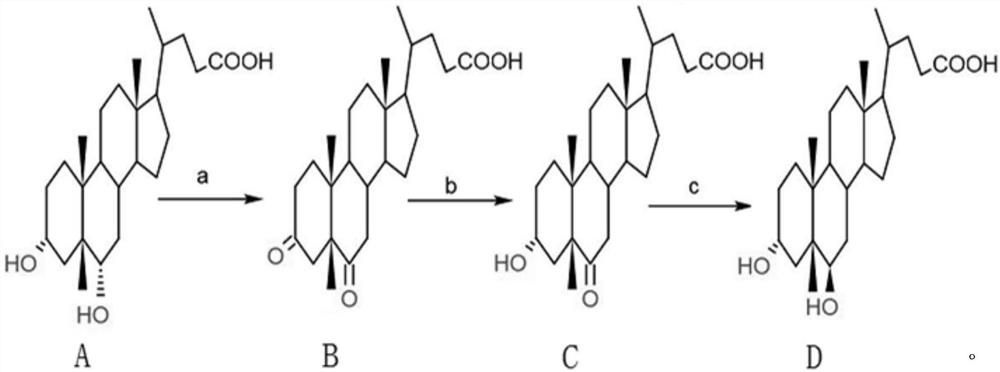
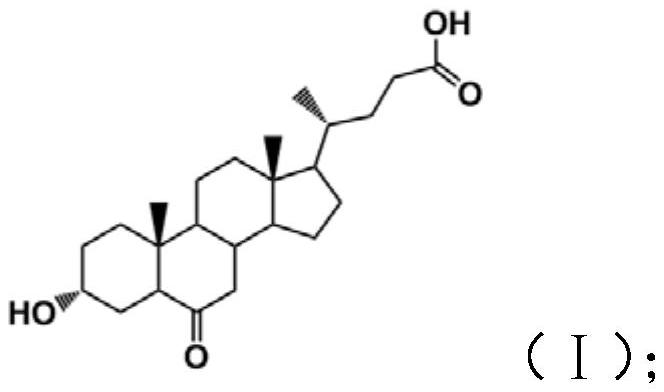
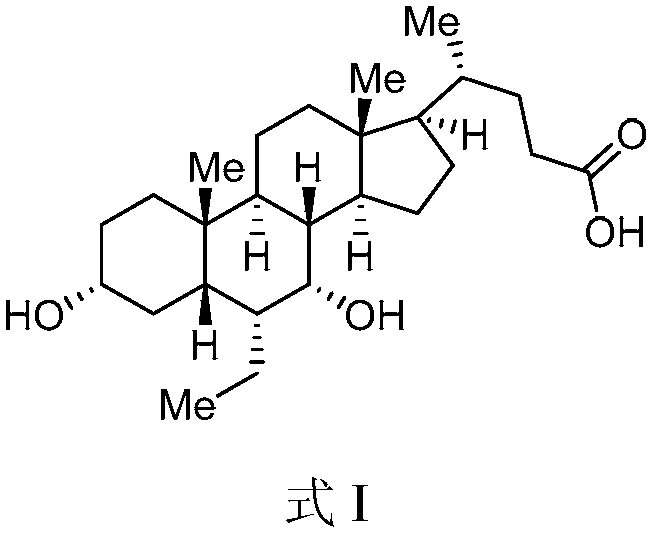
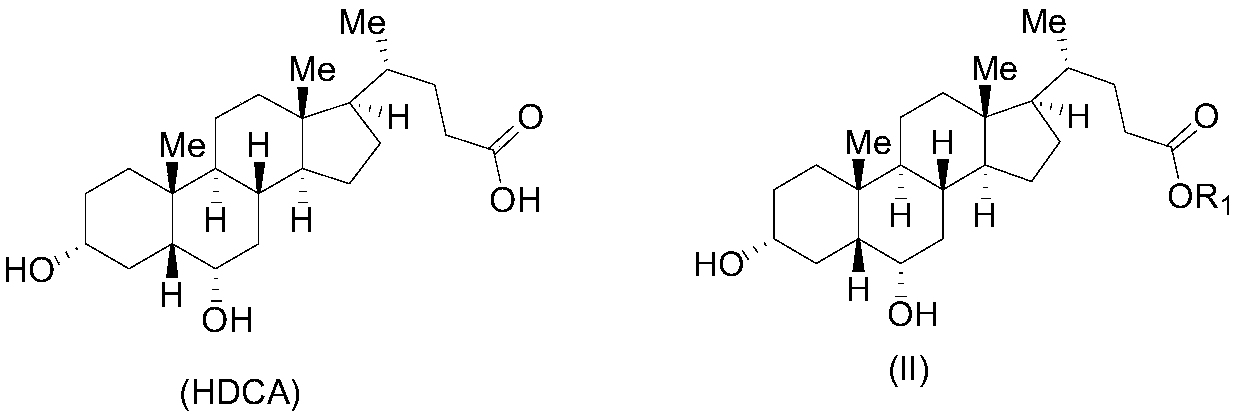
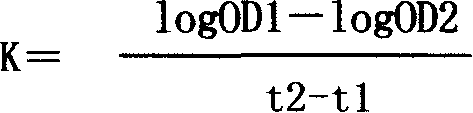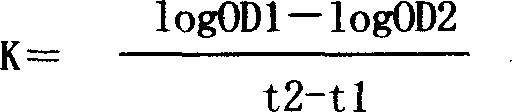Patents
Literature
34 results about "Hyodesoxycholic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
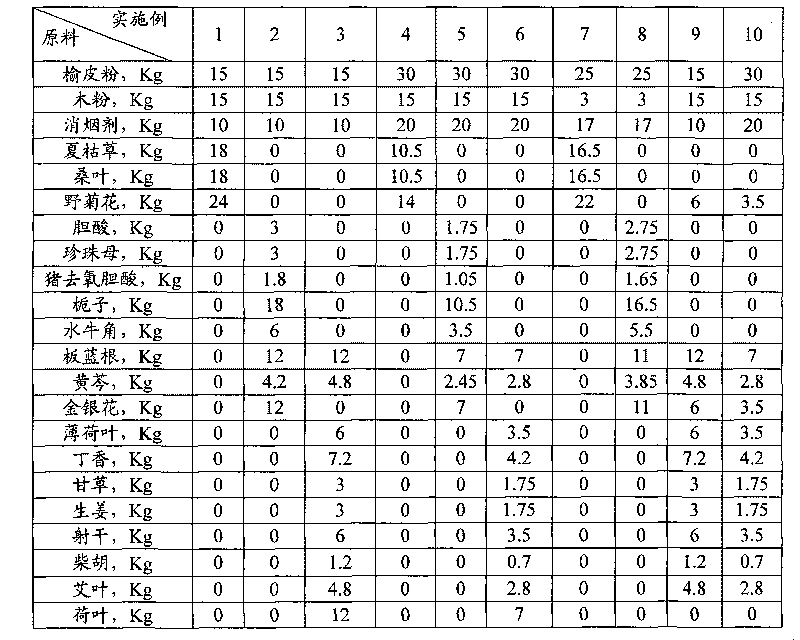
Chinese herbal medicine health-care joss stick
InactiveCN101690503AReduce burning rateSolve the problem of irritating and easily causing drynessBiocidePest repellentsDisinfectantPeppermints
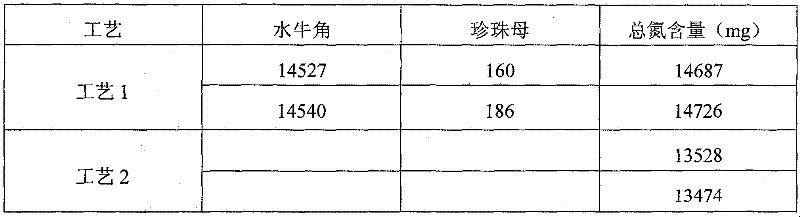
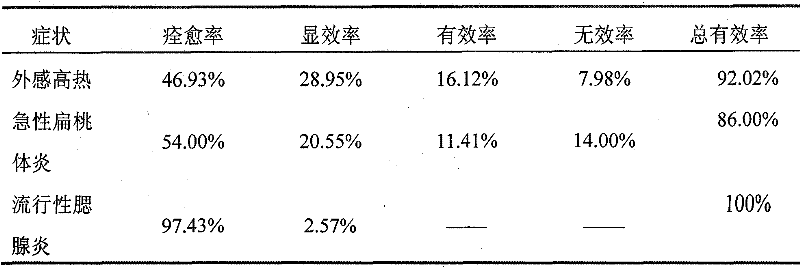
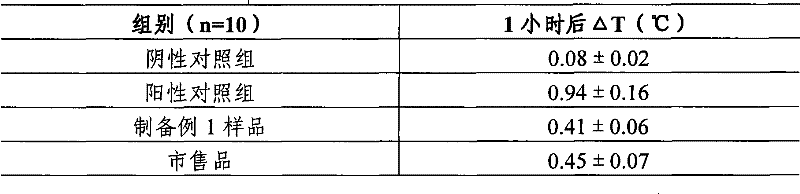
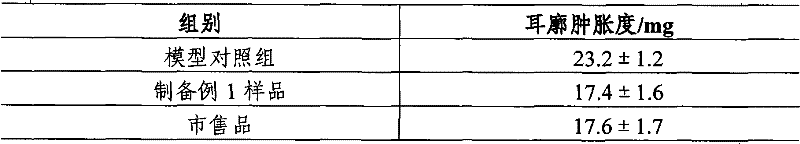
The invention discloses a Chinese herbal medicine health-care joss stick, comprising a substrate prepared by mixing elm bark powder, cellulosine, a smoke eliminating agent and an additive (additives); the additive (additives) is (are) one or a plurality of an air freshener, a disinfectant, a helminthic epidemic-prevention material and an anti-flu material; the air freshener comprises one or a plurality of peppermint leaf, clove, liquorice and radix isatidis; the disinfectant comprises one or a plurality of ginger, blackberry lily, chrysanthemum flower, wild chrysanthemum flower and lotus leaf; the helminthic epidemic-prevention material comprises one or a plurality of artemisia leaf, radix scutellariae and Chinese thorowax; and the anti-flu material is the mixture of selfheal, mulberry leaf and wild chrysanthemum flower, or the mixture of cholic acid, mother-of-pearl, hyodesoxycholic acid, gardenia, cornu bubali, indigowoad root, radix scutellariae and honeysuckle flower. By adding different kinds of Chinese herbal medicine additives, the Chinese herbal medicine health-care joss stick realizes many beneficial functions, such as air refreshing, disinfection, helminthic epidemic-prevention, flu prevention and the like, thereby being an incensing joss stick.
Owner:何丹凤
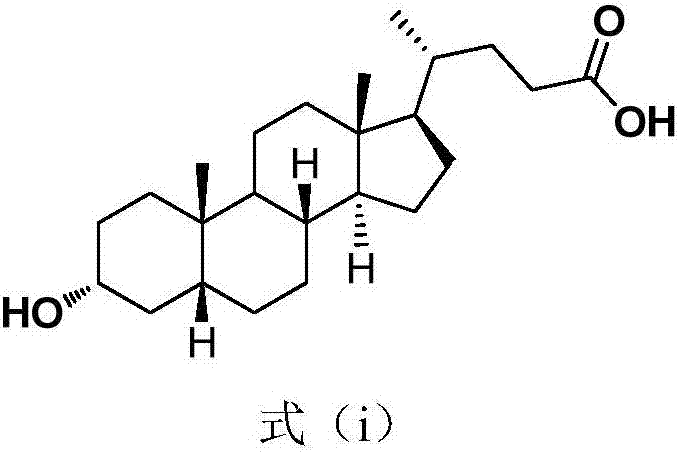
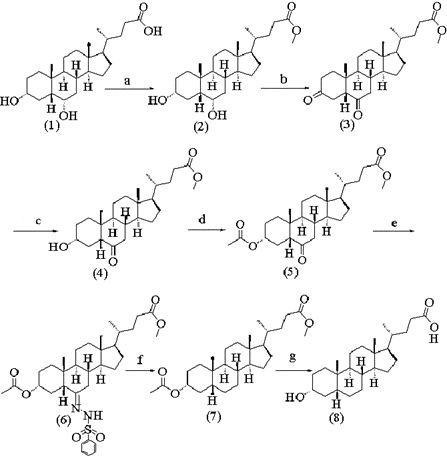
Method for synthesizing lithocholic acid from hyodesoxycholic acid
ActiveCN106977572AReduction reaction with few side reactionsSimple post-processingSteroidsSynthesis methodsHyodeoxycholic acid
The invention discloses a method for synthesizing lithocholic acid, comprising: using hyodesoxycholic acid as a start material, and performing two-step reaction of 6Alpha-OH selective oxidation and Huang Minglon reduction to synthesize the lithocholic acid. The start material herein is low in price and easy to obtain, the synthetic steps are short, posttreatment is simple, few side reactions are employed, and the method is applicable to industrial production.
Owner:JIANGSU JIAERKE PHARMA GRP CORP
Quality detection method for liver protection dropping pill of traditional Chinese medicine preparation
ActiveCN103808842AQuality improvementEfficient detectionComponent separationChlorogenic acidMedicine
The invention relates to a quality detection method for a liver protection dropping pill of traditional Chinese medicine preparation. The quality detection method comprises the following steps: measuring the content of effective components of the liver protection dropping pill, including saikoside a and schizandrin, and identifying schisandra chinensis, pulvis fellis suis and artemisia capillaris in the liver protection dropping pill. The quality detection method also comprises the following detection steps: (1) with the saikoside a as a reference substance, measuring whether a radix bupleuri component is contained in a liver protection dropping pill recipe by adopting a high efficiency liquid chromatography method; (2) with the schizandrin as the reference substance, measuring whether a schizandrin component is contained in the liver protection dropping pill by adopting the high efficiency liquid chromatography method; (3) with the schisandrin b as the reference substance, identifying whether a schisandra chinensis component is contained in the liver protection dropping pill by adopting a thin-layer chromatography method; (4) with hyodeoxycholic acid as the reference substance, identifying whether a pulvis fellis suis component is contained in the liver protection dropping pill by adopting the thin-layer chromatography method; and (5) with chlorogenic acid as the reference substance, identifying whether an artemisia capillaris component is contained in the liver protection dropping pill by adopting the thin-layer chromatography method. The quality detection method disclosed by the invention can be used for effectively and reliably controlling the quality of the liver protection dropping pill, and the method is scientific, feasible and reliable.
Owner:HEILONGJIANG KUIHUA PHARMA
Medicine composition
ActiveCN101773563AReduce allergic reactionsImprove safety with highOrganic active ingredientsNervous disorderChlorogenic acidAdditive ingredient
The invention provides a medicine composition, which is prepared from the following medicine effect raw materials: cholic acid, nacre, hyodesoxycholic acid, gardenia, cornu bubali, radix isatidis, baicalin and honeysuckle. The medicine composition comprises the following medicine effect ingredients in percentage by weight: 1.5 to 4.2 parts of cholic acid type ingredients, 3.5 to 5.5 parts of baicalin, 0.1 to 0.5 part of jasminoidin, 3.5 to 5.5 parts of amino acid, 0.05 to 0.08 part of nucleosides compounds and at most 0.1 part of chlorogenic acid. Experiments show that the medicine composition of the invention has the medicine effect similar to Qingkailing, but the anaphylactic reaction is obviously reduced, and the medicine use safety is improved.
Owner:HEILONGJIANG ZBD PHARMA
Method for measuring content of sodium cholate
ActiveCN104458930AAccurate quantitative analysisShorten the timeComponent separationHyodeoxycholic acidOxygen
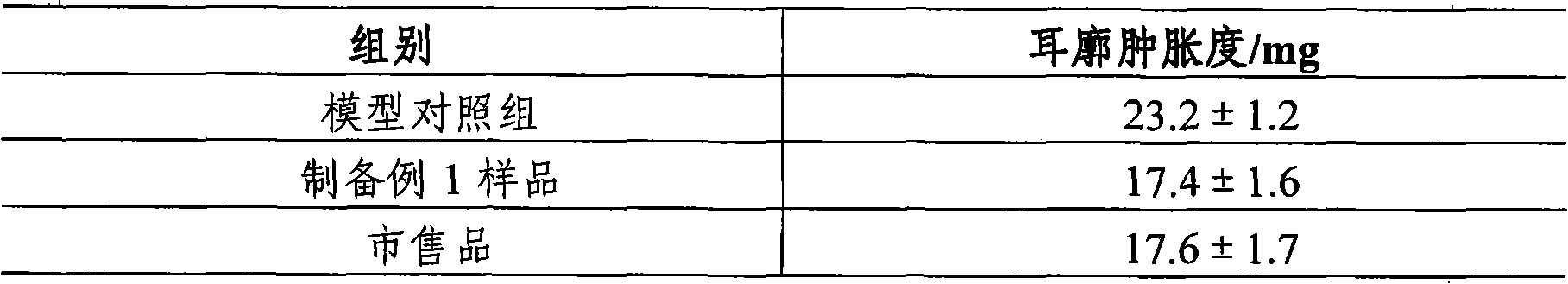
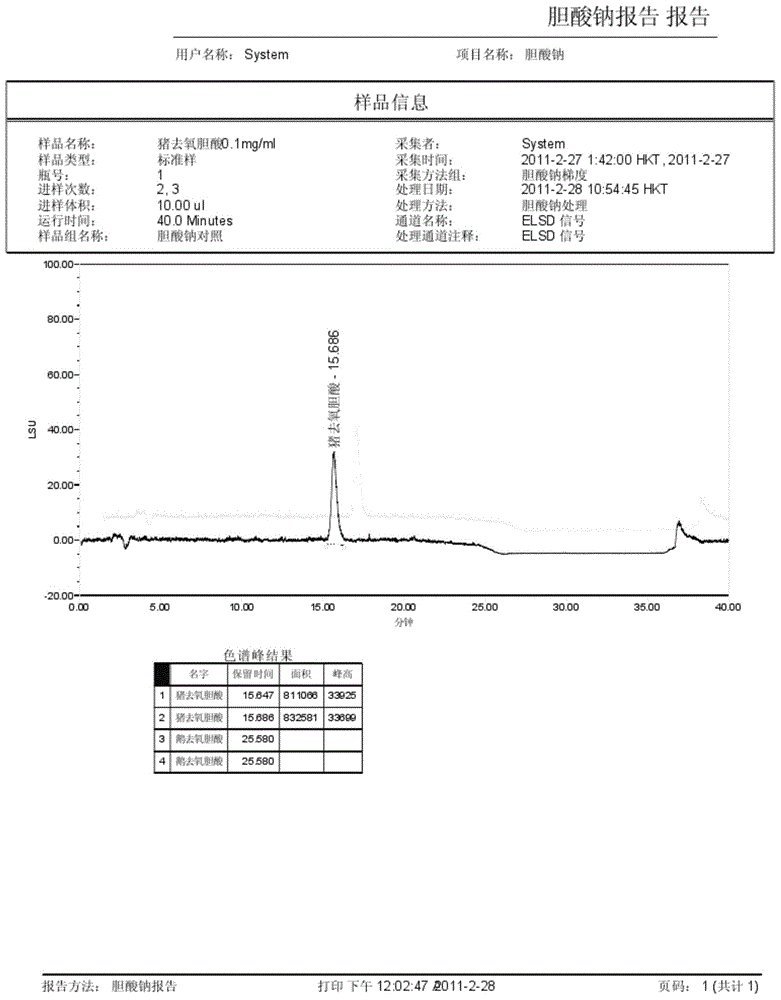
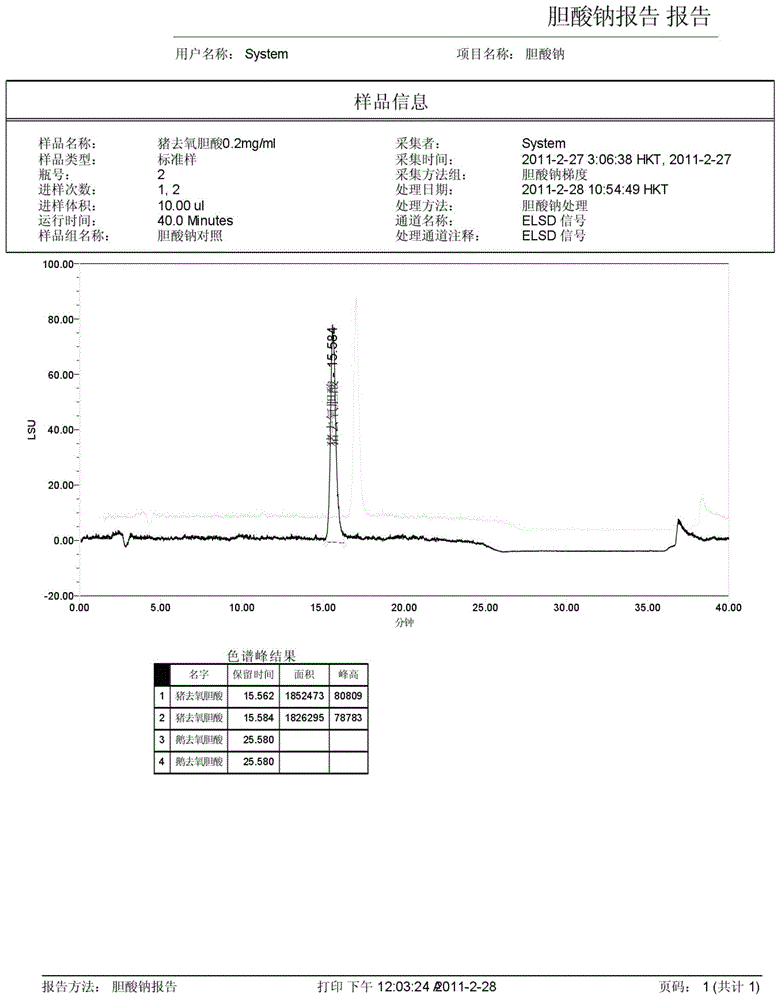
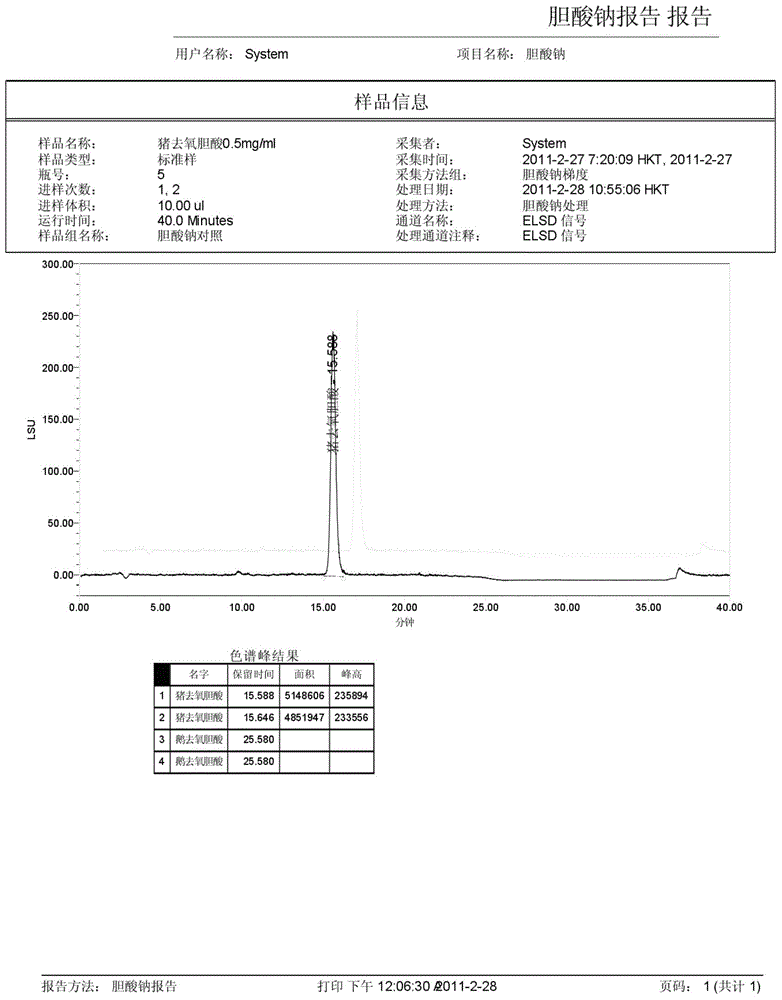
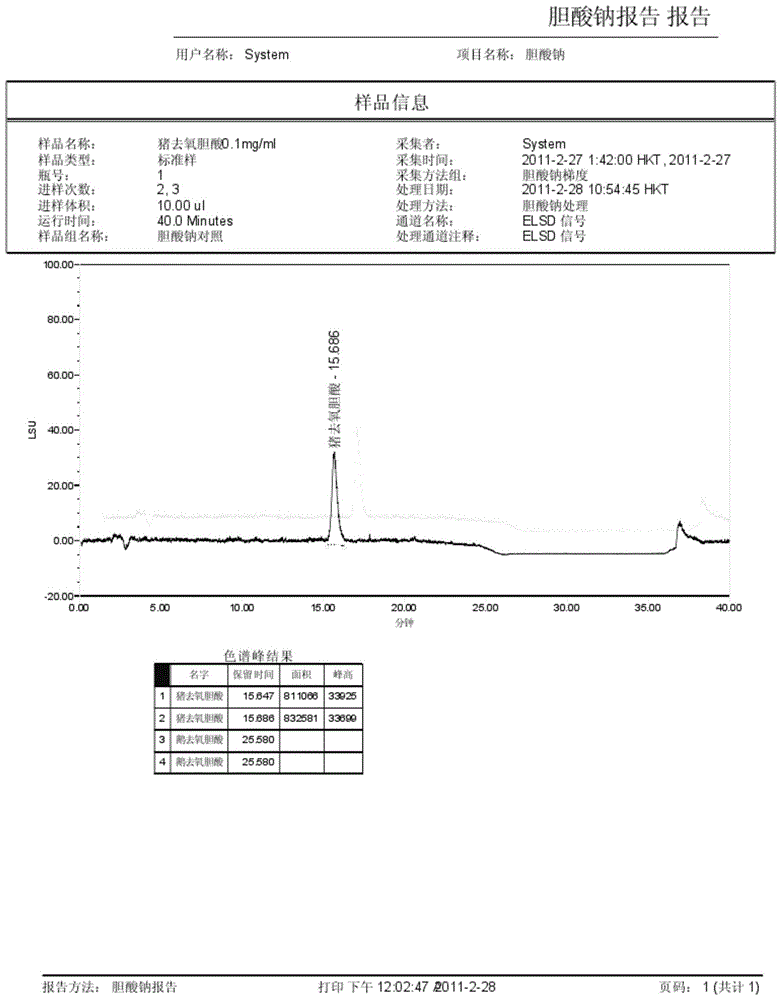
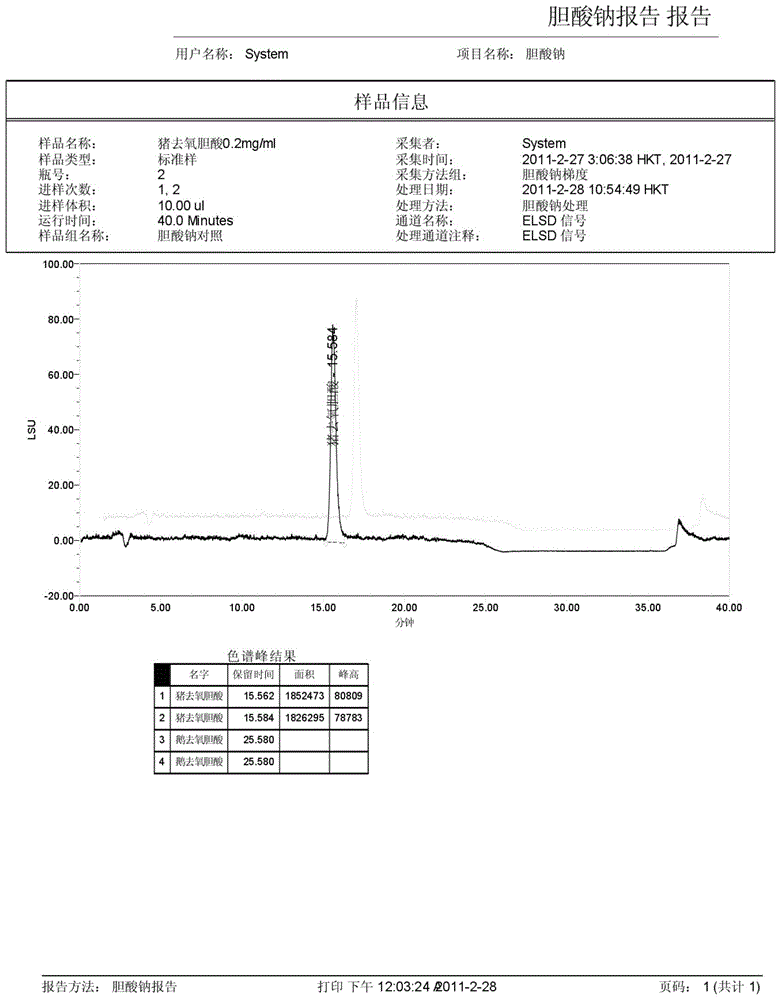
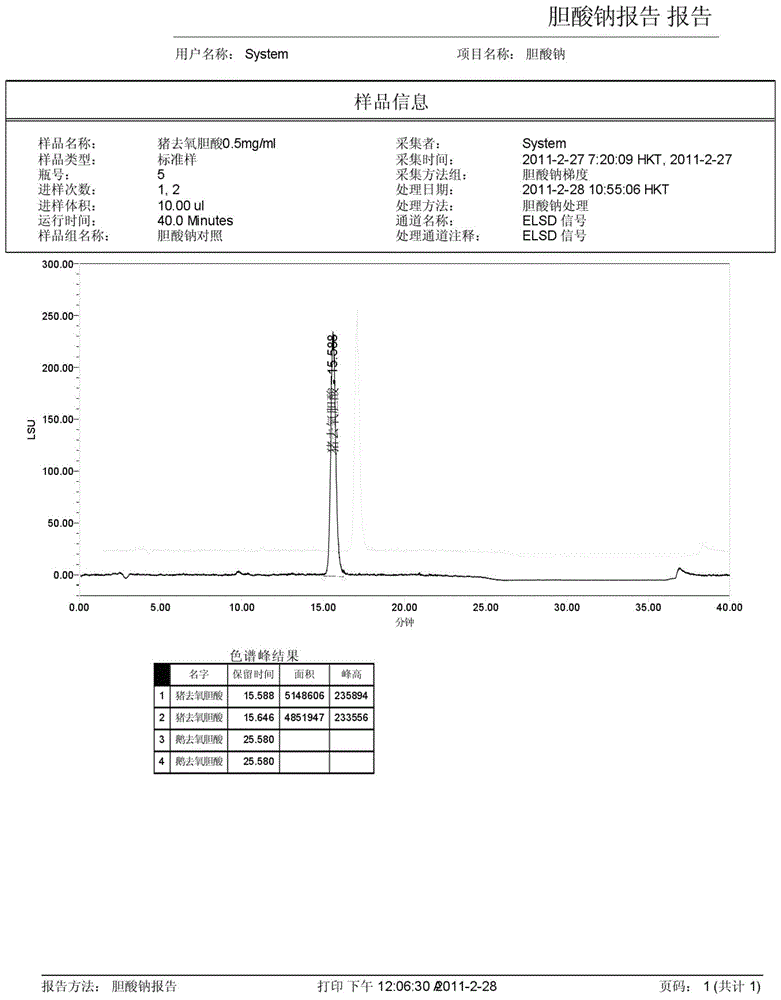
The invention discloses a method for measuring the content of sodium cholate. The method comprises the following steps: (1) preparing a sodium cholate solution to be measured; (2) respectively preparing a standard hyodesoxycholic acid solution and a standard chenodeoxycholic acid solution; and (3) measuring the contents of hyodesoxycholic acid and chenodeoxycholic acid which are contained in the sodium cholate solution to be measured by adopting a high efficiency liquid chromatography method. The method disclosed by the invention has the characteristics of easiness and convenience for operation, accuracy in result, short measurement time and high generalizability and can be widely applied to the technical field of component detection.
Owner:WUHAN BIOCHEM PHARMA
Method for determining content of hyodeoxycholic acid in Beiling capsules through HPLC (High Performance Liquid Chromatography)-ELSD (Evaporative Light Scattering Detector) method
InactiveCN104090056AHigh sensitivitySuitable for quantitative analysisComponent separationTheoretical plateHyodeoxycholic acid
The invention discloses a method for determining the content of hyodeoxycholic acid in Beiling capsules through an HPLC(High Performance Liquid Chromatography)-ELSD (Evaporative Light Scattering Detector) method. The method disclosed by the invention is characterized by comprising the following steps: (1) preparation of a sample, namely preparing hyodeoxycholic acid and Beiling capsule samples, and preparing a test solution from Beiling capsules; (2) HPLC, wherein the flowing phase is acetonitrile-0.05%-0.15% acid solution, the weight percentage of the chromatographic column to the moving phase is (50-75) to (25-50), the flow velocity is 1.0ml.min<-1>, and the sample introduction volume is 20mu l; and (3) detection, wherein an ELSD detector takes air as a carrier gas, the temperature of a drift tube starts from 90 DEG C, once detection is conducted every 5DEG C, the air flow velocity is 1.7-3.0L.min<-1>, and calculation is conducted according to the peak area of hyodeoxycholic acid, the separation degree is not lower than 2.0, and a theoretical plate is not lower than 3000. The method can be used for determining the content of hyodeoxycholic acid, is good in specificity, durability, precision and accuracy, and is applicable to control on the total mass of Beiling capsules.
Owner:SHANGHAI LEIYUNSHANG PHARMA
Preparation method of murine deoxycholic acid
The invention discloses a preparation method of murine deoxycholic acid, which is characterized in that hyodeoxycholic acid (3alpha, 6alpha-dihydroxyl-5beta-cholanic acid) is used as a raw material, and the process comprises the steps of 3, 7-site hydroxyl oxidation, 3-site carbonyl reduction, 7-site carbonyl reduction and purification to obtain high-purity murine deoxycholic acid (3alpha, 6beta-dihydroxyl-5beta-cholanic acid). The murine deoxycholic acid synthesized and prepared through the route is wide in raw material source and sufficient in supply; and the method has the advantages of high yield, high purity and few side reactions, is suitable for large-scale preparation, and can provide data support and standard samples for subsequent related researches.
Owner:ZHONGSHAN BAILING BIOTECHNOLOGY CO LTD
Artificial bezoar and preparation method thereof
InactiveCN101530428ASimple methodEasy to operateUnknown materialsAnhydride/acid/halide active ingredientsAscarisHyodeoxycholic acid
The invention discloses artificial bezoar and a preparation method thereof, and relates to the technical field of production formula and technology of medicine. Sodium bilirubinate, calcium bilirubinate, cholic acid, taurodeoxycholic acid or hyodesoxycholic acid, combined cholesterol or free cholesterol, combined taurine or free taurine or mixture of the combined taurine and the free taurine, ox-bile powder or porcine-bile powder are mixed and grinded into powder. The artificial bezoar contains the sodium bilirubinate, the calcium bilirubinate and other components same as natural bezoar, but does not contain free bilirubin; and the total bilirubin has a constant content of more than 0.1 to 35 percent. The components of each batch of the artificial bezoar are consistent. The artificial bezoar contains no harmful bacteria, virus or ascaris eggs; and the efficacy is higher than the prior artificial bezoar but is equal to the natural bezoar.
Owner:张恒洲
Quality control method of macaque stone bovinebezoar powder
ActiveCN101168009APerfect quality control methodQuality improvementNervous disorderHydroxy compound active ingredientsCinnabarMedicine
The invention discloses a quality control method of monkey bezoar and cow-bezoar powder. The quality control standards of the former monkey bezoar and cow-bezoar powder is revised, lamella distinguish method of bilirubin, monkey bezoar, grassleaf sweelflag rhizome, alpinia zerumbet and licorice is added, lamella check method of hyodesoxycholic acid is also added, and further assay method of cinnabar and cholic acid is added. The quality control method of the monkey bezoar and cow-bezoar powder of the invention has a strong specificity, and the quality of the product is more effectively controlled, further the safety and the efficiency of medicines taking by human bodies are guaranteed.
Owner:GUANGZHOU BAIYUNSHAN QIXING PHARMA
Serum metabolism marker for diagnosing benign and malignant pulmonary nodules and application of the metabolic marker
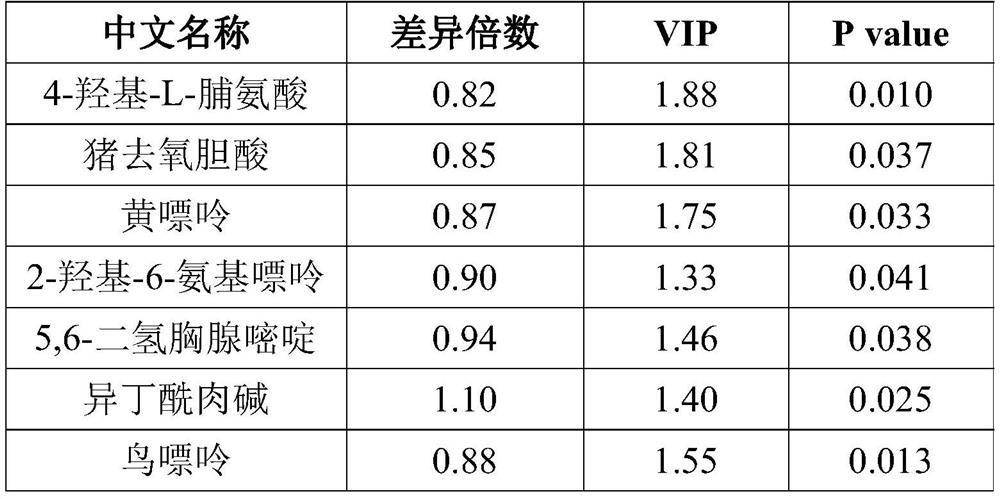
The invention provides a metabolic marker for diagnosing benign and malignant pulmonary nodules and application of the metabolic marker. The metabolic marker is selected from one or combination of 4-hydroxy-L-proline, hyodeoxycholic acid, xanthine, 2-hydroxy-6-aminopurine, 5, 6-dihydrothymine, isobutyryl carnitine, guanine, glutamic acid, asparagine, formononetin, alanine and kynuric acid. The 12 metabolic markers provided by the invention can accurately diagnose and distinguish lung benign and malignant nodules, have high sensitivity and strong specificity, can well replace the existing tissue biopsy and imaging diagnosis mode, reduce trauma and radiation risks, and have clinical use and popularization values.
Owner:SHANGHAI FIRST PEOPLES HOSPITAL
Method for separating hyodeoxycholic acid from pig gall paste by extraction method
The invention relates to a method for separating hyodeoxycholic acid from pig gall paste by an extraction method. The method comprises the following steps: 1, esterification of pig gall paste; 2, concentrating; 3, extraction; 4, concentrating and crystallizing; 5, hydrolytic acidification; 6, drying; 7, crystallizing, filtering and drying to obtain a hyodeoxycholic acid finished product. The method has the advantages that the yield is high and up to 20%-30% (the yield of a traditional method is 10%-15%); the purity is high, and the melting point is 200-204 DEG C (the melting point of the traditional method is 170-190 DEG C); the production period is shortened, and the use amount of an organic solvent is small (the use amount of the traditional method is large); and the product is white powder in appearance(faint yellow or yellow powder in the traditional method).
Owner:ANHUI KEBAO BIOLOGICAL ENG CO LTD
Medicine for treating acute and chronic nasopharyngitis and its preparation process
The present invention discloses one kind of me for treating acute and chronic nasopharyngitis. The medicine is prepared through mixing patchouli oil in 1-30 weight portions and hyodesoxycholic acid in 10-300 weight portions, adding proper amount of supplementary material for orally taken preparation and re-mixing. The medicine of the present invention is used in treating nasopharyngitis and has the outstanding advantages of small dosage, easy taking, high effect and fast acting.
Owner:GUANGZHOU UNIVERSITY OF CHINESE MEDICINE
Pharmaceutical composition for treating cerebral apoplexy and its preparation method
The invention discloses a drug combination and its preparing method, made by taurine, hyodesoxycholic acid, baicalin, Gardenia, and mother-of- pearl, firstly preparing gardenia extract and mother-of-pearl powder hydrolyzate, then adding in taurine and hyodesoxycholic acid and regulating the pH value to obtain a mixed drug solution; making alcohol deposition on the mixed drug solution, then adding in fine baicalin powder, and making the processes of regulating pH value, etc, to obtain a total mixed drug solution; taking the total mixed drug solution to prepare into various preparations. The invention improves Gardenia extracting technique, forming a semi-finished product with Gardenia glycoside as the main component. On the premise of keeping the biological effect of qingkai ling curing cerebral embolism disease, it basically eliminates original-existing bad reactions, achieving the purpose of stabilizing effect and detoxicating, able to be used to develop new medicines for curing cerebral embolism and cerebral hemorrhage diseases.
Owner:BEIJING UNIV OF CHINESE MEDICINE
Medicine for treating acute and chronic nasopharyngitis and its prepn process
InactiveCN1931229APeace of mindStrict compatibilityOrganic active ingredientsRespiratory disorderChronic nasopharyngitisMedicine
The present invention discloses one kind of me for treating acute and chronic nasopharyngitis. The medicine is prepared through mixing patchouli oil in 1-30 weight portions and hyodesoxycholic acid in 10-300 weight portions, adding proper amount of supplementary material for orally taken preparation and re-mixing. The medicine of the present invention is used in treating nasopharyngitis and has the outstanding advantages of small dosage, easy taking, high effect and fast acting.
Owner:GUANGZHOU UNIVERSITY OF CHINESE MEDICINE
Quality control method of macaque stone bovinebezoar powder
ActiveCN101168009BPerfect quality control methodQuality improvementNervous disorderHydroxy compound active ingredientsBiotechnologyCholic acid
The invention discloses a quality control method of monkey bezoar and cow-bezoar powder. The quality control standards of the former monkey bezoar and cow-bezoar powder is revised, lamella distinguish method of bilirubin, monkey bezoar, grassleaf sweelflag rhizome, alpinia zerumbet and licorice is added, lamella check method of hyodesoxycholic acid is also added, and further assay method of cinnabar and cholic acid is added. The quality control method of the monkey bezoar and cow-bezoar powder of the invention has a strong specificity, and the quality of the product is more effectively controlled, further the safety and the efficiency of medicines taking by human bodies are guaranteed.
Owner:GUANGZHOU BAIYUNSHAN QIXING PHARMA
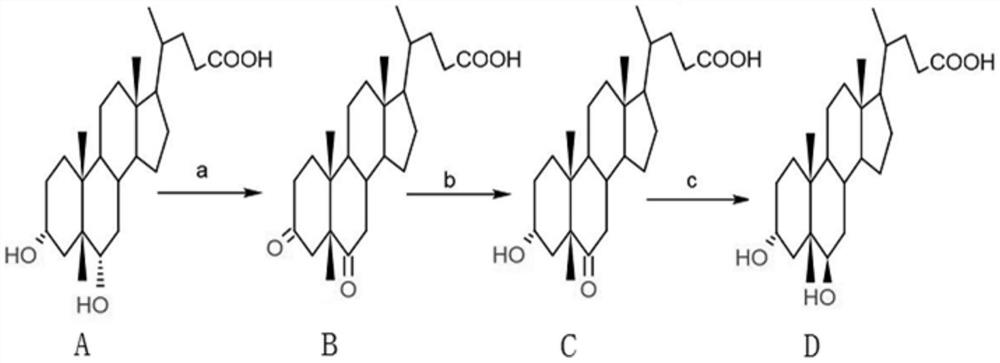
A kind of method utilizing hyodeoxycholic acid to prepare ursodeoxycholic acid
ActiveCN113135973BImprove protectionGood production safetySteroidsFermentationCholic acidChemo enzymatic
Owner:泰安市炜创生物技术中心
Application of Qingkailing composition in preparation of medicine for treating and/or preventing radiation damage
ActiveCN107126474BImprove thymus indexIncrease spleen indexOrganic active ingredientsDermatological disorderCholic acidCerebral hemorrhages
The invention relates to the field of medicine, in particular to application of Qingkailing composition in the preparation of drugs for treating and / or preventing radiation injury; the Qingkailing composition, a traditional Chinese medicine composition, is mainly prepared from cholic acid, nacre mother of pearl, hyodeoxycholic acid, cape jasmine fruit, buffalo horn, isatis root, baicalin, and immature flower of Japanese honeysuckle. There are various forms of Qingkailing at present, including injections, oral liquids, capsules, granules, pills and the like; these drugs are clinically applicable to patients with fever, coma, hemiplegia, obnubilation, acute hepatitis, upper respiratory infections, pneumonia, cerebral thrombosis, and cerebral hemorrhage.
Owner:SHINEWAY PHARMA GRP LTD +2
A chemical-enzymatic method for preparing ursodeoxycholic acid
ActiveCN109154016BReduce manufacturing costReduce process stepsSteroidsFermentationCholic acidSulfohydrazide
A chemical-enzymatic method for preparing ursodeoxycholic acid, comprising adding hyodeoxycholic acid into the first organic solvent, and oxidizing under the action of an oxidizing agent to obtain 6-oxo-lithocholic acid to obtain 6-oxo- Lithocholic acid; the 6-oxo-lithocholic acid and the sulfonyl hydrazide derivative are added to the second organic solvent to make the 6-oxo-lithocholic acid and the sulfonyl hydrazide derivative generate affinity Nucleoaddition-elimination reaction to obtain hydrazone compounds; in an inert gas environment, use a reducing agent to reduce the hydrazone compounds to obtain lithocholic acid; carry out the lithocholic acid under the catalysis of hydroxylase and coenzyme Hydroxylation reaction to obtain ursodeoxycholic acid. The method uses hyodeoxycholic acid as the initial raw material, and obtains ursodeoxycholic acid through a three-step chemical method and a one-step biological enzymatic method. The whole synthesis process has few steps, simple operation, high yield and low cost, and can be widely applied on an industrial scale Production.
Owner:BONTAC BIO ENG SHENZHEN
Method for preparing beta-hyodeoxycholic acid by microbial transformation
InactiveCN112094883ALow costFacilitate the realization of industrial productionBacteriaMicroorganism based processesBiotechnologyMicroorganism
The invention discloses a method for preparing beta-hyodeoxycholic acid by microbial transformation. According to the method, the hydroxyl group at the 3-position of hyodeoxycholic acid is transformedfrom the alpha-position to the beta-position by microbial transformation. The method comprises the following steps: preparing spores and seeds; performing microbial transformation on alpha-hyodeoxycholic acid; and performing separation and purification. The method disclosed by the invention is simple to operate, the alpha-hyodeoxycholic acid with rich resources and low price is taken as a raw material, and the beta-hyodeoxycholic acid is obtained by biological transformation, so that industrial production of the beta-hyodeoxycholic acid is realized, and development and utilization of the beta-hyodeoxycholic acid are promoted.
Owner:CHANGDE YUNGANG BIOTECHNOLOGY CO LTD
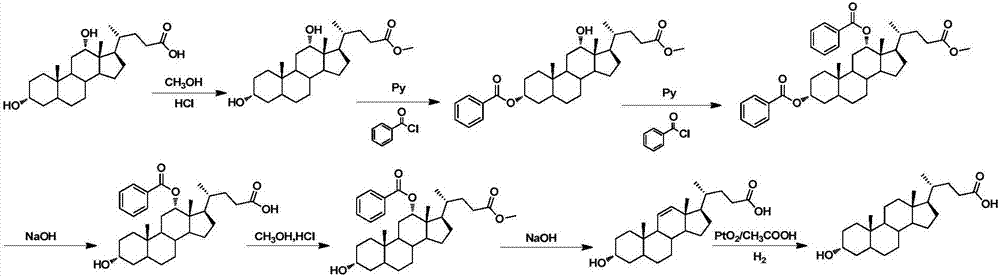
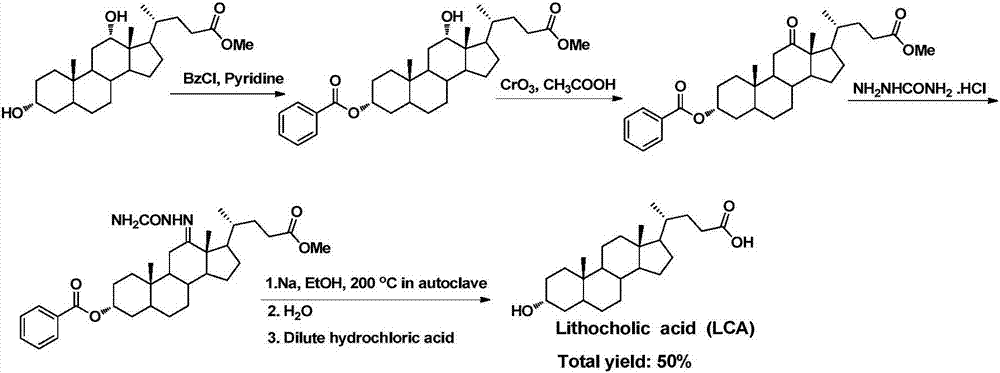
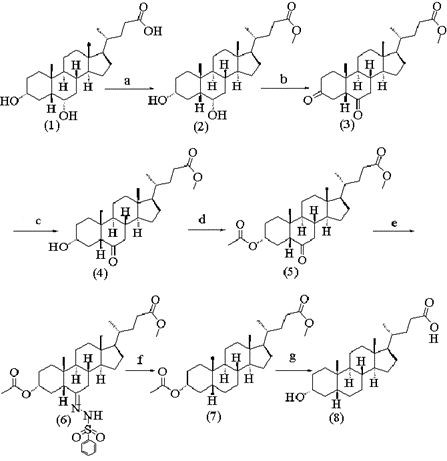
A method for synthesizing lithocholic acid from hyodeoxycholic acid
ActiveCN109134576BSynthetic process conditions are safeSynthetic process conditions are mildSteroidsCholic acidHydrolysis
The invention discloses a method for synthesizing lithocholic acid by using hyodeoxycholic acid as a raw material. The present invention uses hyodeoxycholic acid as a starting material, undergoes 7 steps of 24-carboxyl esterification, 3α-hydroxyl and 6α-hydroxyl oxidation to carbonyl, selective reduction, acylation, hydrazone formation, dehydrazone, and hydrolysis to form stones. cholic acid. The starting material of the invention is cheap and easy to obtain, hydrazine hydrate is not used in the synthesis process, the synthesis process conditions are safe, environmentally friendly and mild, the total yield is high, and the method is suitable for industrial production.
Owner:HEZE RUIZHI TECH DEV
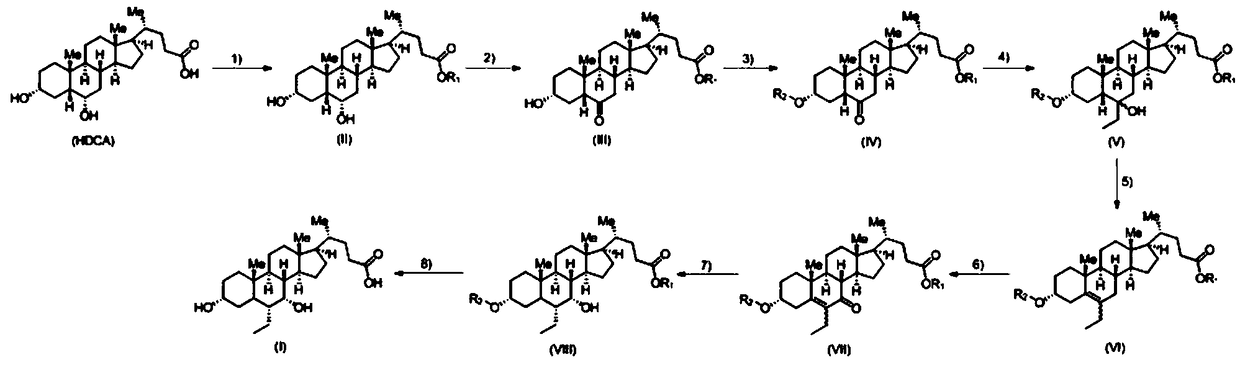
A kind of preparation method of obeticholic acid
The invention belongs to the field of medicine, particularly relates to a preparation method of 6-ethylchenodeoxycholic acid. The method comprises the following steps of using HDCA (hyodesoxycholic acid) as starting raw materials; sequentially performing esterification reaction, oxidation reaction, hydroxy group protection reaction, reaction with ethyl zinc bromide or diethyl zinc reagent, dewatering reaction, oxidation reaction, reduction reaction and hydroxyl removal and carboxyl group protection reaction to obtain the 6-ethylchenodeoxycholic acid. The method provided by the invention has the advantages that the raw material problem can be solved; the severe reaction conditions of strong alkalinity, high temperature and the like can be avoided; the synthesis efficiency of the 6-ethylchenodeoxycholic acid is greatly improved, so that the provided novel preparation method has the advantages that the byproducts are few; the operation is simple and convenient; the reaction conditions are mild; the cost is low; the method is suitable for the large-scale production of the 6-ethylchenodeoxycholic acid.
Owner:青州市欣泰生物制品有限公司
Crystallizing tank for extracting hyodeoxycholic acid
PendingCN114733225AEasy extractionFewer extraction stepsChemical industrySolution crystallizationPhysical chemistryHeat energy
The invention discloses a crystallizing tank for extracting hyodeoxycholic acid, which comprises a reaction tank and a crystallizing cabin mounted below the reaction tank, a plurality of crystallizing plates are obliquely arranged in the crystallizing cabin, hot gas discharged by the reaction tank is conveyed into the crystallizing plates after being secondarily heated, the reaction tank discharges liquid in a water curtain shape, and the crystallizing cabin is communicated with the crystallizing plates. And the liquid flows along the inclined direction of the crystallization plate and is heated to evaporate and crystallize. According to the crystallizing tank for extracting hyodeoxycholic acid provided by the invention, the crystallizing plate is filled with high-temperature steam, so that high temperature is attached to the surface of the crystallizing plate, liquid in contact with the surface of the crystallizing plate can be evaporated in an extremely short time, hyodeoxycholic acid contained in the liquid can form crystals, and the whole crystals are more convenient to extract; the previous tedious crystallization extraction steps are reduced; and the device recycles and heats steam flowing in the reaction tank, the crystallization cabin and the crystallization plate, so that resources consumed by heat energy provided for heating the crystallization plate are reduced.
Owner:福建省南仹生物科技有限公司
A kind of synthetic method of 3α-hydroxyl-5α-cholanic acid
The invention discloses a synthesis method of 3α-hydroxy-5α-cholanic acid. The present invention uses hyodeoxycholic acid as a starting material, and undergoes 4 steps of 24-carboxyl esterification, 3α-OH and 6α-OH oxidation to carbonyl, selective reduction, and Huang Minglong reaction to generate lithocholic acid isomer 3α-hydroxyl -5α-cholic acid fills the gap in the field of lithocholic acid isomer synthesis, and the method of the present invention can stably obtain the lithocholic acid isomer, namely 3α-hydroxyl-5α-cholanic acid, wherein The total yield of lithocholic acid isomers, namely 3α-hydroxy-5α-cholanic acid, was as high as 47%.
Owner:HEZE RUIZHI TECH DEV
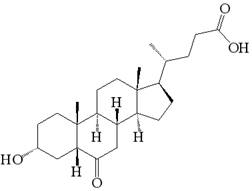
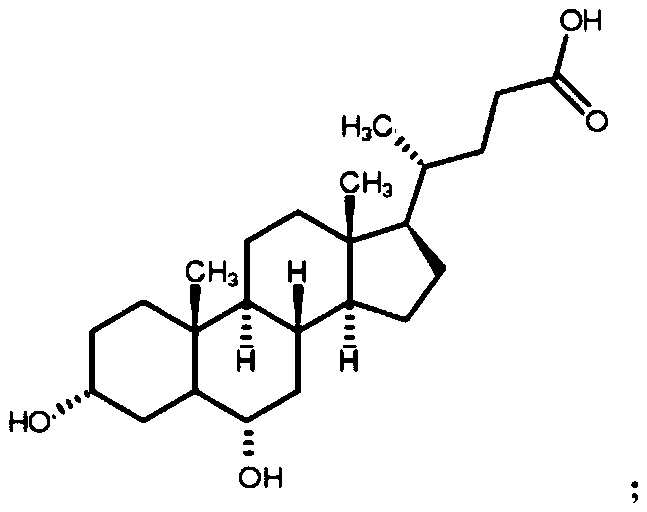
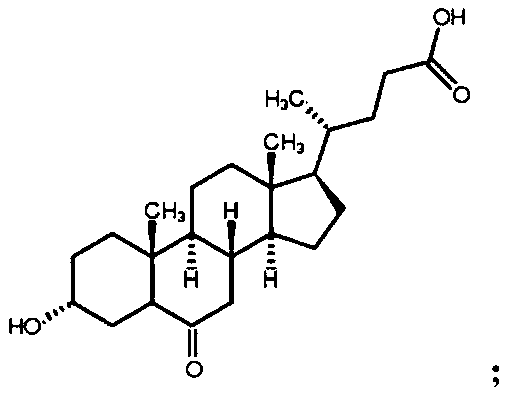
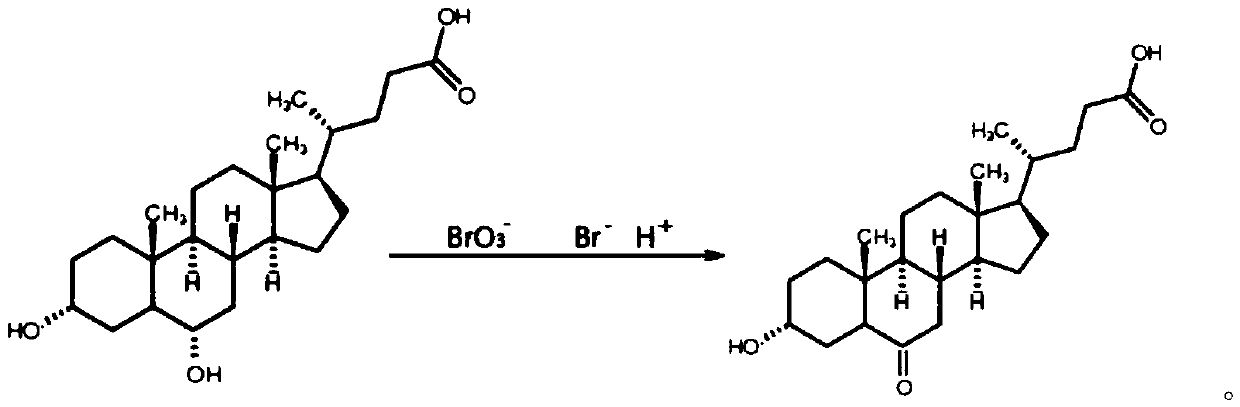
Preparation method of 3-hydroxy-6-ketocholanic acid with low cost and high yield
InactiveCN111233960ASolve unmanageable problemsHigh manufacturing process reproducibilitySteroidsCholic acidAklanonic acid
The invention discloses a preparation method of 3 alpha-hydroxy-6-keto-5 beta-cholestane-24-acid with low cost and high yield, which is characterized by comprising the following steps: adding hyodeoxycholic acid into a mixed solution of an organic solvent and water, and stirring to dissolve; adding bromide and acid, stirring and dissolving; bromate is added for reaction; adding a terminating agent, and stirring to terminate the reaction; adding water to crystallize the product; and carrying out solid-liquid separation, washing a solid product with water for multiple times, and drying to obtainthe 3 alpha-hydroxy-6-keto-5 beta-cholestane-24-acid. According to the method, a common and safe reagent is adopted, the hyodeoxycholic acid is selectively oxidized into the 3 alpha-hydroxy-6-keto-5beta-cholestane-24-acid under a relatively mild condition, the product purity is greater than 98.0%, and the yield is greater than 85%. The method has the advantages of cheap reagents, simple operation, high process reproducibility, simple post-treatment, high product purity and high yield, and can easily implement industrial production.
Owner:上海慈瑞医药科技股份有限公司
Method for preparing ursodesoxycholic acid from hyodeoxycholic acid
ActiveCN113135973AImprove protectionGood production safetySteroidsFermentationCholic acidChemo enzymatic
The invention provides a method for preparing ursodesoxycholic acid by using hyodeoxycholic acid, which comprises the following steps: firstly, oxidizing 3,6-hydroxyl of hyodeoxycholic acid into 3,6-keto by using a chemical method; secondly, reducing the 3-keto group into 3 alpha-hydroxyl by using 3[alpha]-steroid dehydrogenase, and adding hydroxyl to a substrate by using 7[beta]-hydroxylase to generate 7-hydroxyl; and finally, removing 6-keto by using a chemical method to generate UDCA. The chemical-enzyme method is simple in process, mild in reaction condition, environment-friendly, high in reaction substrate concentration and reproducible in coenzyme, the production cost and the environmental protection pressure are greatly reduced, and the chemical-enzyme method provides an environment-friendly, economical and efficient new thought for preparing the UDCA.
Owner:泰安市炜创生物技术中心
Preparation method of qingkailing syrup
ActiveCN101708238BAnti-inflammatoryGood anti-inflammatory effectOrganic active ingredientsNervous disorderSucrosePropyl p-hydroxybenzoate
Owner:哈尔滨一洲制药有限公司
Medicine composition
ActiveCN101773563BReduce allergic reactionsImprove safety with highOrganic active ingredientsNervous disorderChlorogenic acidAdditive ingredient
The invention provides a medicine composition, which is prepared from the following medicine effect raw materials: cholic acid, nacre, hyodesoxycholic acid, gardenia, cornu bubali, radix isatidis, baicalin and honeysuckle. The medicine composition comprises the following medicine effect ingredients in percentage by weight: 1.5 to 4.2 parts of cholic acid type ingredients, 3.5 to 5.5 parts of baicalin, 0.1 to 0.5 part of jasminoidin, 3.5 to 5.5 parts of amino acid, 0.05 to 0.08 part of nucleosides compounds and at most 0.1 part of chlorogenic acid. Experiments show that the medicine composition of the invention has the medicine effect similar to Qingkailing, but the anaphylactic reaction is obviously reduced, and the medicine use safety is improved.
Owner:HEILONGJIANG ZBD PHARMA
Method for extracting hyodeoxycholic acid from pig gall paste
ActiveCN114315948AHigh yieldAvoid quality impactChemical industrySteroidsSodium bicarbonateCholic acid
A method for extracting hyodeoxycholic acid from pig gall paste comprises the following steps: step 1, adding sodium hydroxide and water into the pig gall paste, heating to 100-110 DEG C, stirring and refluxing for 18-20 hours, standing and cooling to room temperature, and removing an upper solution; 2, adjusting the pH value of the lower-layer solution obtained in the step 1 to 8.2-8.3, adding magnesium salt, stirring and crystallizing for 4-6 hours, filtering, and collecting a solid; step 3, adding water into the solid collected in the step 2, heating to 40-50 DEG C, adding an acidic solution to adjust the pH to 6.2-6.3, stirring, and filtering to obtain a crude product; step 4, adding water and sodium carbonate into the crude product prepared in the step 3, stirring, heating to 90-100 DEG C, refluxing for 1 hour, standing to room temperature, and collecting filtrate; and 5, adjusting the pH value of the filtrate obtained in the step 4 to 3.3-3.4, carrying out filter pressing, washing with a sodium bicarbonate solution until the pH value is 6.5-6.6, drying, and crushing to obtain the hyodeoxycholic acid. The yield of the hyodeoxycholic acid prepared by the method is up to 35%, the melting point is 200 DEG C, the preparation steps are simple, no organic solvent is adopted for extraction, and the cost is low.
Owner:福建省南仹生物科技有限公司
A kind of assay method of sodium cholate content
ActiveCN104458930BAccurate quantitative analysisShorten the timeComponent separationHyodeoxycholic acidCholanic acid
The invention discloses a method for measuring the content of sodium cholate. The method comprises the following steps: (1) preparing a sodium cholate solution to be measured; (2) respectively preparing a standard hyodesoxycholic acid solution and a standard chenodeoxycholic acid solution; and (3) measuring the contents of hyodesoxycholic acid and chenodeoxycholic acid which are contained in the sodium cholate solution to be measured by adopting a high efficiency liquid chromatography method. The method disclosed by the invention has the characteristics of easiness and convenience for operation, accuracy in result, short measurement time and high generalizability and can be widely applied to the technical field of component detection.
Owner:WUHAN BIOCHEM PHARMA
Calculus bovis factitius and preparation method thereof
The invention discloses calculus bovis factitius and a preparation method thereof. The calculus bovis factitius comprises the following components in parts by weight: 10-15 parts of taurine, 10-15 parts of cholic acid, 5-10 parts of bilirubin, 15-20 parts of hyodeoxycholic acid, 1-5 parts of cholesterol, 1-5 parts of zinc sulfate, 0.1-3 parts of tricalcium phosphate, 0.1-1 part of ferrous sulfate and 20-50 parts of starch. The prepared calculus bovis factitius is high in the content and purity of effective components, the preparation method is simple, cost is low, and industrial production is easy to achieve.
Owner:四川菲德力制药有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com