Obeticholic acid preparation method
A technology of obeticholic acid and hyodeoxycholic acid, applied in the direction of steroids, organic chemistry, etc., can solve the problems of high cost, reduction, and method practicability, and achieve the effect of easy cost and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
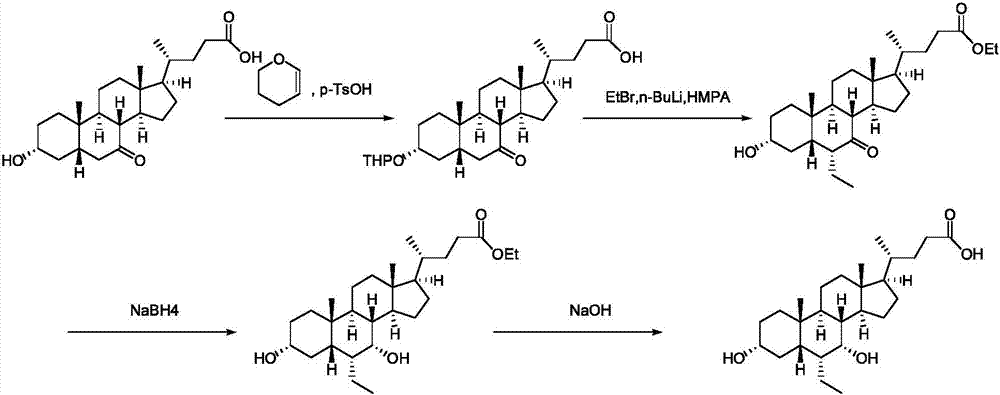
[0048] A preparation method of obeticholic acid, the structural formula of the obeticholic acid is as follows:
[0049]
[0050] Including the following steps:
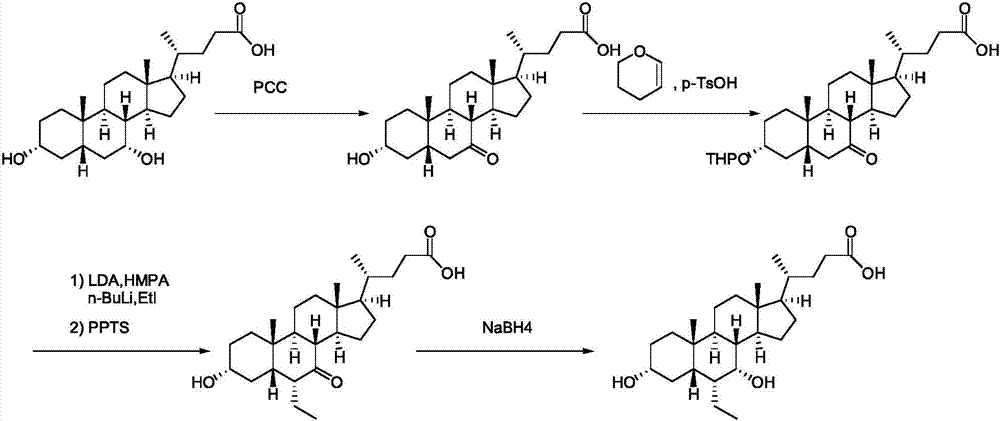
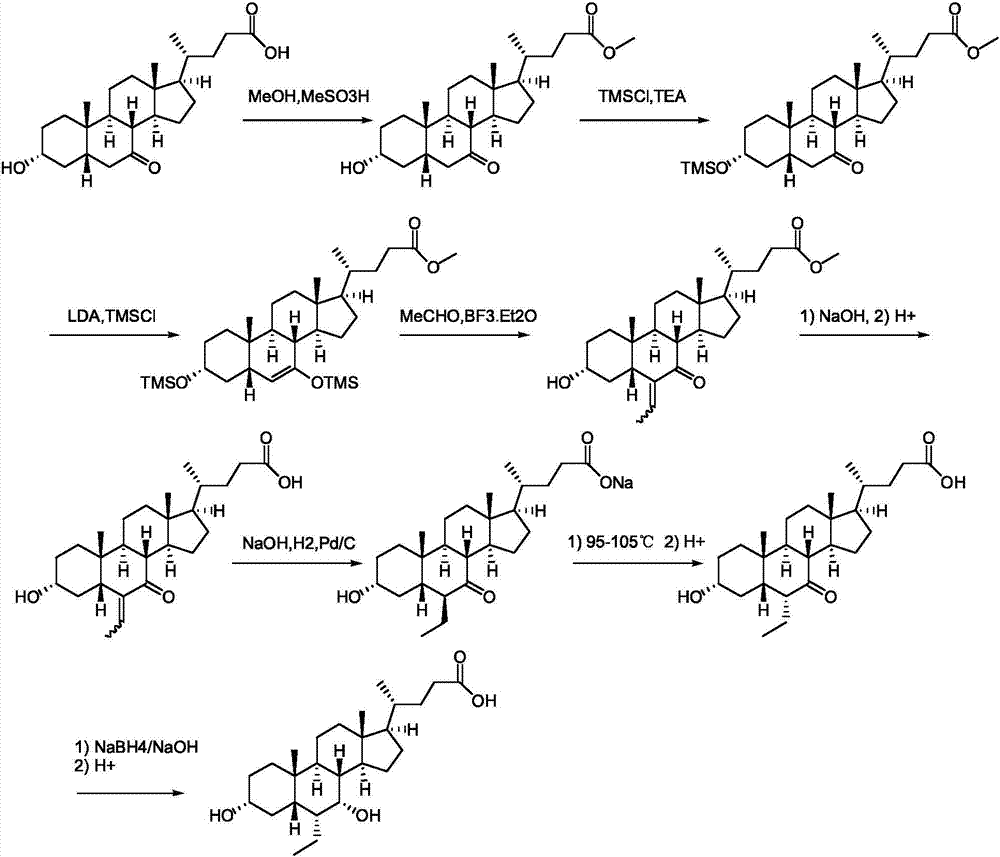
[0051] (1) Hyodeoxycholic acid II reacts with alcohol compound III under the action of a catalyst to generate ester compound IV: add hyodeoxycholic acid II (39.26g, 0.1mol) in 500mL three-necked flask and dissolve it in 100mL methanol, add 0.5 mL of concentrated sulfuric acid was heated and refluxed overnight; the reaction solution was cooled to room temperature, diluted with 400 mL of ethyl acetate, washed with saturated aqueous sodium bicarbonate solution (100 mL*2) and 100 mL of saturated saline, dried over anhydrous sodium sulfate, and suction filtered. The filter cake was rinsed with 50 mL of ethyl acetate, and the filtrates were combined and concentrated to dryness under reduced pressure to obtain ester compound IV (40 g, yield 98.5%): 1 H-NMR (CDCl3): 4.06(m, IH), 3.67(s, 3H), 3.62(m, 1H), 2.36(m, 1H), 2.24...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com