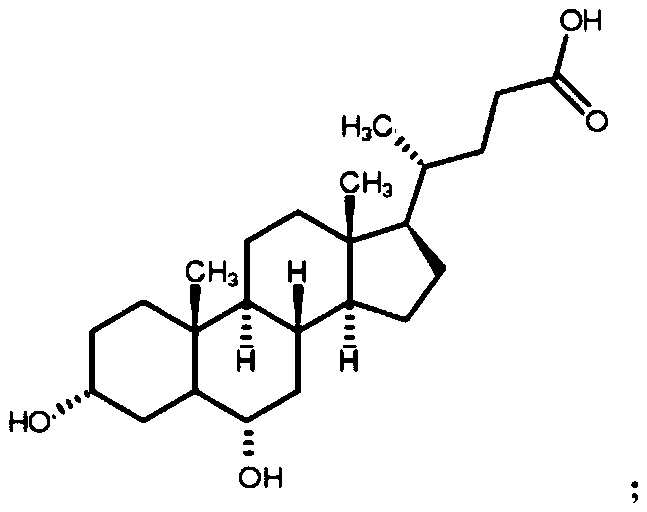
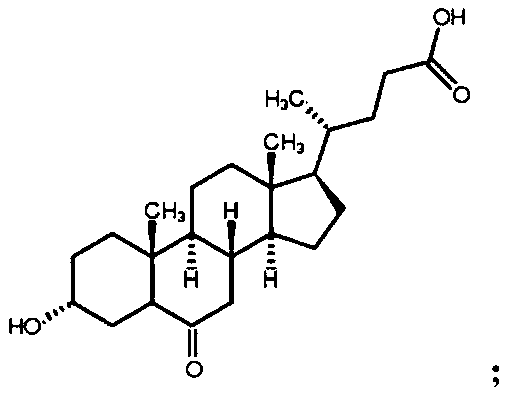
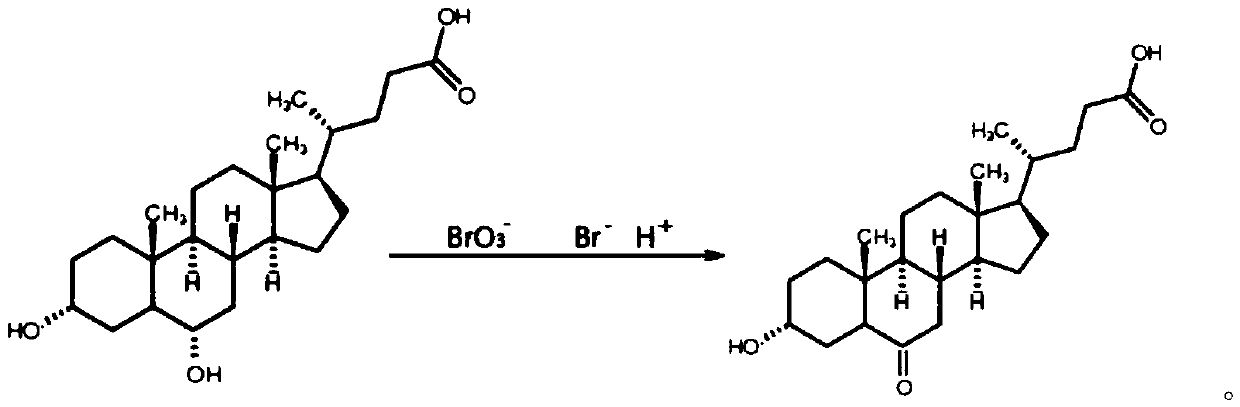
Preparation method of 3-hydroxy-6-ketocholanic acid with low cost and high yield
A high-yield technology of ketocholanoic acid is applied in the field of preparation of 3α-hydroxy-6-keto-5β-cholestane-24-acid, and can solve the problems of hindering the research and application of porcine bile acid synthesis, and the like, Achieve the effect of improving the level of medicine, simple follow-up treatment, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Take 100g of hyodeoxycholic acid, dissolve it in 1.2L of acetonitrile-water (volume ratio 10:1) mixed solvent, add 2.5g of ammonium bromide, add 50mL of 10wt% sulfuric acid, stir evenly, cool down to below 10°C, slowly drop Add 150mL of 10wt% potassium bromate solution, stir at room temperature for 24h, monitor the reaction degree with HPLC; add 15mL of 10wt% potassium bisulfite solution to terminate the reaction, stir for 10min, add 3L of water, stir well; stir and crystallize at room temperature for 0.5h, Suction filtration, washing the crystals with distilled water several times until neutral, suction filtration to obtain a white solid, transfer to 60℃±5℃ and dry for 6h to obtain 3α-hydroxy-6-keto-5β-cholestane-24-acid white powder 86.7g, with a purity of 98.3% and a yield of 86.7% as determined by high performance liquid phase-evaporative light detector (HPLC-ELSD).
Embodiment 2
[0039] Take 100g of hyodeoxycholic acid, dissolve it in 20 times of tetrahydrofuran-methanol-water (volume ratio 1:1:1) mixed solvent 2L, add 12g of potassium bromide, add 80mL of 10wt% hydrochloric acid, stir well, slowly drop Add 100mL of 15wt% sodium bromate solution, stir the reaction at room temperature for 1h, and monitor the reaction degree with HPLC; then add 80mL of 10wt% potassium sulfite solution to terminate the reaction, after stirring for 10min, add 1.2L of water, stir well; stir and crystallize at room temperature 2h, filter with suction, wash the crystals with distilled water several times until neutral, and centrifuge to obtain 3α-hydroxy-6-keto-5β-cholestan-24-acid as a white solid, which is transferred to an oven at 80-100°C for 4 hours to obtain White powder 87.3g, high performance liquid phase - evaporative light detector (HPLC-ELSD) assay purity 99.1%, yield 87.3%.
Embodiment 3
[0041] Take 100g of hyodeoxycholic acid, dissolve it in 1000mL of 10 times acetone-ethanol-water (volume ratio 3:1:1) mixed solvent, add 13g of sodium bromide, then add 2mL of formic acid, stir well, then cool down to 10°C Next, slowly add 130mL of 15wt% sodium bromate solution dropwise, stir at room temperature for 6h, and monitor the reaction degree with HPLC; then add 130mL of 10wt% sodium bisulfite solution to terminate the reaction, after stirring for 10min, add 1000mL of water, and stir evenly; room temperature Stir and crystallize for 1 hour, filter with suction, wash the crystals with distilled water several times until neutral, filter with suction to obtain a white solid, transfer to a 40°C oven and dry for 24 hours to obtain 3α-hydroxy-6-keto-5β-cholestane-24-acid 89.5 g of white powder, with a purity of 98.6% and a yield of 89.5% as determined by high performance liquid phase-evaporative light detector (HPLC-ELSD).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com