Pharmaceutical composition for treating cardiovascular disease and preparation method thereof
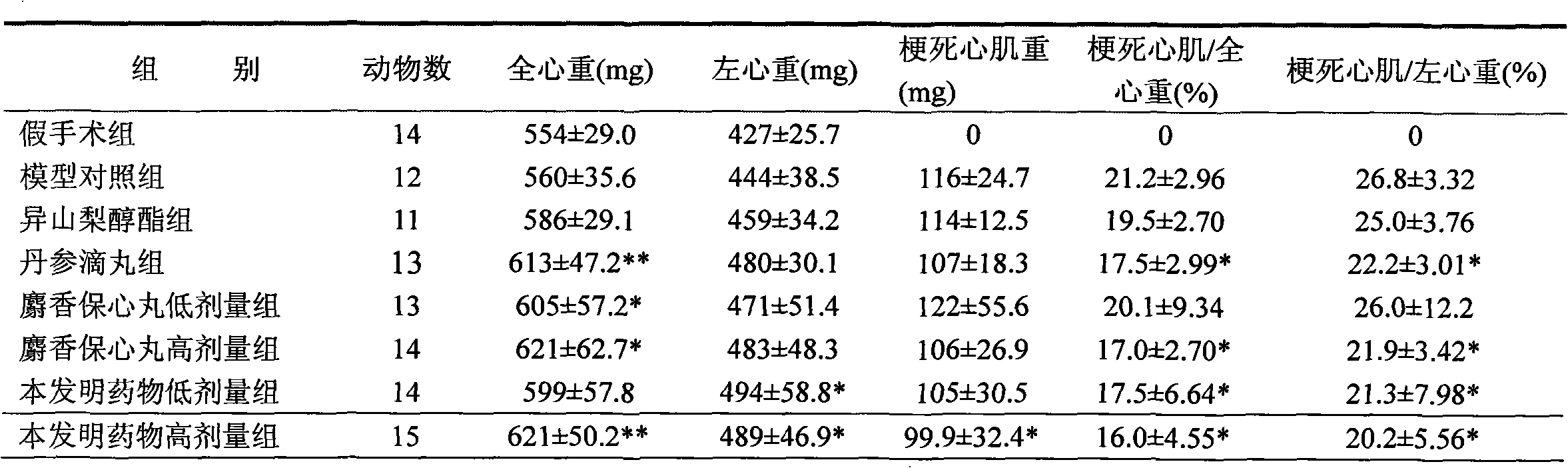
A composition and cardiovascular technology are applied in the field of pharmaceutical compositions for the treatment of cardiovascular diseases, pharmaceutical compositions for the treatment of cardiovascular diseases and their preparation fields, which can solve the problems of complex components of the compound formula, and achieve the reduction of myocardial infarction, the reduction of content, The effect of reducing myocardial infarction size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
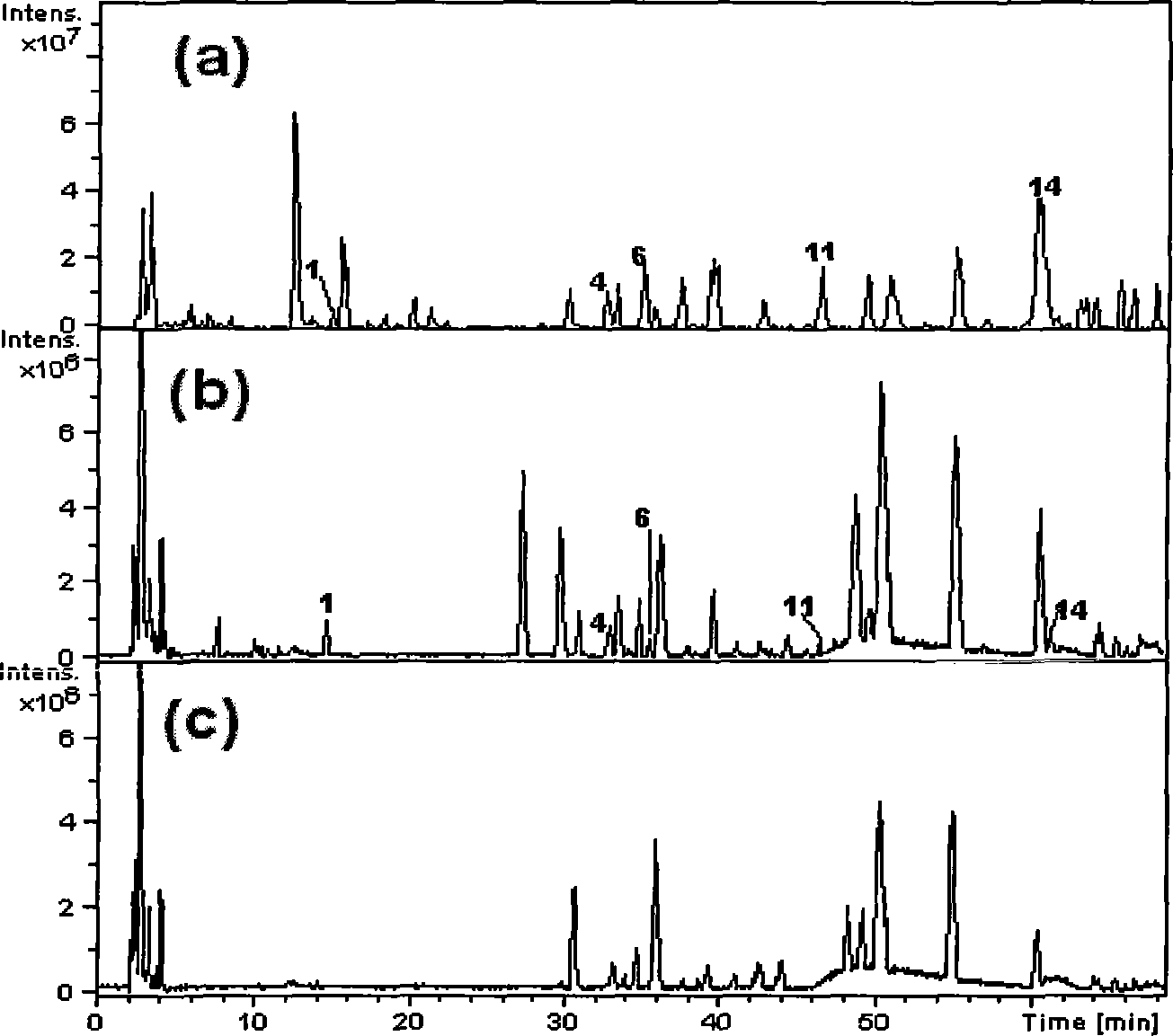
[0042] Take the following ingredients to form the pharmaceutical composition of the present invention: Japanese bufatalin, ginsenoside Re, ginsenoside Ra 1 , 1β-hydroxybufalin, ginsenoside Rb 1 , Recibuadin Alcohol, Ginsenoside Rc, Ginsenoside Rb 2 , Ginsenosides, Ginsenoside Rd, Bufalin, Cholic Acid, Hyodeoxycholic Acid, Cinobufinyl, Chenodeoxycholic Acid, and Deoxycholic Acid.
[0043] Prepare 1000 formulation units.
[0044] Preparation method: mix the above compound and auxiliary materials, and prepare tablets, dispersible tablets, buccal tablets, orally disintegrating tablets, sustained-release tablets, capsules, soft capsules, dropping pills, granules, injections, etc. according to conventional methods.
[0045] in,
[0046] japonica bufatalin 1.0 mg (the preparation process is as follows: toad bufa medicinal material coarse powder (20 mesh), percolation with 95% ethanol ethanol, the residue is reflux extracted with 70% ethanol, the combined extracts are concentrated ...
Embodiment 2
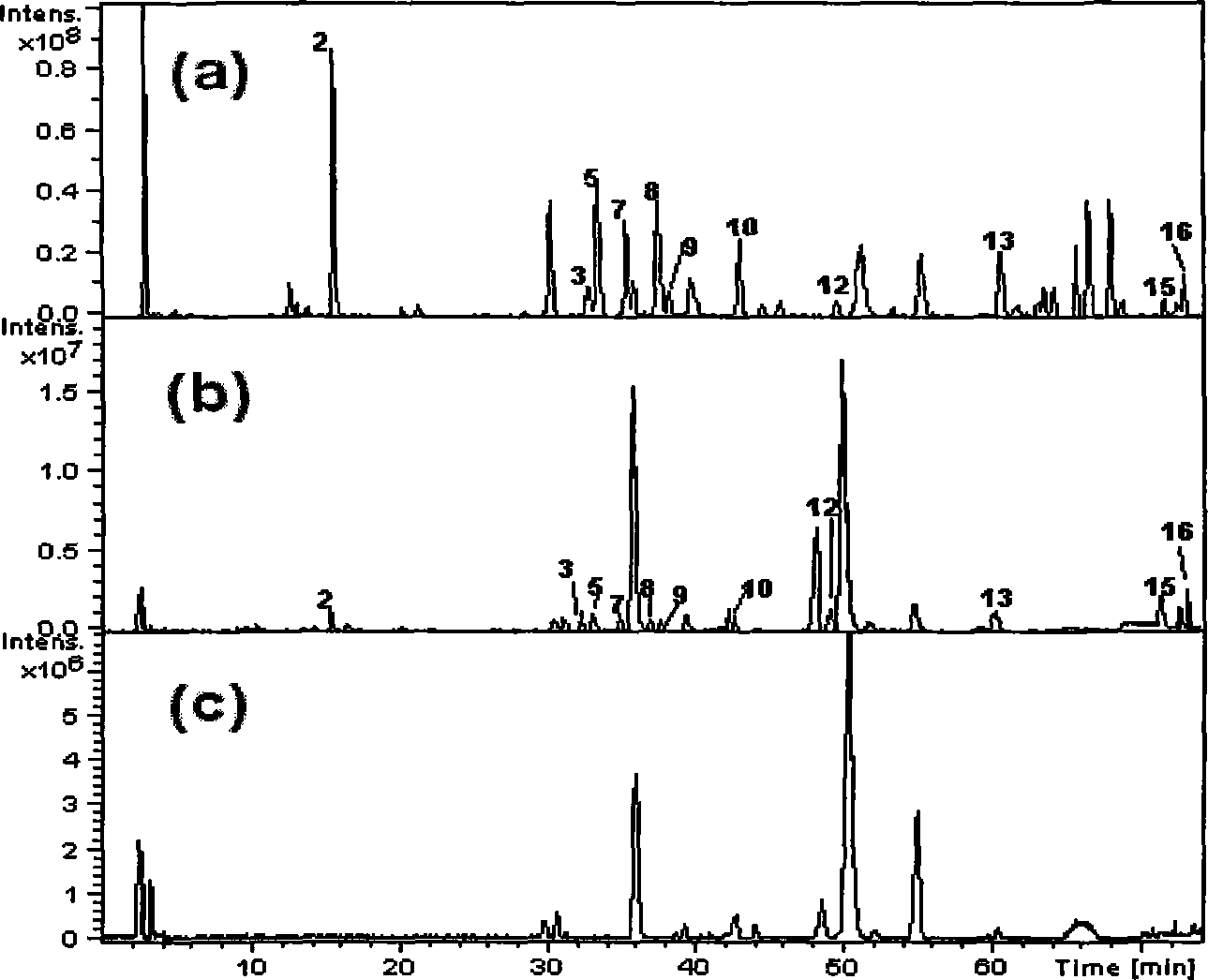
[0063] Take the following components by weight ratio to form the pharmaceutical composition of the present invention: bufatalin japonica 5 mg, ginsenoside Re 10.0 mg, ginsenoside Ra 1 10.0mg, 1β-hydroxybufalin 5mg, ginsenoside Rb 1 10.0mg, reciprocin 10.0mg, ginsenoside Rc 10.0mg, ginsenoside Rb 2 10.0mg, Ginsenoside Rb 3 10.0mg, ginsenoside Rd 10.0mg, bufalin 1.5mg, cholic acid 10.0mg, hyodeoxycholic acid 10.0mg, cinobufaction base 2.5mg, chenodeoxycholic acid 10.0mg, deoxycholic acid 10.0mg. Prepare 1000 formulation units.
[0064] Preparation method: mix the above compound and auxiliary materials, and prepare tablets, dispersible tablets, buccal tablets, orally disintegrating tablets, sustained-release tablets, capsules, soft capsules, dropping pills, granules, injections, etc. according to conventional methods.
[0065] Wherein each component preparation method and source are with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com