Method for synthetizing porcine cholic acid into ursodeoxycholic acid through semi-enzymatic method
A technology for ursodeoxycholic acid and porcine cholic acid, which is applied in the field of synthesizing ursodeoxycholic acid, can solve the problems of abandonment, undeveloped utilization, and short supply of chenodeoxycholic acid, and achieves cost saving, quality improvement and transformation efficiency, reducing environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
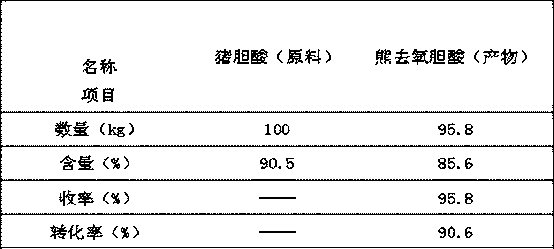
[0027] A method for synthesizing ursodeoxycholic acid from hyocholic acid by a semi-enzymatic method is as follows:
[0028] 1. Oxidation:
[0029] Take 100kg of hyocholic acid and put it into the oxidation reaction kettle, add 600kg of n-butanol, heat to dissolve, add 120kg of water, adjust the pH value to 2.5 with hydrochloric acid, control the temperature within 0°C, add 30kg of calcium hypochlorite in batches under stirring, and react After 1 hour, a sample was taken to detect that the hyocholic acid content was 0.3%, and the reaction was finished. The temperature of the above solution was raised to 60°C, and the lower aqueous phase was separated to obtain a n-butanol solution of 3,6,7 keto-cholanic acid, which was then transferred to a reduction reaction kettle.
[0030] 2. Restoration:
[0031] Add 70kg of glucose to the reduction reactor, add 300kg of 25.3mmol / L phosphate buffer solution, add 50g of NADP (nicotinamide adenine dinucleotide phosphate), 30g of NAD (nicot...
Embodiment 2
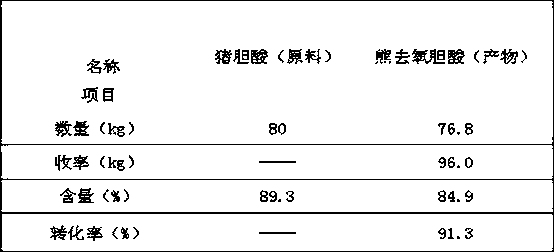
[0039] A method for synthesizing ursodeoxycholic acid from hyocholic acid by a semi-enzymatic method is as follows:
[0040] 1. Oxidation:
[0041] Take 80kg of hyocholic acid and put it into the oxidation reaction kettle, add 500kg of tert-amyl alcohol, heat to dissolve, add 100kg of water, adjust the pH value to 3 with hydrochloric acid, control the temperature within 5°C, add 24kg of bromine in batches under stirring, and react for 1 hour Reaction ends when taking a sample to detect after the hyocholic acid content is 0.36%. The above solution was heated to 60°C, and the lower aqueous phase was separated to obtain a tert-amyl alcohol solution of 3,6,7 keto-cholanic acid, which was then transferred to a reduction reaction kettle.
[0042] 2. Restoration:
[0043] Add 56kg of lactic acid to the reduction reactor, add 240kg of 32.7mmol / L phosphate buffer solution, add 30g of NADP (nicotinamide adenine dinucleotide phosphate), 12g of NAD (nicotinamide adenine dinucleotide), 3...
Embodiment 3
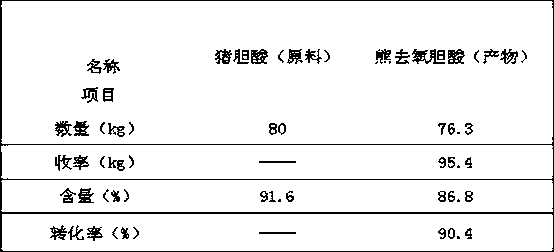
[0051] A method for synthesizing ursodeoxycholic acid from hyocholic acid by a semi-enzymatic method is as follows:
[0052] 1. Oxidation:
[0053] Take 80kg of hyocholic acid and put it into the oxidation reaction kettle, add 500kg of n-butanol, heat to dissolve, add 100kg of water, adjust the pH value to 2.0 with hydrochloric acid, control the temperature within 10°C, add 24kg of calcium hypochlorite in batches under stirring, and react After 1 hour, take a sample and detect that the reaction ends when the hyocholic acid content is 0.45%. The temperature of the above solution was raised to 60°C, and the lower aqueous phase was separated to obtain a n-butanol solution of 3,6,7 keto-cholanic acid, which was then transferred to a reduction reaction kettle.
[0054] 2. Restoration:
[0055] Add 56kg of glucose to the reduction reactor, add 240kg of 48.3mmol / L phosphate buffer solution, add 35g of NADP (nicotinamide adenine dinucleotide phosphate), 12g of NAD (nicotinamide aden...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com