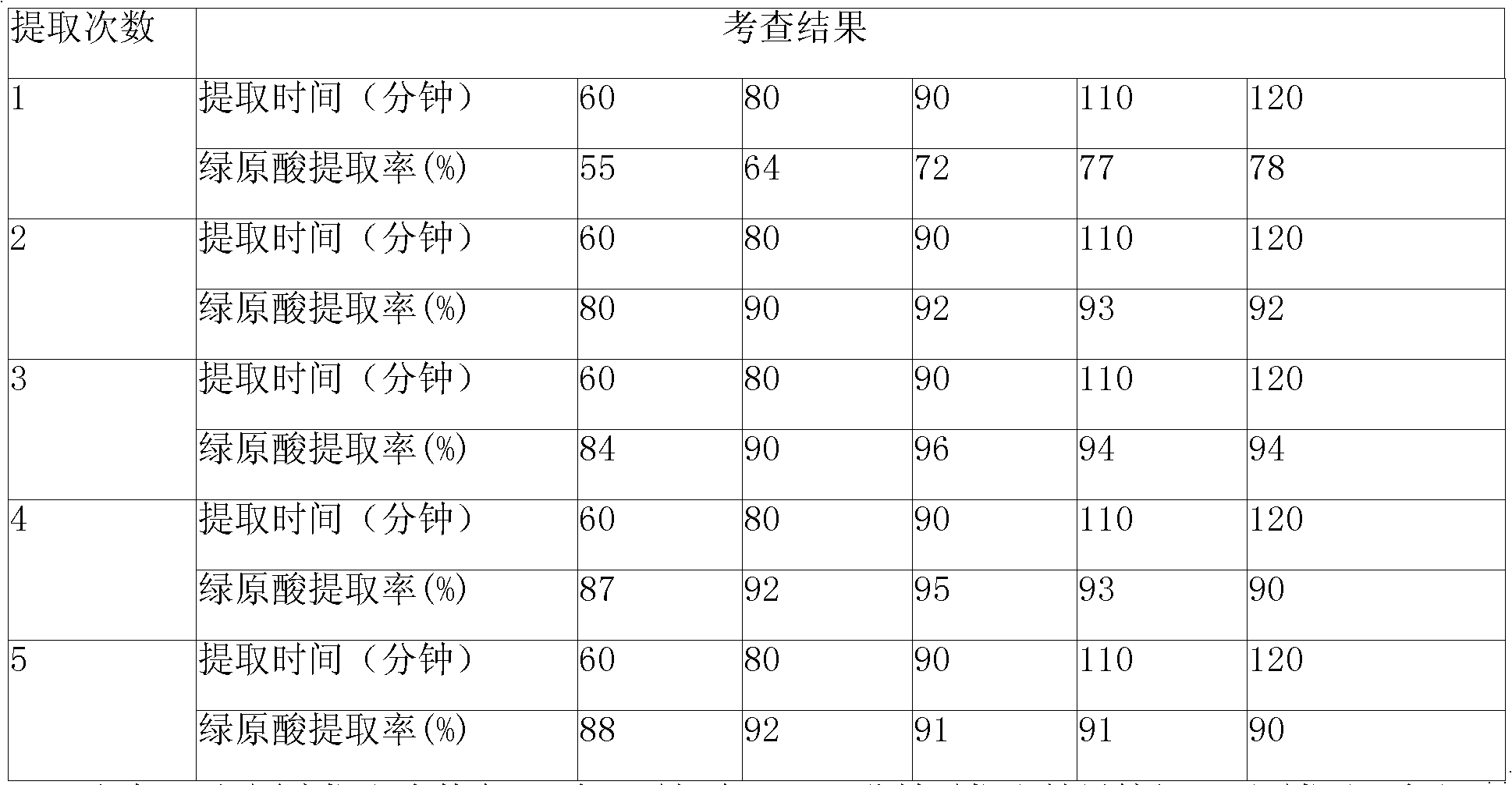
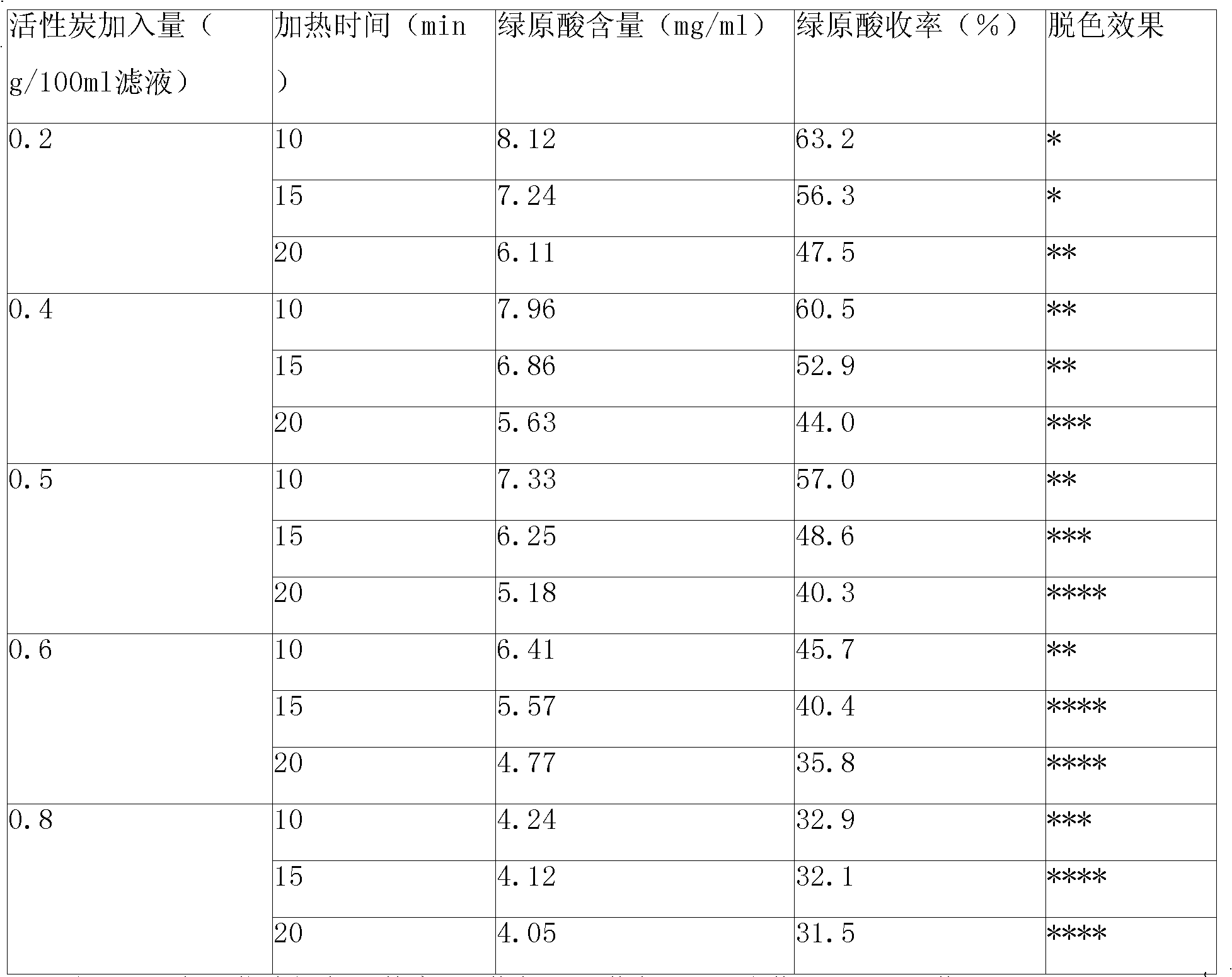
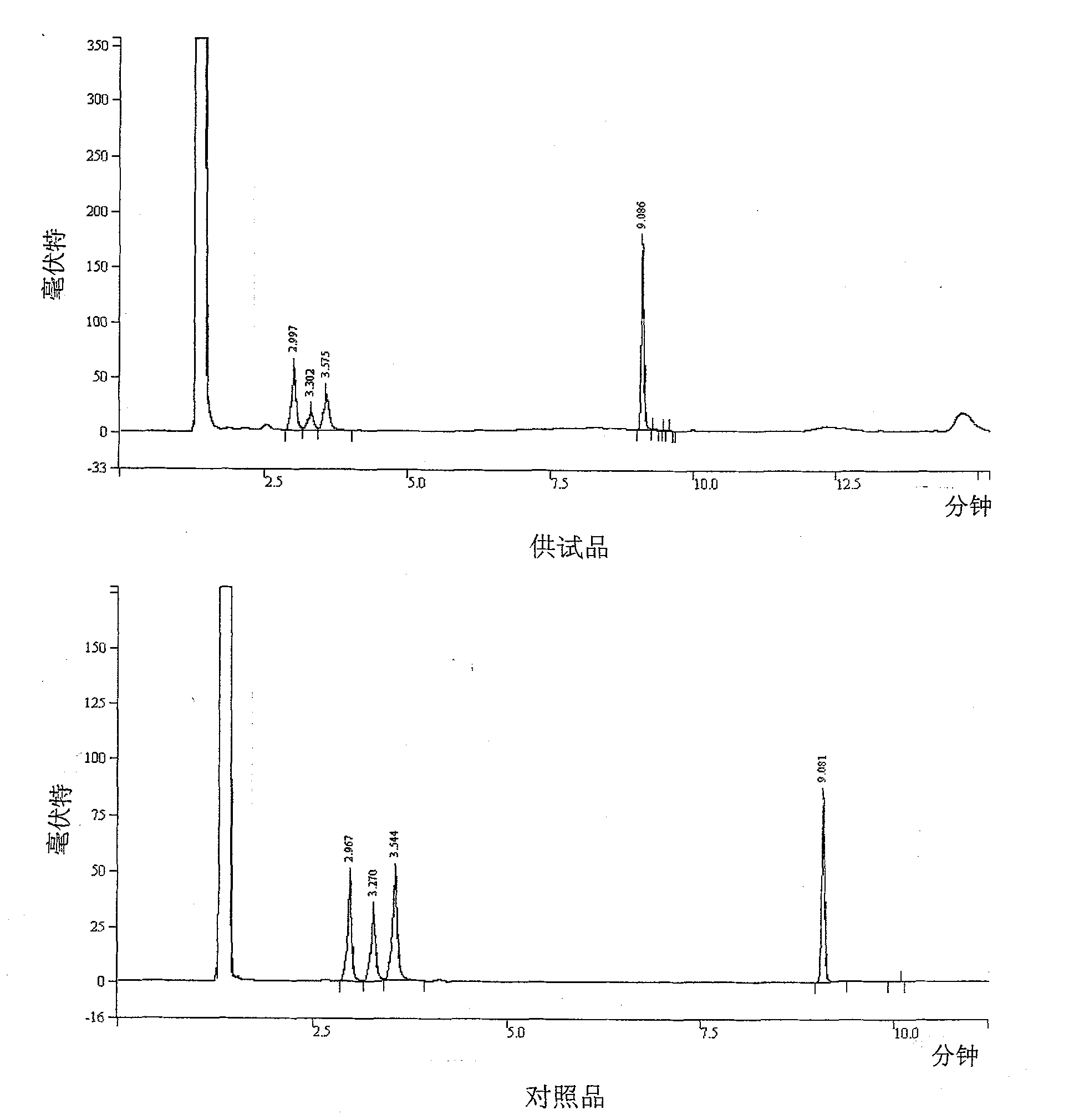
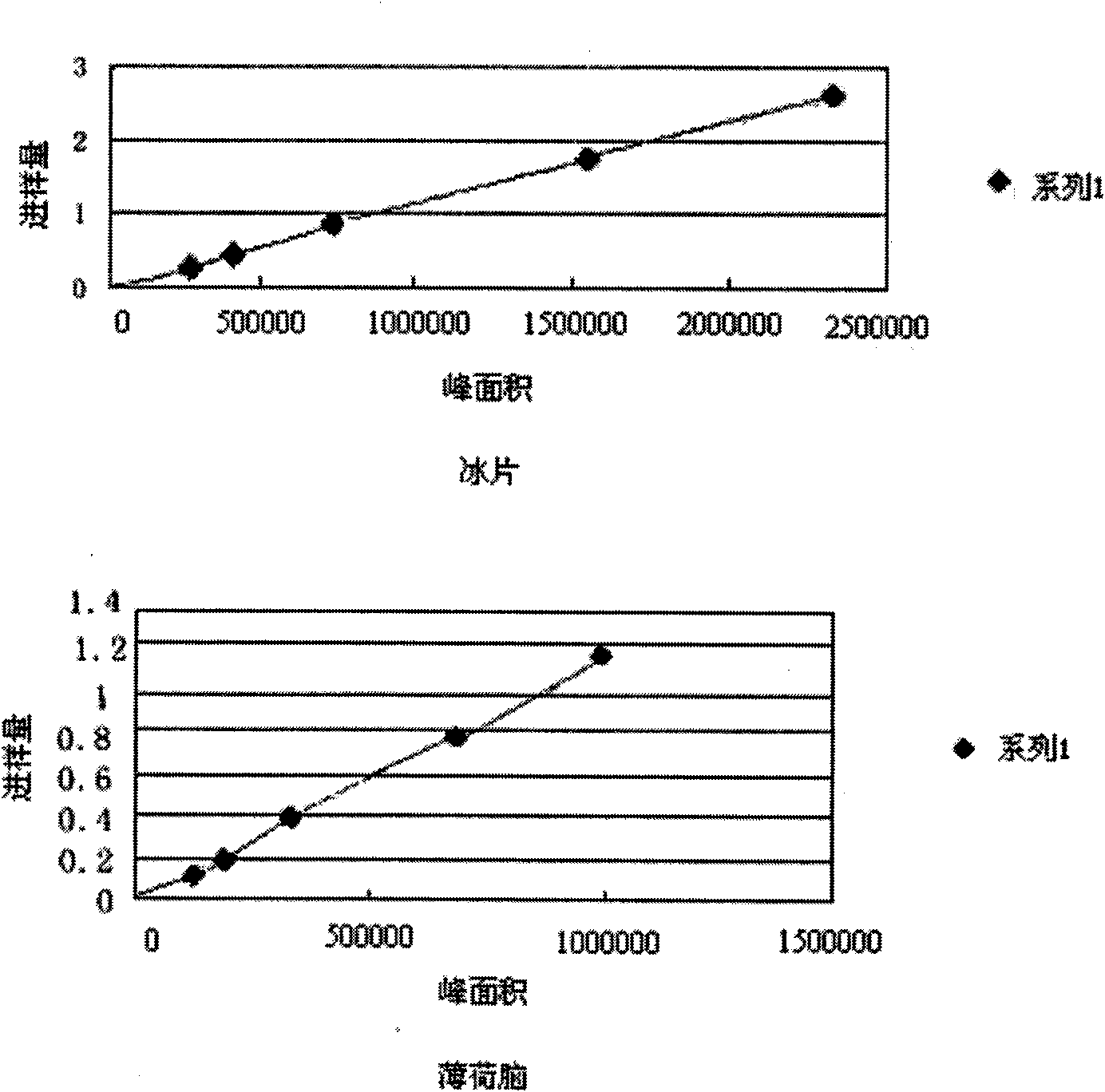
Patents
Literature
142 results about "Hyodeoxycholic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
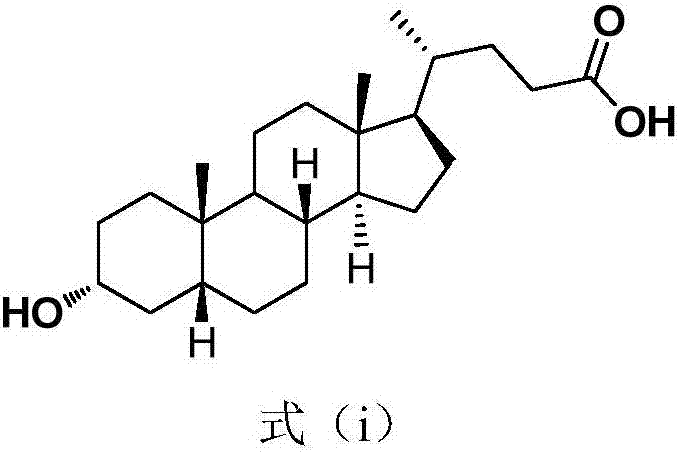
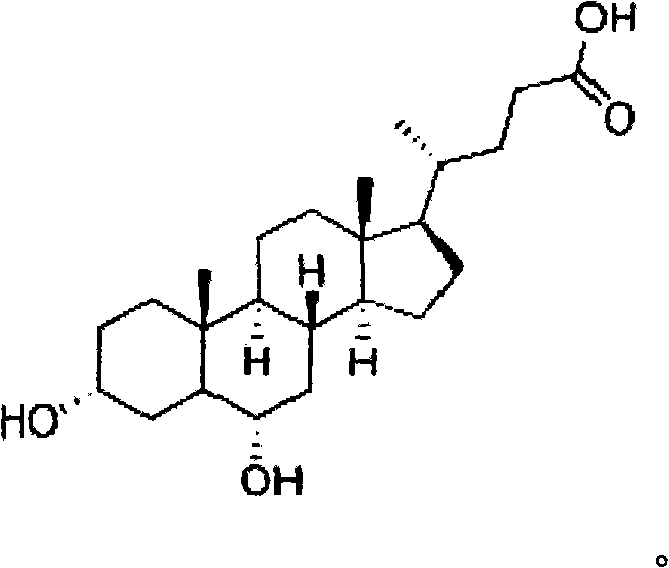
Hyodeoxycholic acid, also known as 3α,6α-Dihydroxy-5β-cholan-24-oic acid or HDCA, is a secondary bile acid, one of the metabolic byproducts of intestinal bacteria. It differs from deoxycholic acid in that the 6α-hydroxyl is in the 12 position in the former. The 6α-hydroxyl group makes HDCA a hydrophilic acid, a property it shares with hyocholic acid. HDCA is present in mammalian species in different proportions. It is the main acid constituent of hog bile, and for this reason it was used industrially as precursor for steroid synthesis before total synthesis became practical.
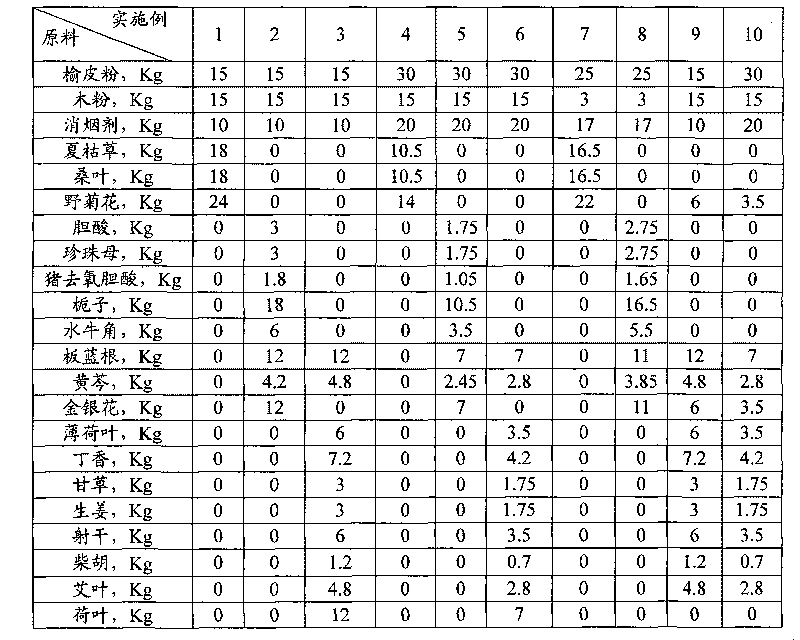
Chinese herbal medicine health-care joss stick
InactiveCN101690503AReduce burning rateSolve the problem of irritating and easily causing drynessBiocidePest repellentsDisinfectantPeppermints
The invention discloses a Chinese herbal medicine health-care joss stick, comprising a substrate prepared by mixing elm bark powder, cellulosine, a smoke eliminating agent and an additive (additives); the additive (additives) is (are) one or a plurality of an air freshener, a disinfectant, a helminthic epidemic-prevention material and an anti-flu material; the air freshener comprises one or a plurality of peppermint leaf, clove, liquorice and radix isatidis; the disinfectant comprises one or a plurality of ginger, blackberry lily, chrysanthemum flower, wild chrysanthemum flower and lotus leaf; the helminthic epidemic-prevention material comprises one or a plurality of artemisia leaf, radix scutellariae and Chinese thorowax; and the anti-flu material is the mixture of selfheal, mulberry leaf and wild chrysanthemum flower, or the mixture of cholic acid, mother-of-pearl, hyodesoxycholic acid, gardenia, cornu bubali, indigowoad root, radix scutellariae and honeysuckle flower. By adding different kinds of Chinese herbal medicine additives, the Chinese herbal medicine health-care joss stick realizes many beneficial functions, such as air refreshing, disinfection, helminthic epidemic-prevention, flu prevention and the like, thereby being an incensing joss stick.
Owner:何丹凤
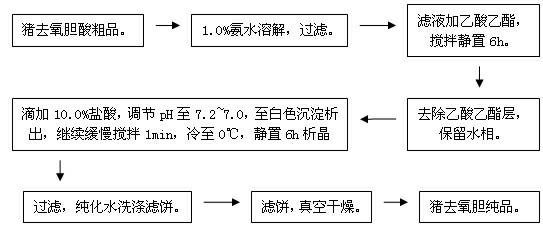
Method for preparing high-purity hyodeoxycholic acid by pig bile
InactiveCN101037463AReduce the difficulty of purificationLow impurity contentDigestive systemUnknown materialsBenzeneHyodeoxycholic acid
The invention relates to a method for extracting the hyodeoxycholic acid from the leftovers of pig bile or extracted bilirubin, including saponify the leftovers with alkali, adjusting pH value, acetic ester extraction, decoloring with active carbon; concentrating the mother liquid to a proper volume, precipitating deposit, separating the deposit and drying to get coarse hyodeoxycholic acid; esterifying the coarse product, then doing an addition reaction with benzene, separating the methylhyodeoxycholanate-benzene addition compound; decomposing the addition compound with alkali, adjust pH value, getting the purified deoxycholic acid. The obtained mother liquid can be further extracted to get precious chenodeoxycholic acid. The craft has a lot of material, a low pollution, safety and no poison, a low cost, a high product purity and is suitable for industry production in a large scale.
Owner:JIANGSU UNIV
Method for preparing chenodeoxycholic acid
The invention relates to a method of distilling the chenodeoxychoilc acid from the offcuts which be gained by distilling the bilirubin from the bile of the pig. With the offcuts of the alkali saponification, the deposit is treated with the oxidation treatment by means of the hydrogen peroxide, the filtrate is treated by the dilute sulfuric acid and decolored by means of the active carbon and solved, crystaled using the ethyl acetate again and again; the coordinate production of the hyodeoxycholic acid is purified, the mother liquor is concentrated to the cream and melted using the alkali and have the salifying treatment with the chloride of barium, so the cholate is formed, the cholate is treated to take off barium when the cholate suspending the aqueous solution containing the sodium carbonate, then it is acidificated with the dilute sulfuric acid and is separated ,deposited and dried to gain the crude chenodeoxycholic acid which is decolored using the active carbon and is melted, rimed and dried using the ethyl acetate. Then the finished production of the chenodeoxycholic acid form.The whole technics has some merits of the abundant material, the amity of the environment, the safety and innocuity, producing many outputs from the single stuff and easy to the mass production. The invention is used for distilling the chenodeoxycholic acid.
Owner:CHANGDE YUNGANG BIOTECHNOLOGY CO LTD
Prepn process of Qingkailing injection and injection powder and its quality control method
The present invention provides a improved preparation process of Qingkailing injection and powder for injection. The improved preparation process includes high speed centrifugal treatment in extracting cape jasmine, isatis root and honeysuckle, merging extractive liquid, ultrafiltering and other technological steps. It can produce Qingkailing injection product and powder product for injection with ever higher stability and ever longer effective period. The present invention also discloses the quality control method of baicalin, cholic acid, hyodeoxycholic acid, jasminoidin and chlorogenic acid in Qingkailing injection and powder for injectino as well as identification method of Qingkailing injection and power for injection.
Owner:GUIZHOU YIBAI PHARMA CO LTD
Preparation method of high-purity hyodeoxycholic acid
The invention discloses a preparation method of high-purity hyodeoxycholic acid. The preparation method comprises the steps: weighing raw materials, adding strong alkaline and water, saponifying at the temperature of 95-100 DEG C for 16-24h, cooling to room temperature, standing, siphoning to remove a supernatant liquid, adding water into paste in the lower layer, stirring for dissolving the paste, adding strong acid to acidize until a congo red test paper turns to be blue, adding ethyl acetate, stirring for extracting for 20-50min, standing for layering, removing a water phase, adding water into the ethyl acetate to wash until the pH value of the water phase is equal to 6-7, adding the ethyl acetate into anhydrous sodium sulfate to dehydrate, carrying out activated carbon decoloration, filtering, concentrating until precipitates are separated out, cooling, filtering or squeezing and drying in vacuum; adding an alcohol solvent, stirring for dissolving the above product, slowly adding an alkaline organic solvent, stirring, cooling, separating out a great number of precipitates, filtering and drying; and adding water and sodium hydroxide, stirring for dissolving, dropwise adding hydrochloric acid with a volume concentration of 1:1, stirring, filtering and drying to obtain the hyodeoxycholic acid. The invention aims to provide the preparation method of the hyodeoxycholic acid with simple process, high yield and high purity.
Owner:ZHONGSHAN BAILING BIOTECHNOLOGY CO LTD
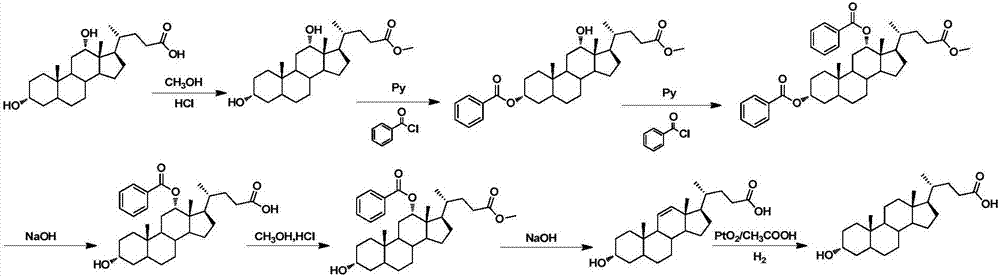
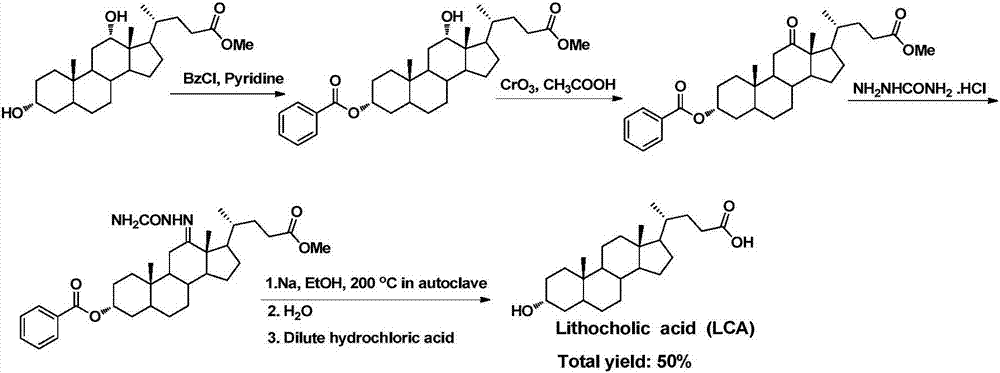
Method for synthesizing lithocholic acid from hyodesoxycholic acid
ActiveCN106977572AReduction reaction with few side reactionsSimple post-processingSteroidsSynthesis methodsHyodeoxycholic acid
The invention discloses a method for synthesizing lithocholic acid, comprising: using hyodesoxycholic acid as a start material, and performing two-step reaction of 6Alpha-OH selective oxidation and Huang Minglon reduction to synthesize the lithocholic acid. The start material herein is low in price and easy to obtain, the synthetic steps are short, posttreatment is simple, few side reactions are employed, and the method is applicable to industrial production.
Owner:JIANGSU JIAERKE PHARMA GRP CORP
Method for extracting hyodeoxycholic acid from pig bile
The invention discloses a method for extracting hyodeoxycholic acid from pig bile, which comprises the following steps: diluting the pig bile, adding sodium hydroxide to perform the saponification reaction and removing yellowish-brown supernatant; adding hydrogen peroxide to carry out decoloring; regulating pH, heating to the temperature of 50 DEG C, adding magnesium salt solution, stirring, crystallizing, filtering and collecting solid; adding sodium carbonate into the solid, stirring to enable the solid to be dissolved, filtering and collecting filtrate; and adding dilute sulphuric acid into the filtrate to regulate pH to 3, carrying out filter pressing, washing a filter cake to pH of 6 to 7, drying and grinding to obtain a hyodeoxycholic acid pure product. According to the invention, the method adopts inorganic salt and magnesium salt to crystallize and separate the hyodeoxycholic acid without adopting an organic solvent to carry out extraction; the use quantity of the magnesium salt is 0.15 time of mass of the pig bile; the use quantity of the solvent is small; cost is low; quality of the hyodeoxycholic acid is prevented from being influenced due to volatilization of the organic solvent; pollution to the environment, which is caused by the organic solvent, is avoided; the method has a simple process and few operating steps and the reaction conditions of each step is easy to control; and the purity of the hyodeoxycholic acid can reach over 99 percent.
Owner:SICHUAN DAXIONG BIOTECH
Quality detection method for liver protection dropping pill of traditional Chinese medicine preparation
ActiveCN103808842AQuality improvementEfficient detectionComponent separationChlorogenic acidMedicine
The invention relates to a quality detection method for a liver protection dropping pill of traditional Chinese medicine preparation. The quality detection method comprises the following steps: measuring the content of effective components of the liver protection dropping pill, including saikoside a and schizandrin, and identifying schisandra chinensis, pulvis fellis suis and artemisia capillaris in the liver protection dropping pill. The quality detection method also comprises the following detection steps: (1) with the saikoside a as a reference substance, measuring whether a radix bupleuri component is contained in a liver protection dropping pill recipe by adopting a high efficiency liquid chromatography method; (2) with the schizandrin as the reference substance, measuring whether a schizandrin component is contained in the liver protection dropping pill by adopting the high efficiency liquid chromatography method; (3) with the schisandrin b as the reference substance, identifying whether a schisandra chinensis component is contained in the liver protection dropping pill by adopting a thin-layer chromatography method; (4) with hyodeoxycholic acid as the reference substance, identifying whether a pulvis fellis suis component is contained in the liver protection dropping pill by adopting the thin-layer chromatography method; and (5) with chlorogenic acid as the reference substance, identifying whether an artemisia capillaris component is contained in the liver protection dropping pill by adopting the thin-layer chromatography method. The quality detection method disclosed by the invention can be used for effectively and reliably controlling the quality of the liver protection dropping pill, and the method is scientific, feasible and reliable.
Owner:HEILONGJIANG KUIHUA PHARMA
Qingkailing oral disintegration tablet and its preparing method
InactiveCN1586587ADisintegrates quicklyQuick effectAntipyreticAnalgesicsCross-linkCarboxymethyl starch
The present invention discloses Qingkailing oral disintegration tablet and its preparation process. The present invention features that the extracted effective parts of Chinese medicinal materials cape jasmine, isatis root, nacre and buffalo horn are mixed with cholic acid, hyodeoxycholic acid, baicalin, honeysuckle extract and medicinal supplementary material to compound the medicine. The present invention features its composite disintegrating agent, which consists of erythritol and chitin, substituted hydroxypropyl methyl cellulose, carboxymethyl starch sodium, cross-linked carboxymethyl starch sodium or insoluble cross-linked polyvinyl pyrrolidone in certain proportion. The erythritol has also the effect of corrective, and this can reduce the amount of the medicinal supplementary material. Pharmaceutical experiment shows that the Qingkailing oral disintegration tablet has fast disintegration speed, fast acting and high pharmacological effect.
Owner:张晴龙
Qingkailing suppositorium
ActiveCN101327262AQuick effectLong duration of actionOrganic active ingredientsNervous disorderHydrolysateThird generation
The invention discloses a Qingkailing suppository, relates to a Chinese medicine compound preparation, the raw materials of which mainly come from plants and animals, in particular to a novel formulation of Qingkailing preparation. The Qingkailing suppository provided by the invention for supplying rectum with medicine adopts the following components of cholic acid, hyodeoxycholic acid, baicalin, buffalo horn, the hydrolysate of mother-of-pearl, gardenia, banlangen, the water purified extract of honeysuckle as Qingkailing combination. The suppository which is 1-3g weight is made from the raw materials and supplementary materials with the following weight proportions of 8-13 proportions of Qingkailing combination, 5-50 proportions of suppository base, 0.1-5 proportions of surfactant, 0.05-3 proportions of sorbefacient, and 0-3 proportions of additional agent. The Qingkailing suppository of the invention not only can compensate the defects that the oral taking preparation is slow to take effect and injection preparation has great toxicity, but also can prolong the duration of the medicine, provides a wider selection range for clinics and sufferers, has simple production technology, is suitable for industrialized batch production, and is suitable to be taken by infants.
Owner:GUANGZHOU PHARMACEUTICAL INDUSTRIAL RESEARCH INSTITUTE +1
Purification method of hyodeoxycholic acid
The invention discloses a purification method of a hyodeoxycholic acid. The purification method of the hyodeoxycholic acid comprises the following steps that: a hyodeoxycholic acid crude product is dissolved with aqueous ammonia to remove insoluble impurities by filtration, and a filtrate is collected; in the collected filtrate, ethyl acetate is added and allowed to keep stand to remove a fat-soluble substance, and the ethyl acetate is removed by siphoning to obtain an aqueous phase liquid; and in the aqueous phase liquid, a hydrochloric acid solution is added dropwise to adjust the pH value, and then crystallization, filtration, water wash and drying are carried out to obtain a hyodeoxycholic acid pure product. According to the purification method provided by the invention, the process route is direct, the operation is simple, the environment is protected, the purity of the obtained product HPLC (High-Performance Liquid Chromatography) is more than or equal to 99.8%, and the defects that the purity of a product obtained by the traditional method is not high, and a toxic carcinogen benzene needs to be used are overcome.
Owner:JIANGSU QINGJIANG PHARMA
Process for preparing hyodeoxycholic acid
The invention discloses a process for preparing hyodeoxycholic acid and belongs to the field of biological pharmacy. The extraction and purification process comprises the following steps of: leftover salting-out, alkaline hydrolysis, dissolution and discoloring, filtering and dewatering, concentrated crystallization and vacuum-drying to obtain a crude product; and methyl esterification, column chromatography separation, alkaline hydrolysis again, purification, finished product vacuum-drying and the like. The process has the advantages that an obtained product has high purity, is free from extraneous odor and is environmentally friendly, a solvent can be recycled, and the process is easy to operate, has low cost, is simple and safe to operate and can be used for industrial production.
Owner:NANJING XINBAI PHARMA +1
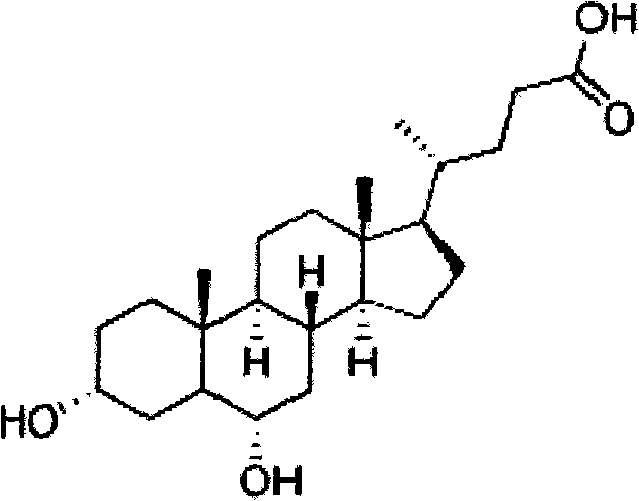
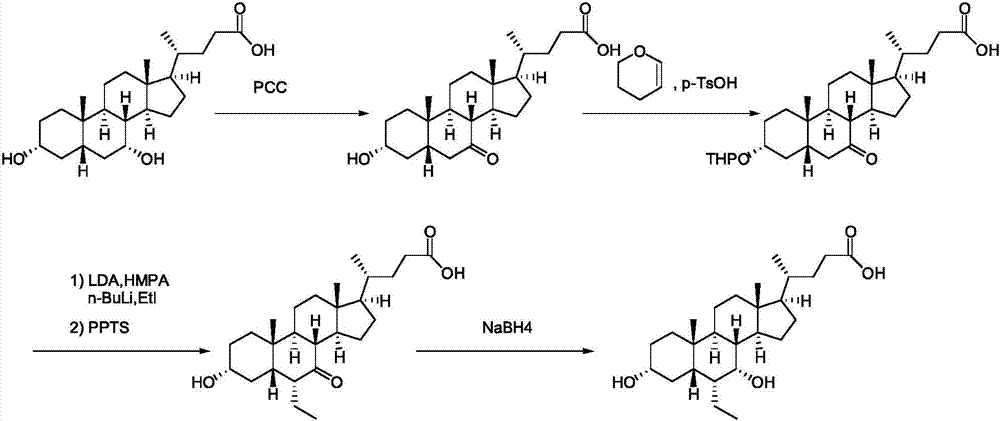
Hyodeoxycholic acid
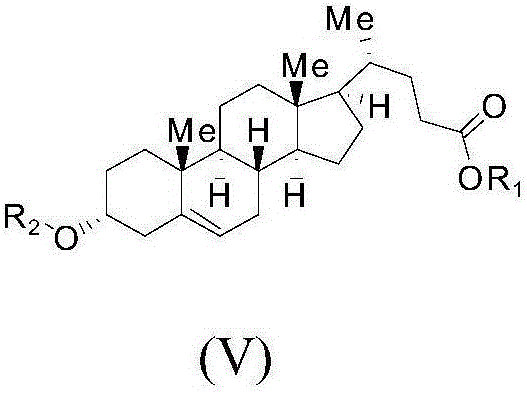
The present invention discloses a hyodeoxycholic acid, which has the following structural formula. According to the present invention, two steps of a crude hyodeoxycholic magnesium extraction part (acidification, saponification, decoloration and precipitation) and a hyodeoxycholic acid purification part (acidification and crystallization) are adopted to obtain the refined hyodeoxycholic acid finished product, wherein a melting point of the hyodeoxycholic acid finished product can be 199-200 DEG C, a melting range is less than 2 DEG C, and content is more than 99%. According to the present invention, a production cycle is greatly shortened, a use amount of the organic solvent is less, and bile acid loss is not substantially generated in the whole process.
Owner:FUJIAN XIANYOU COUNTY NANFENG BIOCHEM
Medicine composition and detection method of preparations of medicine composition
The invention discloses a traditional Chinese medicine Annao pill and a detection method of preparations prepared from the raw materials in the formula of the Annao pill. The detection method disclosed by the invention comprises identifying and / or content measuring methods, wherein the identifying methods are one or more of methods for identifying cholic acid and hyodeoxycholic acid, identifying rhizoma coptidis, identifying geniposide and identifying baicalein; and the content measuring methods are one or more of methods for measuring contents of geniposide, baicalein, rhizoma coptidis, menthol and borneol. The detection method disclosed by the invention has good linear relation, precision, stability, repeatability and recovery and is of great importance for strictly controlling the quality of the Annao pill and the preparations of the Annao pill.
Owner:哈尔滨蒲公英药业有限公司
Preparation method of chenodeoxycholic acid
ActiveCN106831923AImprove monitoring accuracyReduce the ratioSteroidsHyodeoxycholic acidMedicinal chemistry
The invention discloses a preparation method of chenodeoxycholic acid. The preparation method includes following steps: refining hyodeoxycholic acid; preparing a methyl hyodeoxycholate; eliminating 6-bit hydroxyl; preparing chenooxycholic acid. The preparation method is simple in steps, raw materials used in the method are wide and sufficient in source, and the preparation method is high in yield of the chenodeoxycholic acid.
Owner:眉山市新功生物科技有限公司
Medicine composition
ActiveCN101773563AReduce allergic reactionsImprove safety with highOrganic active ingredientsNervous disorderChlorogenic acidAdditive ingredient
The invention provides a medicine composition, which is prepared from the following medicine effect raw materials: cholic acid, nacre, hyodesoxycholic acid, gardenia, cornu bubali, radix isatidis, baicalin and honeysuckle. The medicine composition comprises the following medicine effect ingredients in percentage by weight: 1.5 to 4.2 parts of cholic acid type ingredients, 3.5 to 5.5 parts of baicalin, 0.1 to 0.5 part of jasminoidin, 3.5 to 5.5 parts of amino acid, 0.05 to 0.08 part of nucleosides compounds and at most 0.1 part of chlorogenic acid. Experiments show that the medicine composition of the invention has the medicine effect similar to Qingkailing, but the anaphylactic reaction is obviously reduced, and the medicine use safety is improved.
Owner:HEILONGJIANG ZBD PHARMA
Chenodeoxycholic acid and preparation method of intermediate of chenodeoxycholic acid
The invention discloses chenodeoxycholic acid and a preparation method of an intermediate of the chenodeoxycholic acid. In the invention, commercial hyodeoxycholic acid is used as a raw material, andthe preparation method comprises the following steps: performing side chain carboxylmethylation,recrystallization,6 alpha-hydroxyl selective oxidation, and introducing into beta-bromine at the position 7 to obtain an intermediate III with the total yield of 16%; and reducing and hydrolyzing the intermediate III to obtain the chenodeoxycholic acid. The method is short in reaction route, is free ofreagents, such astrimethylchlorosilane and highly-toxic p-toluenesulfonylhydrazide, and is relatively low in environmental pollution; and the synthesized intermediate III plays an important role in the synthesis of cholic acid drugs.
Owner:SHANDONG RUIYING PIONEER PHARMA +1
Method for preparing qingkailing granules
InactiveCN1513505AHigh embedding rateLow viscosityNervous disorderAntipyreticHyodeoxycholic acidAqueous solution
A medicine 'Qingkailing particles' for treating diabetes is prepared through adding the aqueous extract of honeysuckle flower, capejasmine fruit and isatis root, the hydrolyzed liquid of buffalo horn powder and nacre powder, ox-chololic acid, hyodeoxycholic acid, and baicalin to the aqueous solution of starch at 60-90 deg.C, stirring, emulsifying spray drying, mixing with additive and granulating.
Owner:增城市广增药物研究所
Obeticholic acid preparation method
The invention discloses an obeticholic acid preparation method, which comprises that (1) hyodeoxycholic acid II reacts with an alcohol compound III under the action of a catalyst to generate an ester compound IV; (2) the ester compound IV is subjected to PDC oxidation in dichloromethane to generate a compound V; (3) the compound V and trimethyl chlorosilane are subjected to a reaction at a temperature of -70 to -20 DEG C in tetrahydrofuran by using lithium diisopropylamide as an alkali to generate a silyl enol ether compound VI; (4) the silyl enol ether compound VI is subjected to m-chloroperoxybenzoic acid oxidation and deprotection in dichloromethane to generate a compound VII: (5) the compound VII and Yield generated from ethyltriphenylphosphonium bromide under the action of a strong alkali are subjected to a Wittig alkenylation reaction at a temperature of 0-70 DEG C to convert the ketone into the vinyl so as to generate a compound VIII; (6) the double bond of the compound VIII is subjected to catalytic hydrogenation reduction in a mixing solvent to generate a compound IX; and (7) the compound IX is hydrolyzed under an alkaline condition to generate the obeticholic acid.
Owner:XIAMEN HALOSYNTECH CO LTD
Method for measuring content of sodium cholate
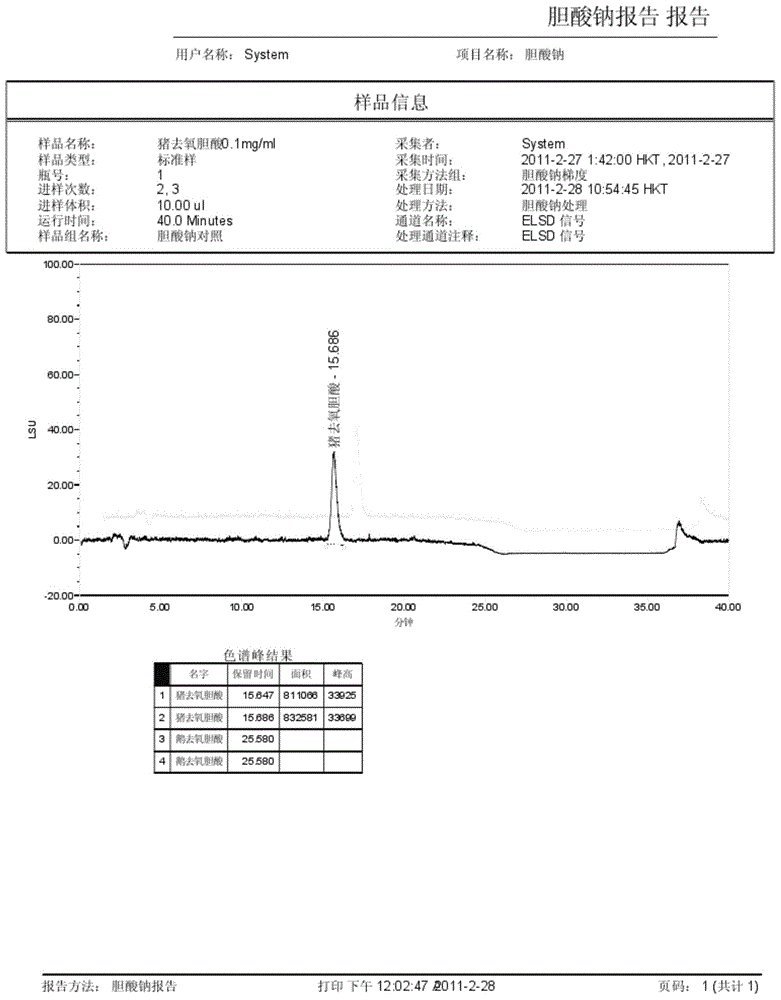
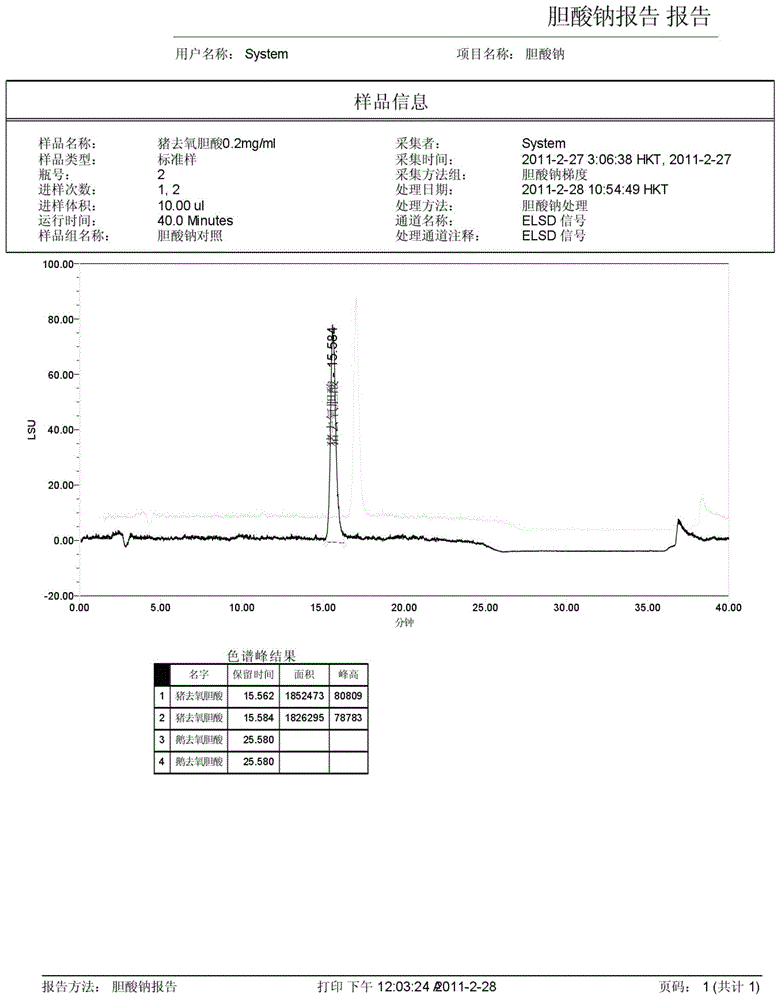
ActiveCN104458930AAccurate quantitative analysisShorten the timeComponent separationHyodeoxycholic acidOxygen
The invention discloses a method for measuring the content of sodium cholate. The method comprises the following steps: (1) preparing a sodium cholate solution to be measured; (2) respectively preparing a standard hyodesoxycholic acid solution and a standard chenodeoxycholic acid solution; and (3) measuring the contents of hyodesoxycholic acid and chenodeoxycholic acid which are contained in the sodium cholate solution to be measured by adopting a high efficiency liquid chromatography method. The method disclosed by the invention has the characteristics of easiness and convenience for operation, accuracy in result, short measurement time and high generalizability and can be widely applied to the technical field of component detection.
Owner:WUHAN BIOCHEM PHARMA
Method for synthetizing porcine cholic acid into ursodeoxycholic acid through semi-enzymatic method
InactiveCN109722463AExpand processing raw materialsHigh yieldFermentationResource utilizationHyodeoxycholic acid
The invention discloses a method for synthetizing porcine cholic acid into ursodeoxycholic acid through a semi-enzymatic method. The method includes process steps of oxidation, reduction, Huang-Minlonreaction, extraction into salt neutralization, etc. The method uses a scrap material porcine cholic acid after chenodeoxycholic acid and hyodeoxycholic acid are extracted from pig galls as a raw material, and synthetizes a ursodeoxycholic acid crude product through a mode of combining a chemical method and a biological enzymatic method; and the method is simple in technology, low in cost, high inresource utilization rate, high in yield and suitable for large-scale production.
Owner:CHANGDE YUNGANG BIOTECHNOLOGY CO LTD
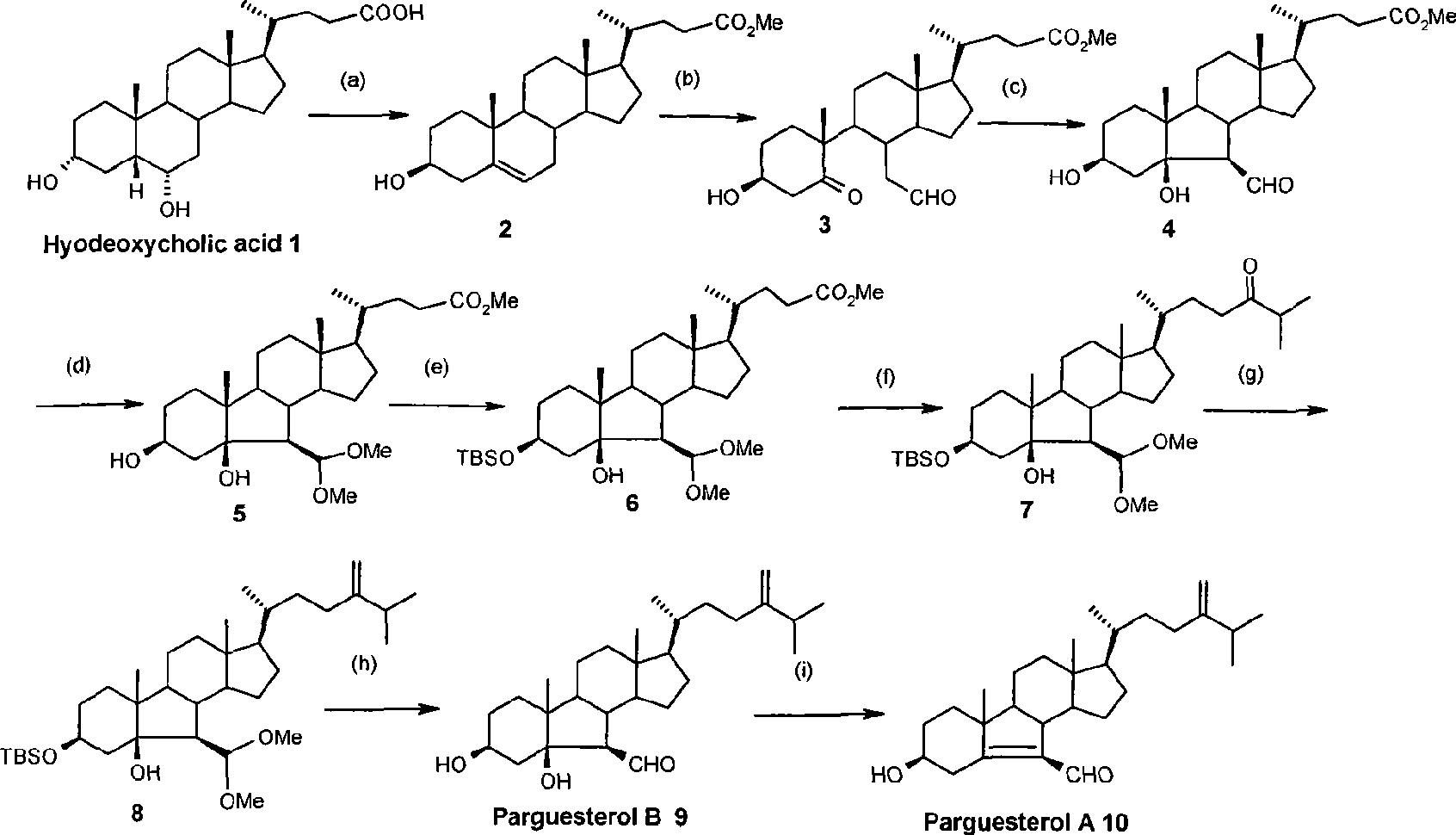
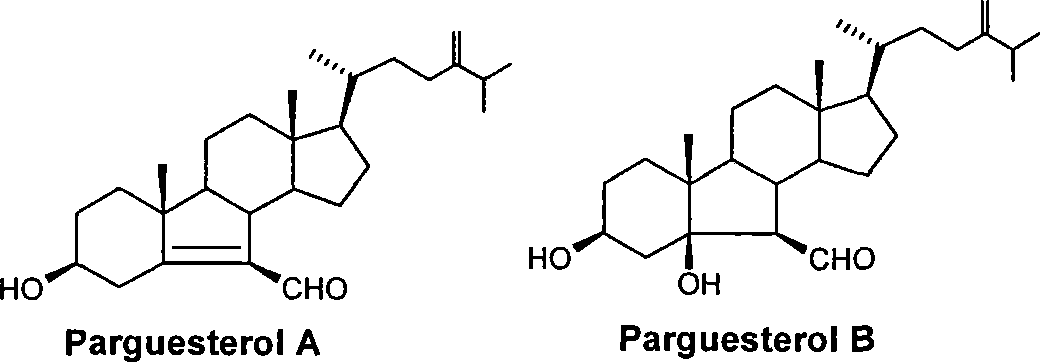
Synthesis of tuberculosis resistant compound of arguesterol
InactiveCN101508717ARaw materials are cheap and easy to getHigh yieldAntibacterial agentsSteroidsHyodeoxycholic acidTreating tuberculosis
The invention relates to a method for synthesizing parguesterol A and parguesterol B. The method comprises the following steps: performing reactions of methyl esterification, p-toluene sulfonic acid esterification, SN2 nucleophilic substitution, E1 elimination reaction by a three-step one-pot process, ozonization, intramolecular aldol condensation, methylal protection, dimethyl tert-butyl silicon-based protection, isopropylation, olefination reaction, deprotection, intramolecular dehydration and the like on hyodeoxycholic acid to synthesize the parguesterol A and the parguesterol B. The method has the advantages of cheap and available raw materials, short synthetic route, high yield, low cost and mild reaction condition, and being suitable for industrialized production. The parguesterol A and the parguesterol B have effect of inhibiting mycobacterium tuberculosis and can be used for treating tuberculosis.
Owner:ARMY MEDICAL UNIV
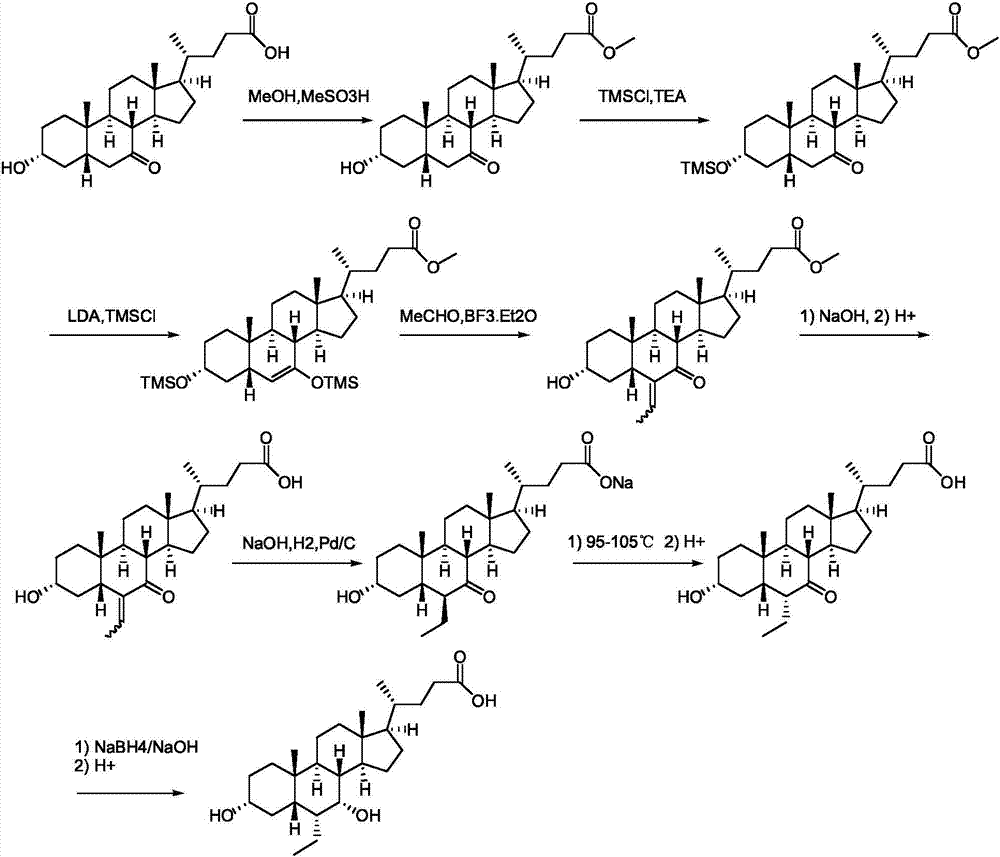
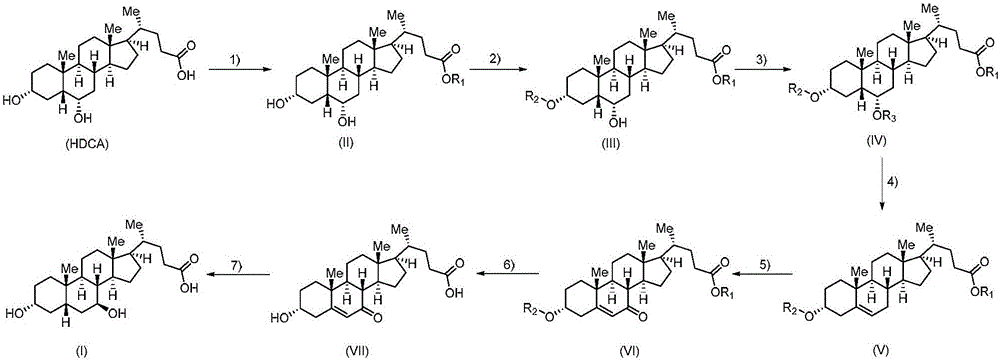
Method for preparing ursodeoxycholic acid
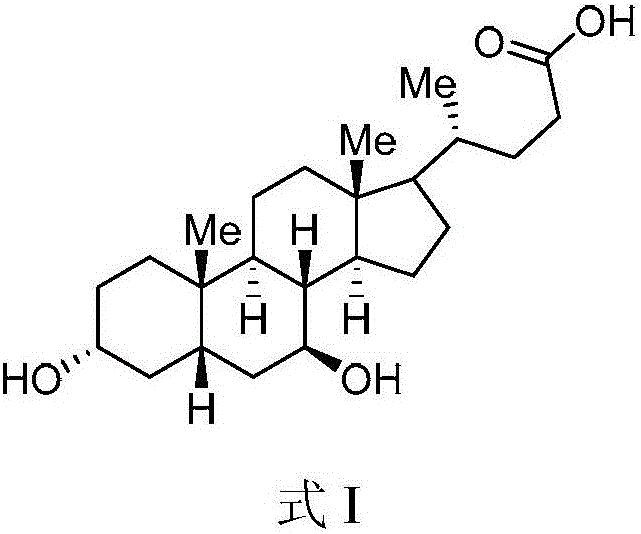
The invention belongs to the field of medicine, and particularly relates to a method for preparing an ursodeoxycholic acid. The method comprises the following steps: performing esterification reaction to obtain a compound shown in formula II by taking an HDCA (hyodeoxycholic acid) as a starting raw material; performing selective hydroxy protection reaction to obtain a compound shown in formula III; performing hydroxy protection reaction to obtain a compound shown in formula IV; performing elimination reaction to obtain a compound shown in formula V; performing oxidation reaction on the compound shown in formula V under the action of tert-butyl hydroperoxide and pyridinium chlorochromate to obtain a compound shown in formula VI; performing hydrolysis to obtain a compound shown in formula VII; performing reduction to obtain the ursodeoxycholic acid shown in formula I. According to the method, the ursodeoxycholic acid is prepared by taking the HDCA as the starting raw material, so that not only can the problem of raw material shortage be solved, but also the method is convenient to operate, low in byproduct rate and cost, mild in reaction condition and suitable for large-scale production of the ursodeoxycholic acid.
Owner:青州市欣泰生物制品有限公司
Qingkailing Prepn for great transfusion and its prepn process
InactiveCN101019950AOvercoming low clarityOvercome stabilityOrganic active ingredientsAntipyreticHyodeoxycholic acidChemistry
The Qingkailing preparation for great transfusion is prepared with cholic acid 1.2-2.0 weight portions, hyodeoxycholic acid 1.4-2.4 weight portions, buffalo horn 9-16 weight portions, baicalin 1.8-3.2 weight portions, nacre 18-32 weight portions, prepared cape jasmine extract liquid 9-16 weight portions, prepared isatis root extract liquid 75-125 weight portions, and prepared honeysuckle extract liquid 23-38 weight portions. The present invention also discloses the preparation process of the Qingkailing preparation for great transfusion. The Qingkailing preparation for great transfusion has high clarity, high stability, high safety and other advantages.
Owner:TSINGHUA UNIV
Pig gall powder preparing process
ActiveCN104473967AReduce spoilageImprove qualityPowder deliveryMetabolism disorderBile JuiceAdditive ingredient
The invention discloses a pig gall powder preparing process. The process comprises the steps that pig bile is fetched and conveyed into a first drying device, and steam is led into the first drying device to heat the pig bile to 50-70 DEG C; the air pressure in the first drying device is adjusted to be lower than the atmospheric pressure for drying under reduced pressure; when the relative density of materials reaches 0.8-1.25, the pig bile is cooled down to below 30 DEG C, the materials are taken out, placed into a heated air circulation constant-temperature drying oven for drying at the temperature ranging from 40 DEG C to 70 DEG C with the traying thickness ranging from 1 cm to 2 cm, smashed and filtered through a screen with the mesh ranging from 80 to 100 to obtain pig gall powder medicinal slices, and then the pig gall powder medicinal slices are packaged into finished products. Pig gall powder prepared from the pig bile is excellent in quality, and the major component of the pig gall powder is taurohyodeoxycholic acid sodium hydrate which has the effects of clearing heat, moistening dryness, relieving cough and asthma and detoxifying; the pig gall powder is rich in cholanic acid, can effectively inhibit forming of bile acid, dissolve fat and reduce cholesterol and triglyceride in blood and has a good curative effect on various types of hyperlipemia.
Owner:四川神农药业有限公司
Preparation of qinkailing freeze-dried injection
ActiveCN101259181ALoose textureGood lookingOrganic active ingredientsPowder deliveryForeign matterClinical efficacy
The invention provides the preparation method of the freeze-dried powder injection of qingkailing, which is prepared by cholic acid, nacre, hyodeoxycholic acid, gardenia, buffalo horn, isatis root, baicalin and honeysuckle, which undergo the processes of extraction, alternative precipitation and multipole impurity removal to obtain extraction solution in which absolute ethyl alcohol is added. Compared with prior art, the invention increases the content of effective composition of the medicine, shortens the time of freeze drying, reduces production cost and optimizes processing technique. In addition, the examinations of insoluble particle and visible matter are greatly improved, so the bioavailability and stability of the medicine are improved. The preparation of the freeze-dried injection of qingkailing has the advantages of loose texture, good formability, low water content, good stability, and good appearance and color, reducing insoluble particles and being easy to store, so the clinical curative effect of the preparation can be ensured.
Owner:GUIZHOU YIBAI PHARMA CO LTD
Medicinal composition and detection method for preparation thereof
The invention discloses Chinese medicinal tranquilizing pills and a detection method for a preparation which is prepared from raw materials of a formula of the pills. The detection method comprises an authentication method and / or an assay method, wherein the authentication method is one or more of methods for authenticating cholic acid, hyodeoxycholic acid, golden thread, geniposide and baicalin;and the assay method is one or more of methods for assaying geniposide, baicalin, golden thread, menthol crystal and borneol. The detection method has good linear relationship, precision, stability, repeatability and recovery rate, and has great significance to strict control over quality of the tranquilizing pills and the preparation thereof.
Owner:哈尔滨蒲公英药业有限公司
Qingkailing dispersing tablet
ActiveCN1682836AHas the function of clearing heat, detoxifying, calming and calming the nervesAntipyreticAnalgesicsDiseaseAcute Pharyngitis
Owner:哈药集团三精千鹤制药有限公司
Method for controlling quality of injection liquid or powder of
A process for preparing the liquid injection or powder injection of 'Qingkailing' is an improvement to existing process in such areas that high-speed centrifugal technique is used to prepare the liquid extracts of capejasmine fruit, isatis root and honeysuckle flower, and the ultra-filter technique is used for the collected liquid extract, resulting in high curative effect and long effective period. Its quality control method and product discriminating method are also disclosed.
Owner:GUIZHOU YIBAI PHARMA CO LTD
Chinese medicine eye drops and its making method
InactiveCN1939456AEasy to useEnsure safetyOrganic active ingredientsSenses disorderCholic acidVitamin C
A Chinese medicine in the form of eyedrops is prepared proportionally from cholic acid, nacre, deoxychlolic acid of pig, capejasmine fruit, buffalo horn, isatis root, scutellaria root, honeysuckle flower, and auxiliary chosen from endrate disodium, VC sodium, tegosept M and propyl parasept.
Owner:JINLING PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com