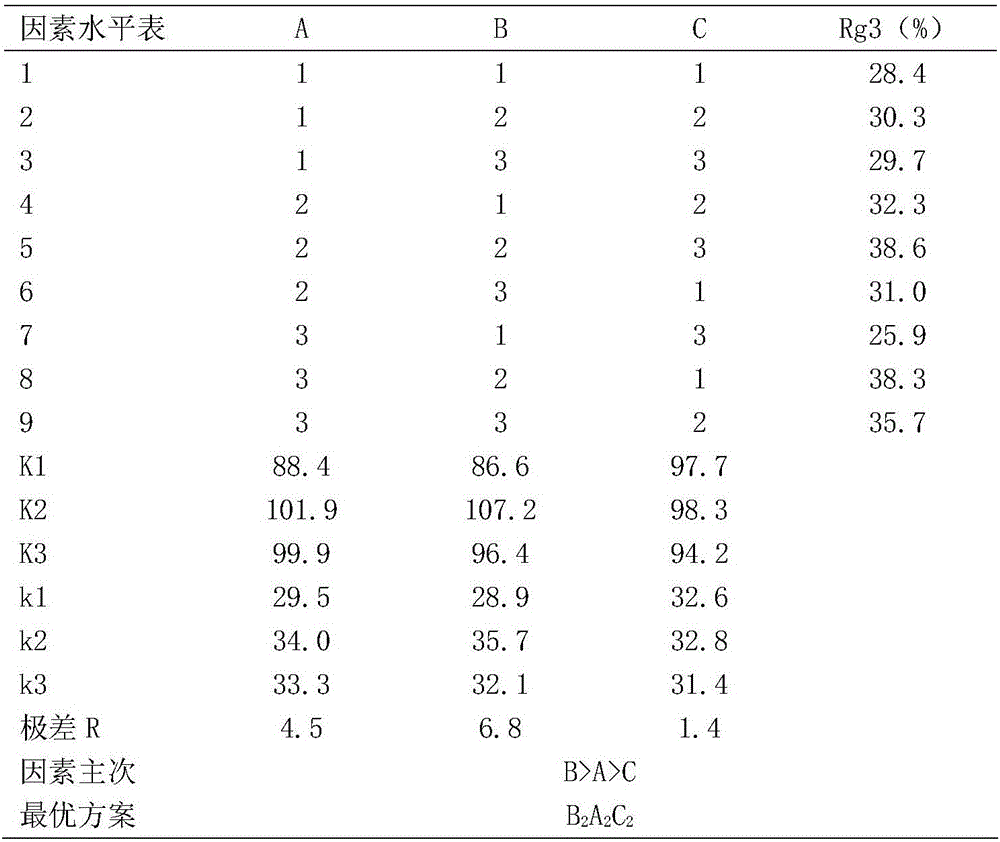
The invention relates to total ginsenoside rich in ginsenoside Rg3 and an extracting method thereof. The extract comprises, by mass, 1.0-3.0% of ginsenoside Rb1, 0.2-1.0% of ginsenoside Rb2, 0.5-2.5% of ginsenoside Rb3, 0.5-2.5% of ginsenoside RC and 28-40% of ginsenoside Rg3. The extracting method comprises the following steps that ethyl alcohol is used as an extracting solvent for extracting ginseng through a microwave extracting method, a certain amount of vitamin C is added into the extracting solution, a crude extracting solution of the total ginsenoside is extracted through microwave heating within a certain period of time, and the crude extracting solution of the total ginsenoside is further enriched and purified through a macroporous resin adsorption process to obtain the extract of total ginsenoside.
Owner:江西樟树市庆仁保健品有限公司
Method for producing ginseng fruit and ginseng flower stalk with high content of ginsenoside
InactiveCN101181328AIncrease incomeEnergy modified materialsPlant ingredientsGinsenoside RcAdditive ingredient
Disclosed is a method for producing ginseng fruits and flower stalks with high content of ginsenoside. More particularly, the present invention provides a method for producing ginseng fruits and flower stalks with high contents of ginsenoside Rb1, ginsenoside Rb2, ginsenoside Rc, ginsenoside Rd, ginsenoside Re and other ginsenoside ingredients. According to the present invention, the ginseng fruits and flower stalks have considerably increased contents of ginsenoside Re, Rd and Rb2 compared with general red ginseng roots and / or black ginseng roots. In addition, the present invention can provide ginseng fruits and flower stalks based products with high contents of, especially, ginsenoside Rb1, ginsenoside Rb2, ginsenoside Rc, ginsenoside Rd and / or ginsenoside Re by processing the fruits and flower stalks.
Owner:成仁焕
Panax notoginseng saponin composition and preparation method and application thereof
ActiveCN105816471AClear validityClear contentOrganic active ingredientsBlood disorderGinsenoside RdLow toxicity
The invention provides a panax notoginseng saponin composition and a preparation method and application thereof.The composition is prepared from, by weight, 30-50% of ginsenoside Rg1, 25-40% of ginsenoside Rb1, 7-16% of notoginsenoside R1, 2.7-8% of ginsenoside Re, 0.5-7.0% of ginsenoside Rd, 0.5-2% of ginsenoside Rf, 0.3-2.0% of ginsenoside Rh2, 1-3% of ginsenoside Rc, 0.3-2.5% of ginsenoside Rb3 and 0.5-2.5% of ginsenoside Rg3, wherein ginsenoside Rg1, ginsenoside Rb1 and notoginsenoside R1 account for 70-95% of the total weight of the active components, and the sum of the contents of ginsenoside Rb1 and notoginsenoside R1 is not smaller than 8 times of the content of ginsenoside Rd.Compared with commercially available Xueshuantong injections and Xueshuantong preparations, the panax notoginseng saponin composition has a more obvious arterial and venous thrombosis resistant effect, low toxicity and high safety.
Owner:HARBIN ZHENBAO PHARMA
Method for measuring content of Shenqi blood sugar reducing preparation and application thereof in overall quality control
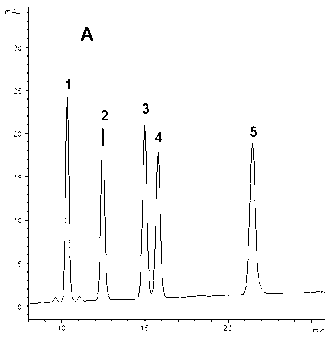
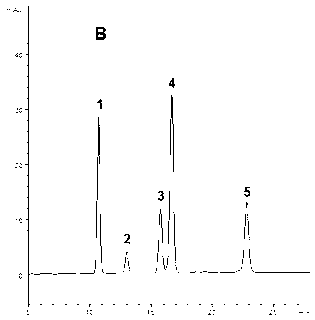
The invention provides a method for measuring the content of a Shenqi blood sugar reducing preparation. The method is used for measuring the content of 10 active ingredients in the Shenqi blood sugar reducing preparation: ginsenoside Rb1, ginsenoside Rc, ginsenoside Rd, ginsenoside Rg1, ginsenoside Re, astragaloside, deoxyschizandrin, schisandrol A, schisandrol B. In the invention, with the guidance of blood sugar reducing activity of a compound, multiple active substances in the compound are detected, and the quality level of the whole compound preparation can be reflected better; meanwhile, a Q-MS ion monitoring mode is selected for content measurement, and the mass spectrum quantification has high accuracy, specificity and sensitivity and is superior to traditional HPLC analysis method; and moreover, fingerprint is established according to the liquid phase diagrams of different batches of Shenqi blood sugar reducing preparations obtained by the method, the similarity is evaluated, the inherent quality of the Shenqi blood sugar reducing preparation product is evaluated more scientifically and comprehensively, and a basis is provided for establishing a quality standard of higher level.
Owner:ZHEJIANG UNIV OF TECH
Application of panaxadiol saponins fraction in preparing medicine for preventing epilepsia
ActiveCN102743401AOvercome curative effectOvercome securityOrganic active ingredientsNervous disorderMedicinal herbsAntiepileptic Agents
The invention provides application of a panaxadiol saponins fraction in preparing a medicine for preventing epilepsia. The component comprises the following main constituents: ginsenoside Rb1, ginsenoside Rb2, ginsenoside Rb3, ginsenoside Rc and ginsenoside Rd, and the medicine is prepared from active constituents in the single panaxadiol saponins fraction or from the active constituents in the single panaxadiol saponins fraction and other medicines. By taking one of five panaxadiol saponins: ginseng, American ginseng rhizome medicinal materials, ginseng stems and leaves, American ginseng stem and leaf medicinal materials and total extractives or total saponins of the ginseng, the American ginseng rhizome medicinal materials, the ginseng stems and leaves and American ginseng stem and leaf medicinal materials as a starting raw material independently or taking a mixture which consists of two or more than two different raw materials as the starting raw material comprehensively, the medicine is prepared by establishing the chromatographic separation and purification technology. According to the medicine prepared from the panaxadiol saponins fraction provided by the invention, an anti-epileptic effect can be greatly improved, and moreover, the toxic and side effects, especially dermatitis and intelligence toxicity, of traditional anti-epileptic medicines can be resisted, therefore, long-standing defects that the anti-epileptic medicines are not enough in curative effect and low in safety are overcome. The structural formula of the panaxadiol saponin is shown in specification.
Owner:ZHEJIANG UNIV
Separation method of monoterpene and saponins components in traditional Chinese medicine composition vegetable drug midbody
ActiveCN104072550AGuaranteed curative effectQuality assuranceSugar derivativesSteroidsKetoneMethyl palmoxirate
The invention discloses a separation method of monoterpene and saponins components in a traditional Chinese medicine composition vegetable drug midbody. The method comprises the following steps of: extracting and purifying by macroporous adsorption resin to obtain 30 percent and 70 percent of ethanol elution parts, separating the 30 percent of ethanol elution part to obtain a white paeony root R1 pure product and a paeoniflorin pure product; separating the 70 percent of ethanol elution part to obtain a paeoniflorin lactone pure product, a peony ketone glycosides pure product, a benzoyl paeoniflorin pure product, a gallate paeoniflorin pure product, a methyl paeoniflorin pure product, a ginsenoside Rg1 pure product, a ginsenoside Rc pure product, a ginsenoside Rb1 pure product, a ginsenoside Rb2 pure product and the like. According to the separated effective component, the administration dosage of the drugs can be shortened, and the quality of the drugs can also be guaranteed.
Owner:HEBEI YILING MEDICINE INST
Method for preparing rare ginsenoside CK through gene combination transformation and application
PendingCN112481280AStrong specificityHigh catalytic efficiencyBacteriaAntibody mimetics/scaffoldsGinsenoside CKGinsenoside Rc
The invention discloses a method for preparing rare ginsenoside CK through gene combination transformation and application of the rare ginsenoside CK. According to the invention, glycosidase generatedby using genes of bacillus subtilis str.168 and bifidobacterium breve 689b can be used, so that the ginsenoside Rc can be converted into the rare ginsenoside CK efficiently in vitro.
Owner:HUNAN INSTITUTE OF ENGINEERING
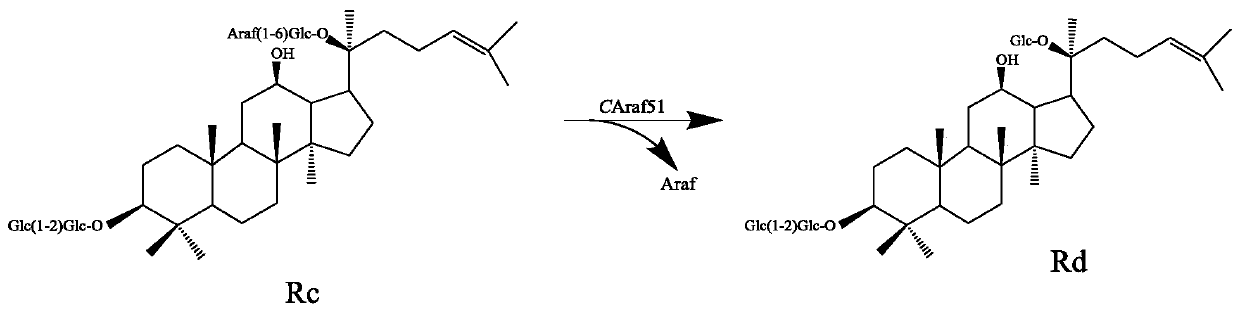
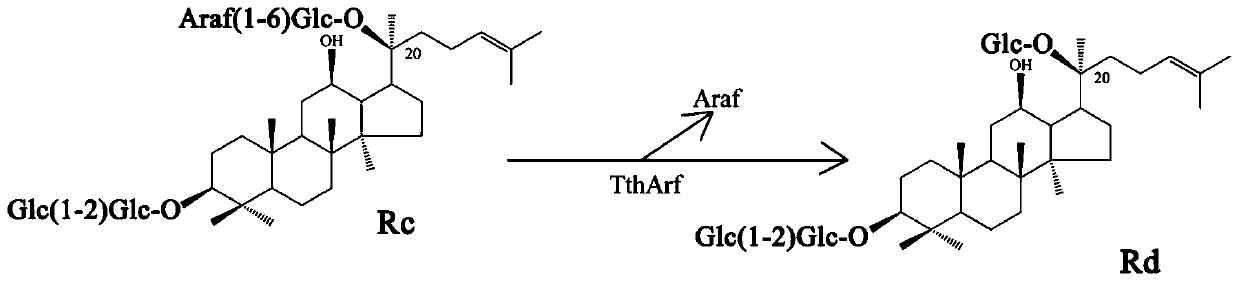
Alpha-L-arabinfuranosidease and coding gene and application thereof
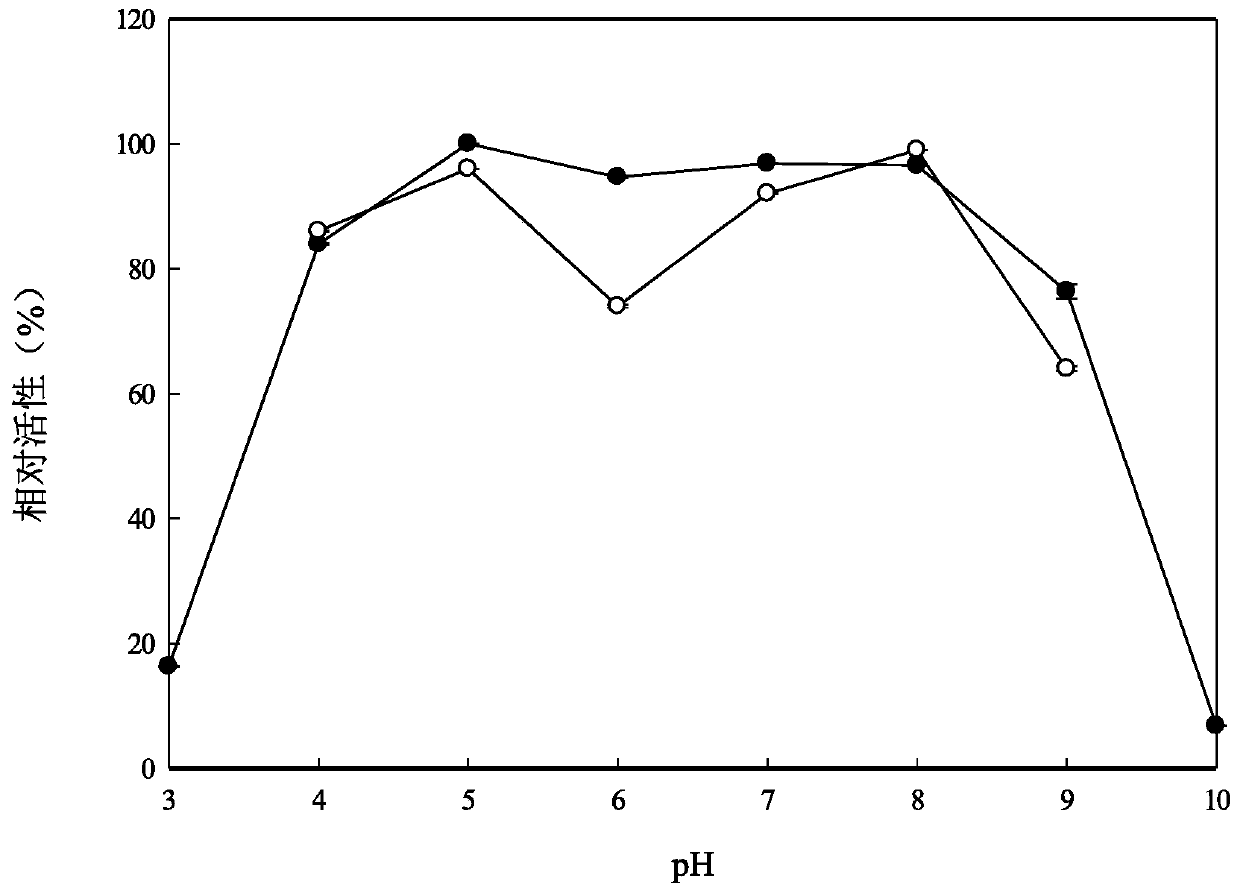
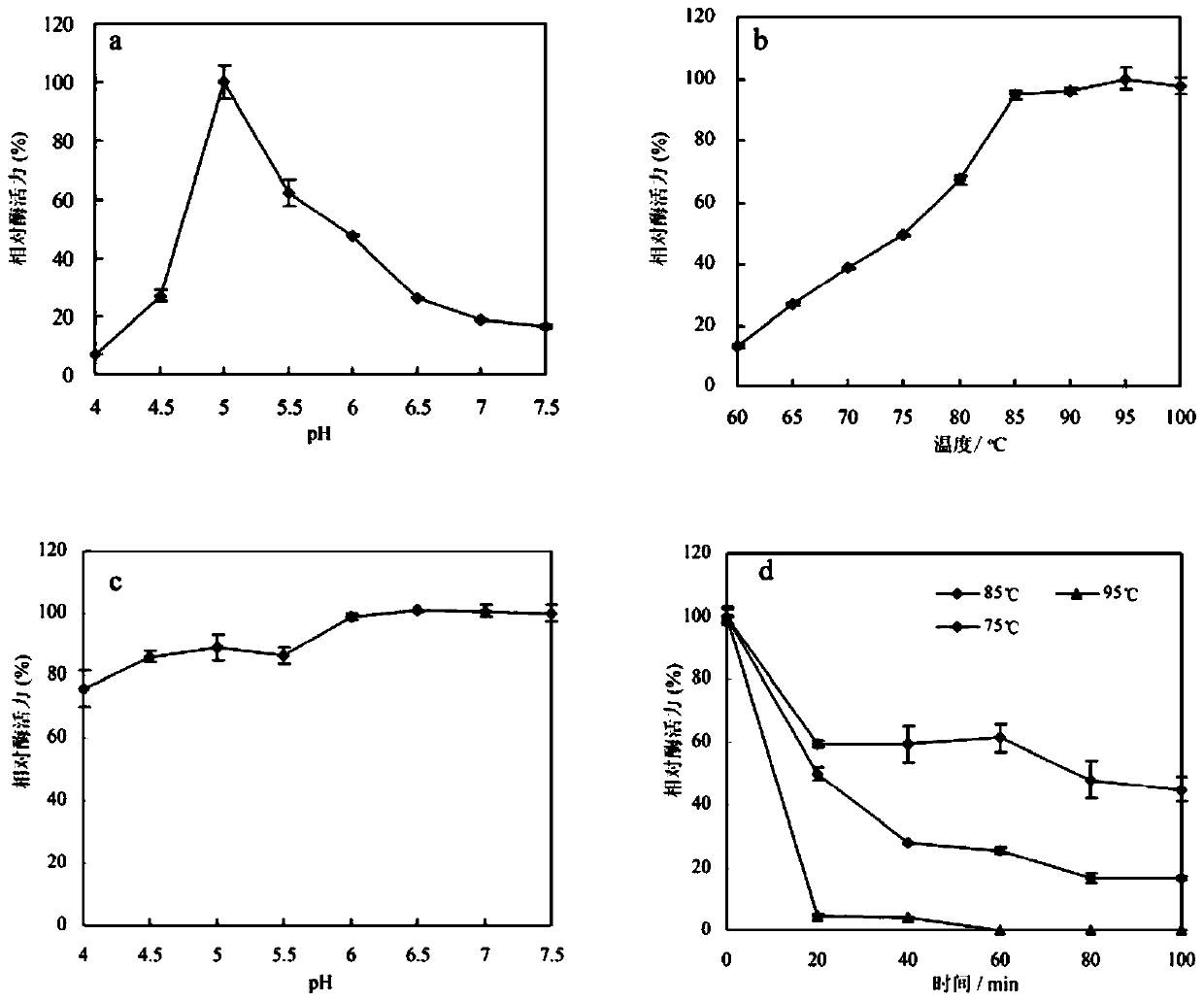
ActiveCN110157696AImprove toleranceIncrease enzyme activityBacteriaMicroorganism based processesBiotechnologyGinsenoside Rc
The invention relates to the fields of genetic engineering technology and biomedicine, discloses alpha-L-arabinfuranosidease and a coding gene and application thereof, and specifically provides the alpha-L-arabinfuranosidease and the coding gene thereof, a recombinant vector, an expression cassette and a transgenic cell line or a recombinant strain which contain the coding gene and application ofthe alpha-L-arabinfuranosidease in preparing ginsenoside Rd. The alpha-L-arabinfuranosidease can more efficiently catalyze the reaction of ginsenoside Rc to prepare the ginsenoside Rd, and the enzymeactivity is higher; the higher activity can be exhibited both in a wider range of pH and temperature, and the stability is strong; the alpha-L-arabinfuranosidease has higher tolerance against arabinose and glucose, and facilitates the synergistic effect with other ginsenoside hydrolases.
Owner:KUNMING NOVOGINSENG BIOENG
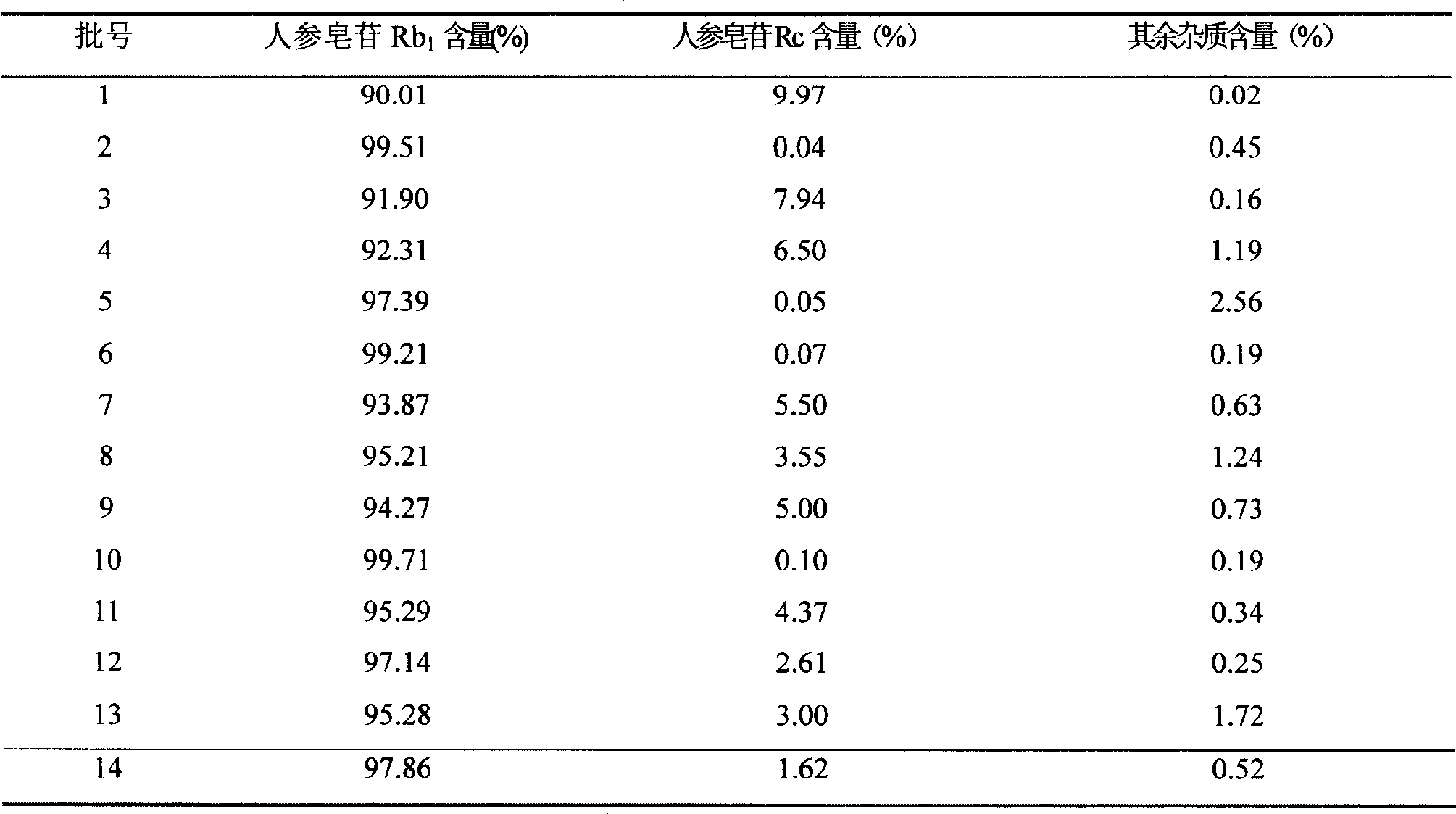
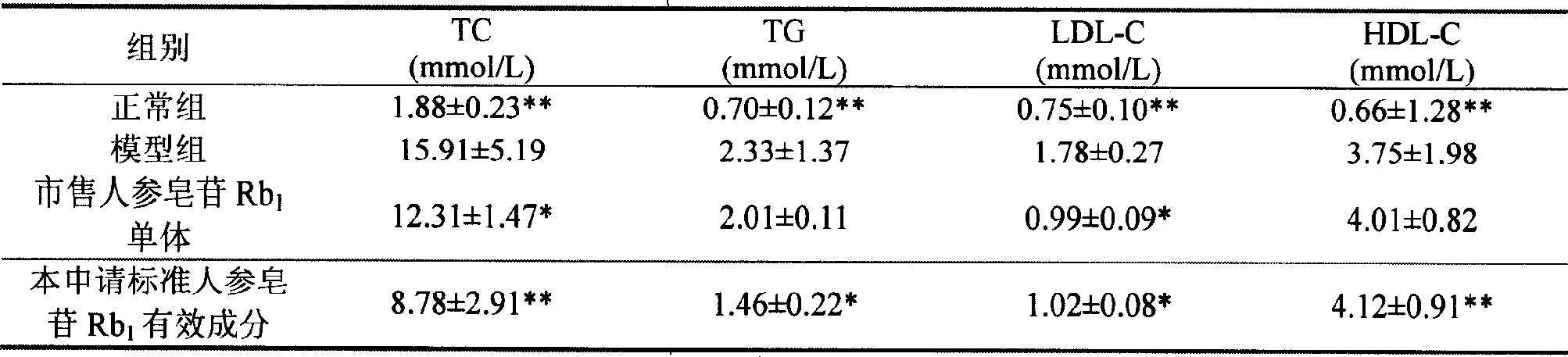
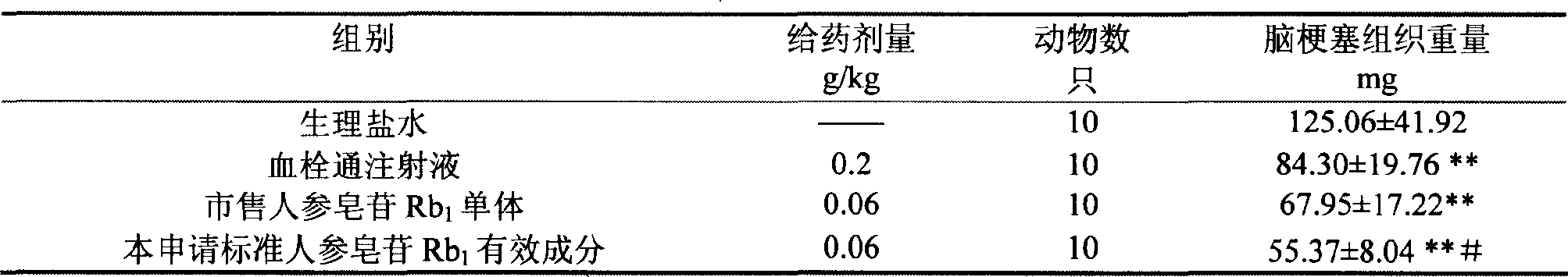
Ginsenoside Rb1 containing impurity ginsenoside Rc
The invention discloses a standard ginsenoside Rb1 active ingredient, containing ginsenoside Rb1 with the content more than or equal to 90% and less than 100%, is also characterized in that the active ingredient contains ginsenoside Rc with the content more than 0% and less than or equal to 10%; or the content of impurity ginsenoside Rc is more than or equal to 0.05% and less than or equal to 6.5%; or the content of ginsenoside Rc is more than or equal to 0.1% and less than or equal to 5.0%; the content of impurity ginsenoside Rc is more than or equal to 0.1% and less than or equal to 3.0%; Pharmacological experiments show that the standard active ingredient of the application has good pharmacological effects and can be prepared into pharmaceutical preparations in Pharmacy.
Owner:PHARMA RES INST OF BENCAO TIANYUAN OF BEIJING
Panax notoginseng flower total saponin, and preparation method and application thereof
InactiveCN110898090AImprove the yield of active ingredientsEasy to operateOrganic active ingredientsNervous disorderPANAX NOTOGINSENG ROOTGinsenoside Rc
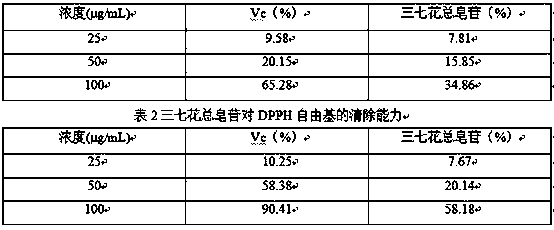
The invention discloses a Panax notoginseng flower total saponin, and a preparation method and an application thereof, and belongs to the field of biological medicines. The Panax notoginseng flower total saponin contains notoginsenoside Fc, ginsenoside Rc, ginsenoside Rb2, ginsenoside Rb3 and ginsenoside Rd in any proportion, and is prepared from Panax notoginseng flowers through the processes ofultrahigh-pressure extraction, filtration, concentration, drying and the like. The preparation method is carried out at normal temperature, is simple to operate and has a high efficiency, and the obtained Panax notoginseng flower total saponin has strong hydroxyl radical and DPPH free radical scavenging capacity, and can be used for preparing antioxidant drugs.
Owner:KUNMING UNIV OF SCI & TECH
Method for producing ginseng fruit and ginseng flower stalk with high content of ginsenoside
Disclosed is a method for producing ginseng fruits and flower stalks with high content of ginsenoside. More particularly, the present invention provides a method for producing ginseng fruits and flower stalks with high contents of ginsenoside Rb1, ginsenoside Rb2, ginsenoside Rc, ginsenoside Rd, ginsenoside Re and other ginsenoside ingredients. According to the present invention, the ginseng fruits and flower stalks have considerably increased contents of ginsenoside Re, Rd and Rb2 compared with general red ginseng roots and / or black ginseng roots. In addition, the present invention can provide ginseng fruits and flower stalks based products with high contents of, especially, ginsenoside Rb1, ginsenoside Rb2, ginsenoside Rc, ginsenoside Rd and / or ginsenoside Re by processing the fruits and flower stalks.
Owner:SEONG IN HWAN
Ginsenoside-ophiopogonin D composition for treating cardiovascular and cerebrovascular diseases
ActiveCN103393708AQuality is easy to controlDrug effect is not easy to controlOrganic active ingredientsBlood disorderDiseaseReperfusion injury
A ginsenoside-ophiopogonin D composition for treating cardiovascular and cerebrovascular diseases comprises the components by weight percent: 28-32% of ginsenoside Rb1, 18-22% of ginsenoside Rg1, 14-16% of ginsenoside Rb2, 14-16% of ginsenoside Rc, 8-12% of ginsenoside Re, and 14-16% of ophiopogonin D. The advantages are that: the provided ginsenoside-ophiopogonin D composition has fixed and scientific components, controllable and stable quality, substantial drug effect and higher security, same or slightly better drug effects on (myocardial, cerebral and lung) ischemia-reperfusion injury resistance, thrombosis resistance, arteriosclerosis resistance, arrhythmia resistance and the like than shenmai injection, and more safety on acute toxicity, abnormal toxicity, hemolysis, coacervation and the like than shenmai injection. The ginsenoside-ophiopogonin D composition has the advantages of clear and fixed components, more stable and controllable quality, substantial treatment effect and higher security.
Owner:DALI PHARMA
Method for preparing extract fraction reinforced with ginsenosides Rg1 or Rb1 from ginseng
The present invention provides a method for preparing an extract fraction reinforced with ginsenoside Rg1 or Rb1 from ginseng. The method for preparing an extract fraction reinforced with ginsenoside Rg1 comprises the steps of: concentrating a spirit extract of ginseng and then adsorbing the extract diluted in water by adding to an adsorption resin; passing distilled water through the adsorption resin, then eluting and removing unadsorbed ingredients; and adding 30-40 v / v% spirits to the adsorption resin to obtain an eluate. The method for preparing an extract fraction reinforced with ginsenoside Rb1 comprises the steps of: concentrating a spirit extract of ginseng and then adsorbing the extract diluted in water by adding to an adsorption resin; passing distilled water through the adsorption resin, then eluting and removing unadsorbed ingredients; and adding 50-80 v / v% spirits to the adsorption resin and then eluting.
Owner:CJ WELLCARE CORPORATION
Ginsenoside cyclodextrin inclusion compound and preparation method thereof
InactiveCN105770908AEasy to prepareMild conditionsOrganic active ingredientsNervous disorderGinsenoside RcEther
The invention discloses a ginsenoside cyclodextrin inclusion compound and a preparation method thereof.The compound comprises ginsenoside and cyclodextrin, wherein the molar ratio of ginsenoside to cyclodextrin is (1:2)-(1:20), the ginsenoside is ginsenoside F1 or ginsenoside Re or ginsenoside Rd or ginsenoside Rb2 or ginsenoside Rc, and the cyclodextrin is hydroxypropyl-beta-cyclodextrin or sulfobutyl ether-beta-cyclodextrin; the compound is prepared through a saturated solution method or an ultrasonic method; after ginsenoside and cyclodextrin form the compound, the solubility of the compound in water is greatly improved. The preparation method is simple and convenient to implement, mild in condition, easy to implement and capable of being used for developing novel prepartions of ginsenoside.
Owner:KUNMING UNIV OF SCI & TECH
Pharmaceutical composition for treating cardiovascular disease and preparation method thereof
ActiveCN103156870AImprove protectionSignificant effectAnhydride/acid/halide active ingredientsCardiovascular disorderLactate dehydrogenaseCreatine kinase
The invention belongs to a field of medicament preparation, and relates to a pharmaceutical composition for treating cardiovascular disease and a preparation method thereof, especially to the pharmaceutical composition which contains drug-efficacy component groups capable of being absorbed into blood. The drug-efficacy component groups comprise: Japanese bufotaline, ginsenoside Re, ginsenoside Ra1, 1[beta]-hydroxy bufalin, ginsenoside Rb1, resibufogenin fine alcohol, ginsenoside Rc, ginsenoside Rb2, ginsenoside, ginsenoside Rd, bufalin, cholic acid, hyodeoxy cholic acid, cinobufagin, chenodeoxycholic acid and deoxycholic acid. The medicament has clear in vivo targets, has characteristics and advantages of multi-component multi-target effects, is good in protective effects for acute myocardial ischemia, and can reduce area of myocardial infarction and content of Lactate dehydrogenase and creatine kinase in serum.
Owner:HEHUANG PHARMA SHANGHAI +1
Xuesaitong pharmaceutical composition and preparation method, preparation and application thereof
ActiveCN108434166AReduce myocardial infarct sizeImprove heart indexOrganic active ingredientsSenses disorderDiseaseTreatment effect
The invention discloses a Xuesaitong pharmaceutical composition and a preparation method, a preparation and application thereof. The Xuesaitong pharmaceutical composition is prepared from the following components in percentage by mass: 0.001 to 99.996% of ginsenoside Rb2, 0.001 to 99.996% of ginsenoside Rb1, 0.001 to 99.996% of ginsenoside Rg1, 0.001 to 99.996% of ginsenoside Rc, and 0.001 to 99.996% of notoginsenoside R1. The Xuesaitong pharmaceutical composition and the preparation have the advantages that the obvious treatment effect on ischemic cardiovascular and cerebrovascular diseases of myocardial infarction, cerebral apoplexy, cerebral infarction, brain hemiplegia and the like, and the retinopathy or optic nerve injury disease due to ischemic cardiovascular and cerebrovascular diseases, diabetes and the like is realized, and the application prospect is broad; compared with the prior art and the product, the treatment effect is better, the safety is higher, and the requirementof clinical medicine usage can be well met; the technology of the preparation method is simple, the conditions are mild, the operation is convenient, the quality is stable and controllable, and the preparation method is suitable for industrialized large-scale production.
Owner:KPC PHARM INC
Method for determining content of ginsenoside in panax traditional Chinese medicine
ActiveCN113433232AHigh sensitivityAccurate quality controlComponent separationBiotechnologyPseudoginsenoside F11
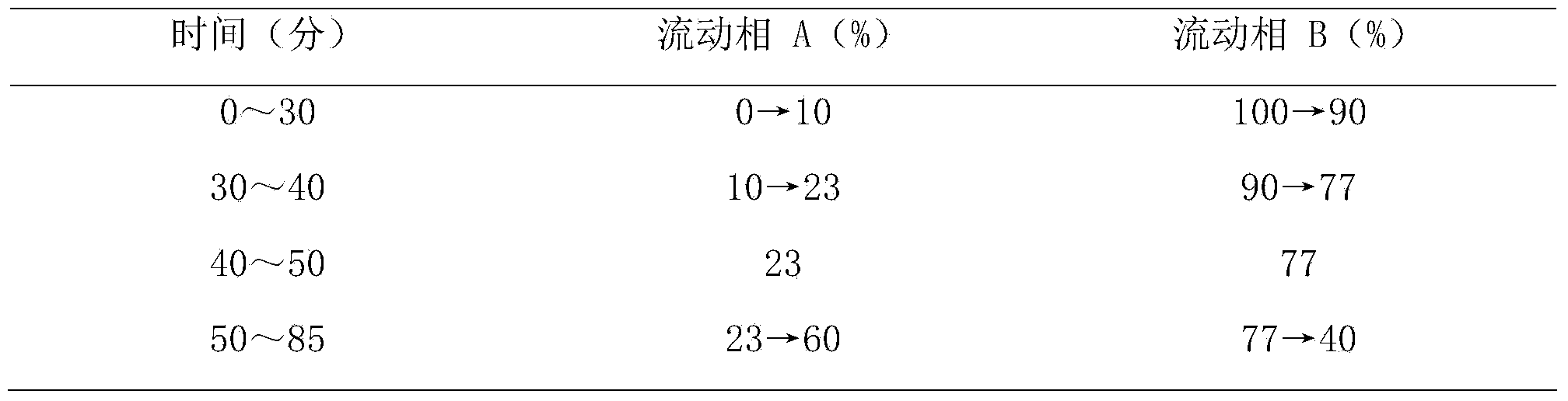
The invention provides a method for determining the content of ginsenoside in a panax traditional Chinese medicine, which adopts an ultra-high performance liquid chromatography electrospray detector method to simultaneously determine the content of 15 kinds of ginsenoside in the panax traditional Chinese medicine. The 15 kinds of ginsenoside comprise notoginsenoside R1, ginsenoside Rg1, ginsenoside Re, 24 (R)-pseudoginsenoside F11, ginsenoside Rf, ginsenoside Ra2, ginsenoside Rb1, ginsenoside Rc, ginsenoside Ro, ginsenoside Rb2, ginsenoside Rb3, panax japonicus saponin IV, ginsenoside Rd, panax japonicus saponin IVa and 20 (R)-ginsenoside Rg3. By adopting the method disclosed by the invention, the contents of 15 kinds of ginsenoside in various panax traditional Chinese medicines can be simultaneously determined by reasonably selecting chromatographic conditions and detection conditions, and the method has the advantages of simplicity, convenience, accuracy, high sensitivity, strong specificity and the like, so that the method can be credibly, comprehensively and accurately used for quality control of the panax traditional Chinese medicines.
Owner:TIANJIN UNIV OF TRADITIONAL CHINESE MEDICINE
Pseudo-ginseng extract product, and applications thereof in preparation of cosmetics
InactiveCN108992379AReduce dosageHigh purityCosmetic preparationsAntipyreticEvaporationGINSENG EXTRACT
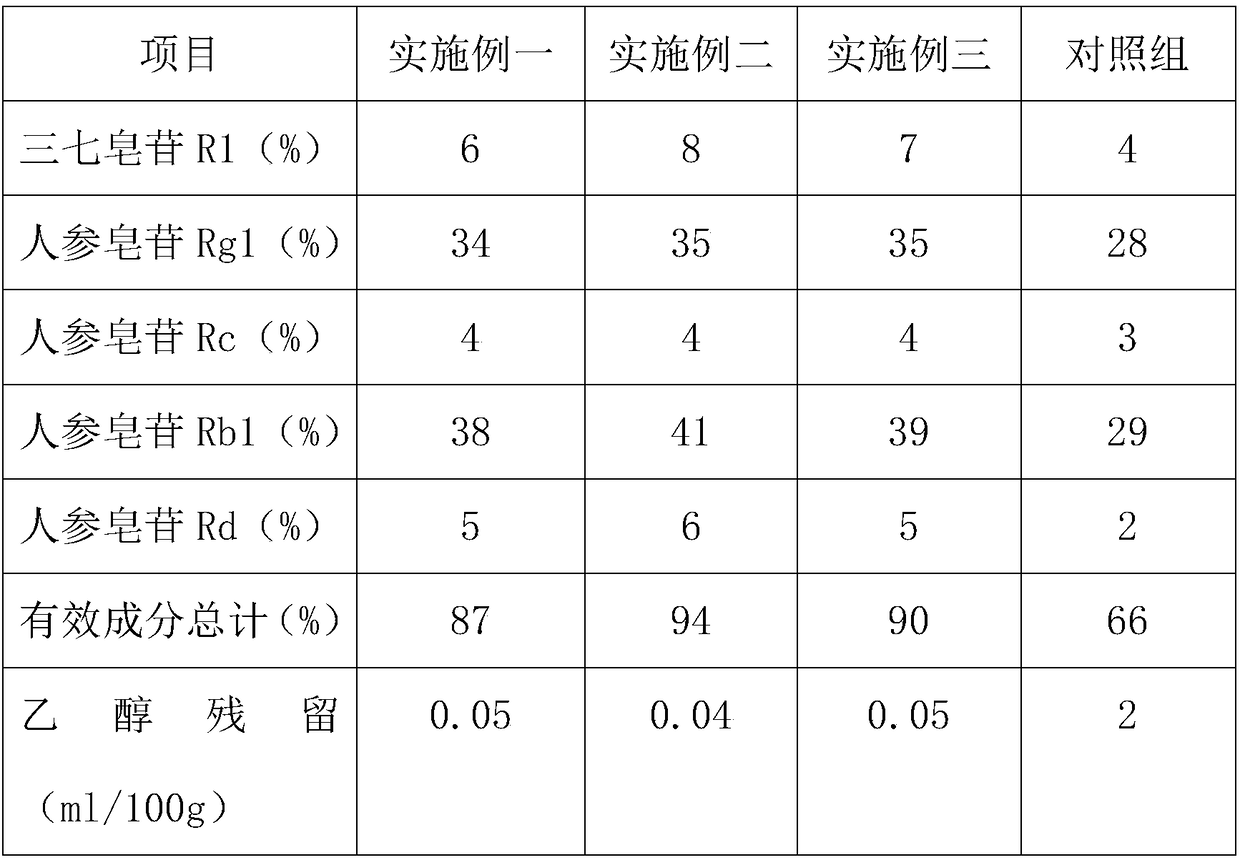
The invention discloses a pseudo-ginseng extract product, and applications thereof in preparation of cosmetics. The pseudo-ginseng extract product comprises 6 to 8% of notoginsenoside R1, 33 to 36% ofginsenoside Rg1, 3 to 5% of ginsenoside Rc, 36 to 41% of ginsenoside Rb1, and 4 to 7% of ginsenoside Rd. A preparation method of the pseudo-ginseng extract product comprises following steps: 1, freshpseudo-ginseng raw material is washed, and the washed pseudo-ginseng raw maerial and purified water are introduced into a pulverizer for crushing until a slurry mixture, which is a pseudo-ginseng slurry, is obtained; 2, the pseudo-ginseng slurry is introduced into an extraction vessel for supercritical fluid extraction so as to obtain a crude pseudo-ginseng extract product; and 3, the crude pseudo-ginseng extract product is heated with stirring, evaporation is adopted to remove a residual entrainer so as to obtain the pseudo-ginseng extract product. According to the preparation method, supercritical fluid extraction technology is adopted for pseudo-ginseng extraction, so that the using amount of organic solvents such as ethanol is reduced greatly, the purity of the pseudo-ginseng extractproduct is higher, and it is more safe for the pseudo-ginseng extract product to be used in cosmetics.
Owner:GUANGZHOU JINSME FINE CHEM CO LTD
Application of ginsenoside Rc in preparation of medicine for treating alcoholic fatty liver
PendingCN114533749AAvoid damageInhibits oxidative stressOrganic active ingredientsDigestive systemGinsenoside RcOxidative stress
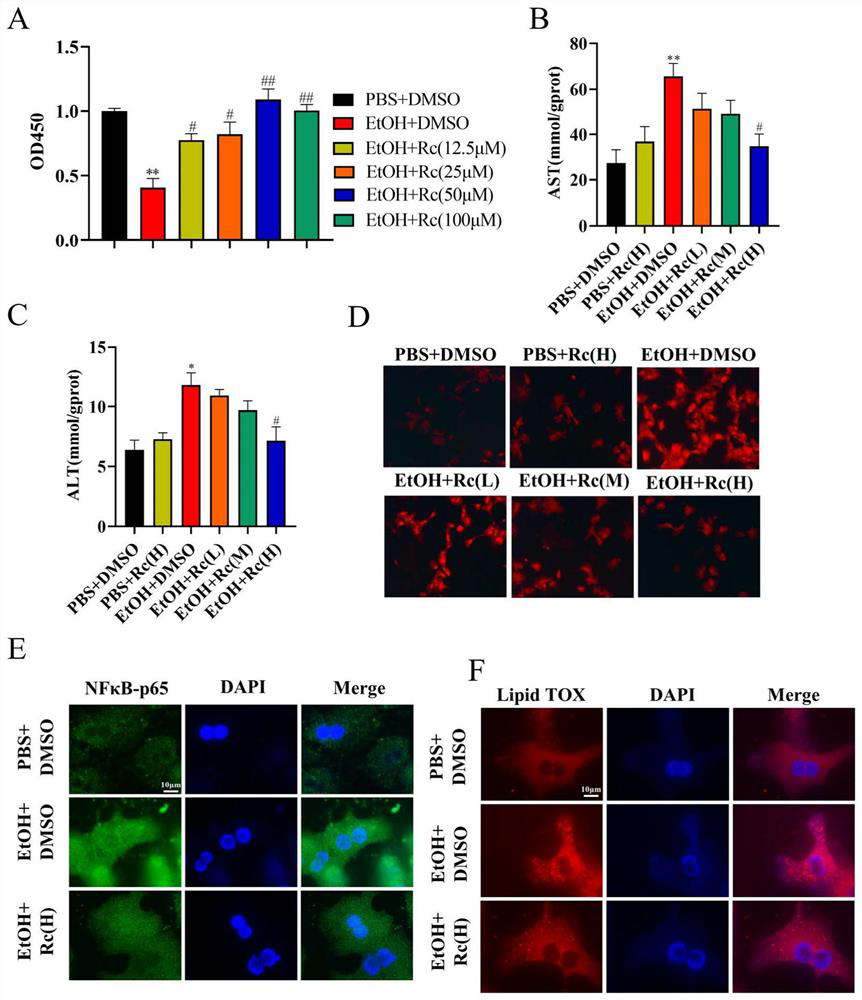
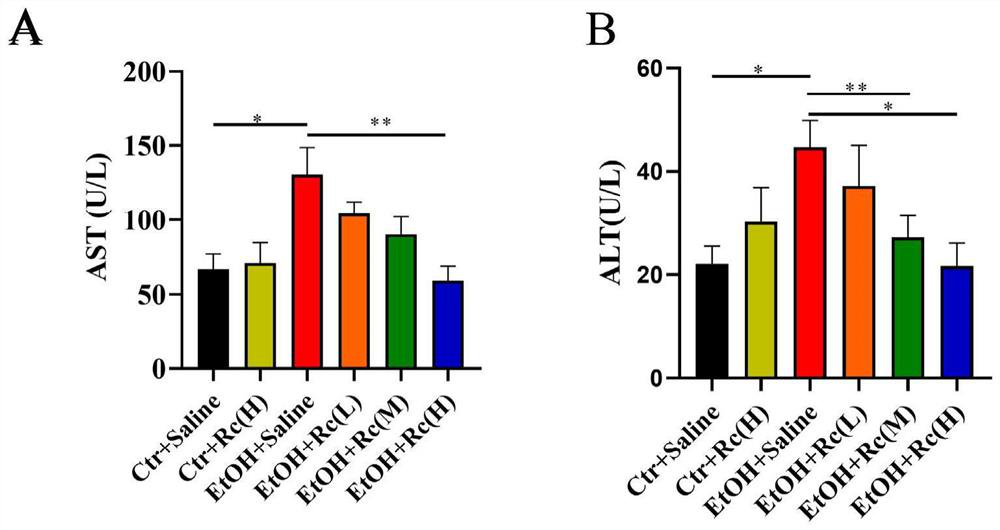
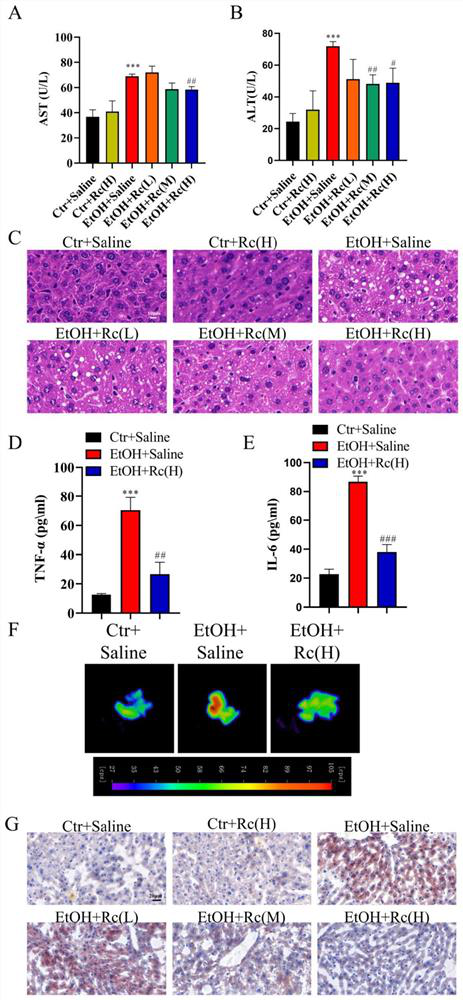
The invention provides application of ginsenoside Rc in preparation of a medicine for treating alcoholic fatty liver. Researches show that ginsenoside Rc can effectively inhibit liver injury, lipid accumulation, oxidative stress reaction and inflammatory reaction, so that acute / chronic alcoholic fatty liver is effectively treated, and a new material source is provided for the medicine for treating the alcoholic fatty liver.
Owner:GUANGZHOU UNIVERSITY OF CHINESE MEDICINE
α-l-arabinofuranosidase and its application in the preparation of ginsenoside rd
ActiveCN104651336BImprove conversion abilityImprove toleranceFermentationGlycosylasesGinsenoside RcD-Glucose
The invention discloses alpha-L-arabinofuranosidase and application thereof in preparing ginsenoside Rd, and belongs to the field of gene engineering techniques and biological medicines. The amino acid sequence of alpha-L-arabinofuranosidase is as shown in SEQ ID NO.1. The alpha-L-arabinofuranosidase disclosed by the invention is high in conversion property for ginsenoside Rd, and after alpha-L-arabinofuranosidase and ginsenoside Rd are cultured for a certain time, the detection shows that ginsenoside Rc is nearly completely converted into ginsenoside Rd. Alpha-L-arabinofuranosidase is relatively high in resistance to arabinose and is not inhibited by glucose feedback.
Owner:NANJING FORESTRY UNIV
A kind of notoginseng total saponins composition and its preparation method and application
ActiveCN105816471BClear contentImprove effectivenessOrganic active ingredientsBlood disorderLow toxicityPANAX NOTOGINSENG ROOT
The invention provides a panax notoginseng total saponin composition and a preparation method and application thereof. The Panax notoginseng saponin composition provided by the invention contains 30-50% of ginsenoside Rg1, 25-40% of ginsenoside Rb, 7-16% of notoginsenoside R1, 2.7-8% of ginsenoside Re, Saponin Rd 0.5-7.0%, ginsenoside Rf 0.5-2%, ginsenoside Rh2 0.3-2.0, ginsenoside Rc 1-3, ginsenoside Rb3 0.3-2.5 and ginsenoside Rg 30.5-2.5%; Ginsenoside Rb1 and Panax notoginsenoside R1 account for 70-95% of the total weight of the active ingredients, and the sum of the content of ginsenoside Rb1 and notoginsenoside R1 is not less than 8 times the content of ginsenoside Rd. Compared with the commercially available Xueshuantong and Xuesaitong preparations, the Panax notoginseng saponin composition provided by the invention has more obvious anti-arteriovenous thrombosis effect and low toxicity and safety.
Owner:HARBIN ZHENBAO PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com