Synthesis of tuberculosis resistant compound of arguesterol
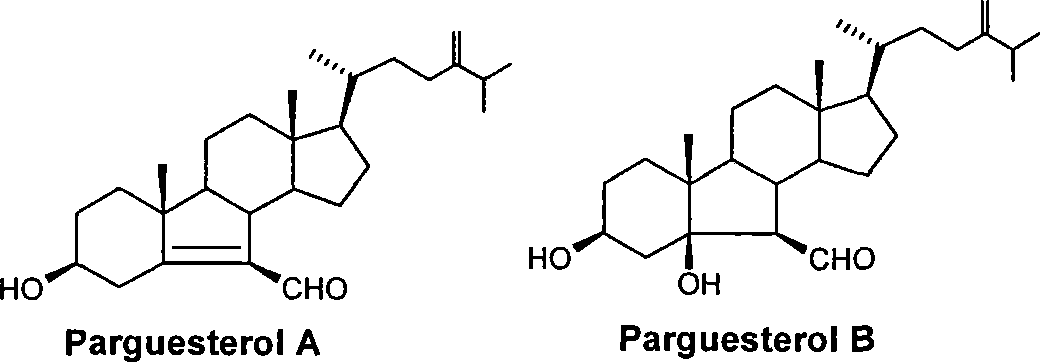
A technology for the synthesis of Pugsterol and its synthesis method, which is applied in the direction of steroids, organic chemistry, antibacterial drugs, etc., and can solve the problem that the synthesis of Pugsterol A and Pugsterol B has not been reported, strepsterol is not easy to obtain, and cannot be synthesized in large quantities and other issues, to achieve the effect of easy access, cheap raw materials, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
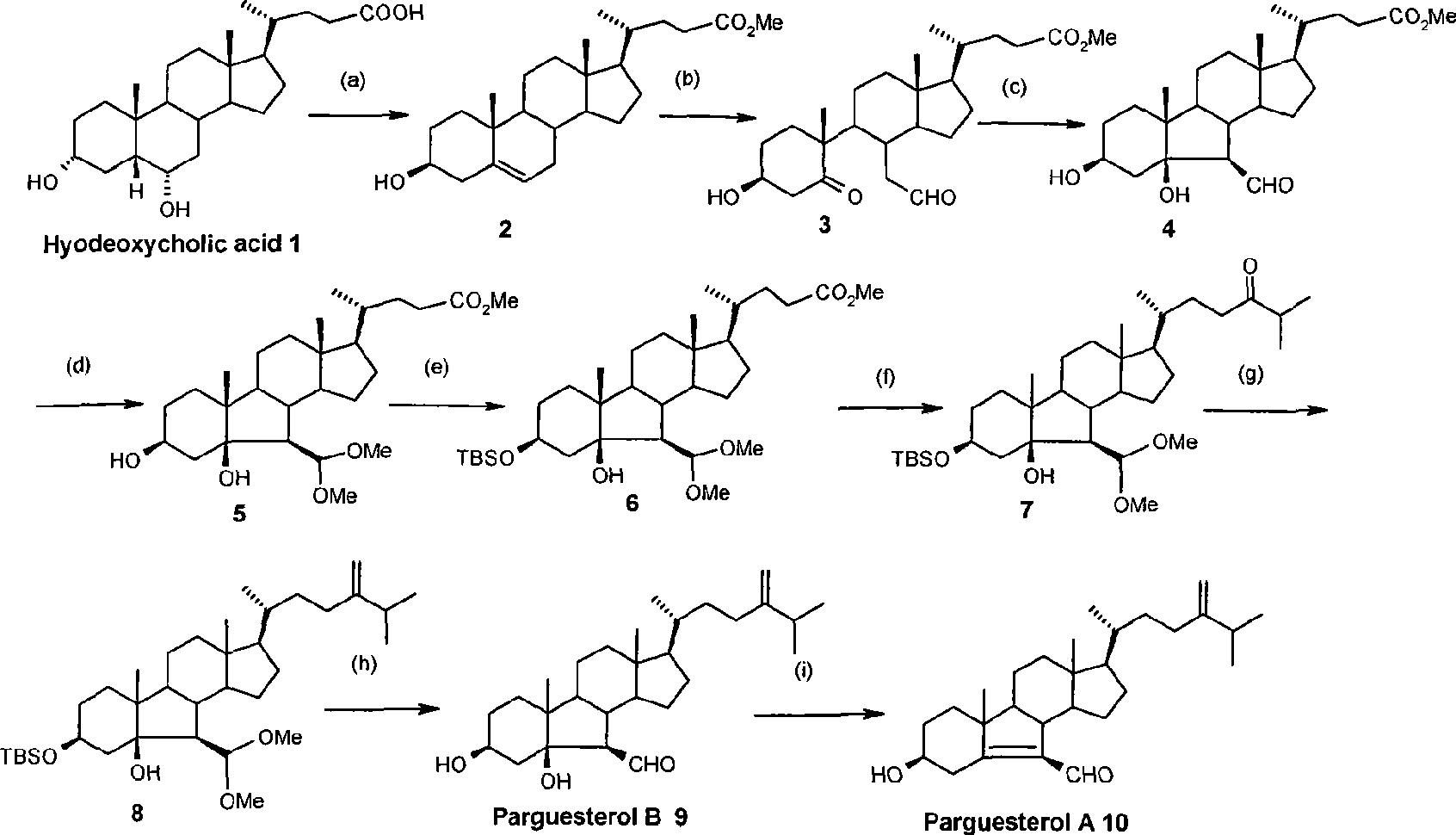
[0067] Preparation Δ 5 Methyl-3β-hydroxycholanate:
[0068]
[0069] Using hyodeoxycholic acid 1 as a raw material, compound Δ was synthesized in 94% yield through three steps of methyl esterification, p-toluenesulfonyl chloride esterification, nucleophilic substitution-trans elimination 5 - Methyl 3β-hydroxycholanate. Melting point: 143.4°C-144.3°C. [α] D 20 -42.3°.
[0070] The product is detected by nuclear magnetic resonance, and the data are as follows: 1 H NMR (400MHz, CDCl 3 )δ ppm : 0.679(s, 3H, 18-H 3 ), 0.925 (d, 3H, J=6.4Hz, 21-H 3 ), 1.006(s, 3H, 19-H 3 ), 3.526(m, 1H, 3α-H), 3.665(s, 3H, COOCH 3 ), 5.359 (d, 1H, J=3.6Hz, 6-H). Detected by mass spectrometry, the data are as follows: EI-MS (m / z): 388 (M), 370 (M-H 2 O). After infrared detection, the data is as follows: IR (cm -1 ): 3489 (OH), 1716 (COOCH 3 ).
Embodiment 2
[0072] Preparation of methyl 5,6-broken-3β-hydroxy-5-keto-6-aldehyde cholanate: (ozonation reaction)
[0073]
[0074] Add 3.635g of Δ to a 250ml three-necked bottle 5 -3β-Hydroxycholic acid methyl ester (9.368mmol), under nitrogen protection, add dichloromethane and anhydrous methanol mixture 150ml (CH 2 Cl 2 :MeOH=4:1), placed in a low-temperature reaction tank at -78°C. Introduce ozone for about 30 minutes, the system appears light blue, stop the infusion of ozone, wash the reaction system with nitrogen for 40 minutes, add dropwise a mixture of 8ml dimethyl sulfide and 10ml dichloromethane. After the dropwise addition was completed, it was allowed to rise to room temperature naturally, and stirred overnight. Concentrate to obtain 5.325 g of crude product. Flash column chromatography (Pet:EtOAc=1.75:1) gave 3.355 g of a colorless oily substance, yield 85%. [α] D 20 : +82.3° (C, 0.53, CHCl 3 ).
[0075] The product is detected by nuclear magnetic resonance, and th...
Embodiment 3
[0077] Preparation of B-carbo-3β, 5β-dihydroxy-6β-formaldehyde methyl cholanate: (Adol condensation reaction)
[0078]
[0079] Add 191mg of 5,6-broken-3β-hydroxyl-5-ketone-6-formaldehyde cholanoic acid methyl ester (0.453mmol) in 100ml eggplant-shaped bottle, dissolve with 8ml of dry benzene, add 1.923g of neutral alumina (18.85 mmol), stirred overnight at room temperature, filtered, washed with ethyl acetate, and concentrated to give 203 mg of crude product. Flash column chromatography (Pet:EtOAc=2.5;1) gave 177 mg of colorless crystals, yield 92%. Melting point: 93.3-94.4°C (literature mp: 95-96°C); [α] D 20 : +31.5°(C, 0.53, CHCl 3 )(Document[α] D 20 : +34.6° (C, 1.0, CHCl 3 )).
[0080] The product is detected by nuclear magnetic resonance, and the data are as follows: 1 HNMR (400MH Z , CDCl 3)δ ppm : 0.717(s, 3H, 18-H 3 ), 0.925 (d, 3H, J=6.4Hz, 21-H 3 ), 0.93(s, 3H, 19-H 3 ), 3.636(s, 1H, 6-H), 3.660(s, 3H, COOCH 3 ), 4.119 (m, 1H, 3α-H), 9.698 (d, J=3...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com