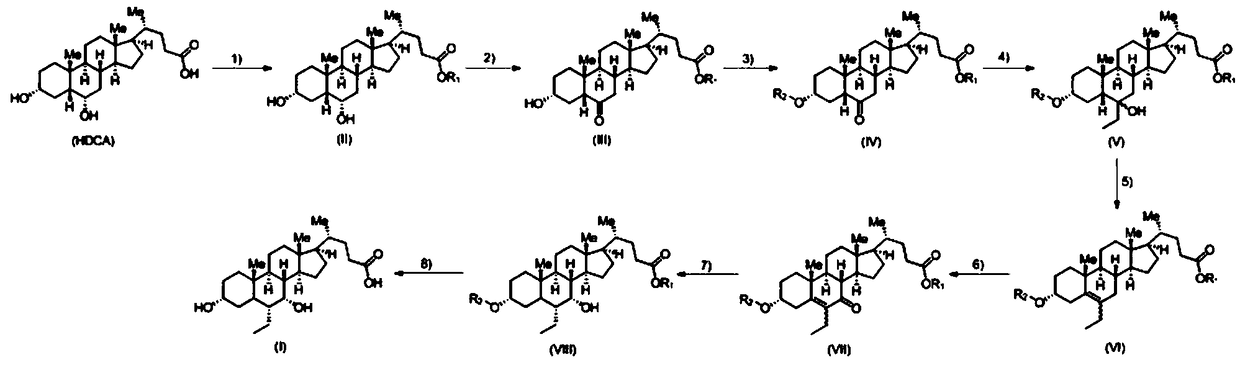
A kind of preparation method of obeticholic acid
A technology of obeticholic acid and hyodeoxycholic acid, which is applied in the field of medicine, can solve the problems of difficult scale-up production, low total yield, high cost, etc., avoid strong alkalinity and high temperature, mild reaction conditions, and improve synthesis efficiency Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0064] The present invention will be described below through specific embodiments, but the present invention is not limited thereto.
[0065] The experimental methods used in the following examples are conventional methods unless otherwise specified; the reagents and materials used in the following examples, unless otherwise specified, can be obtained from commercial sources.
[0066] Hyodeoxycholic acid (HDCA) used in the following examples was purchased from Sichuan Guanghan Bencao Plant and Chemical Co., Ltd., and its trade name is hyodeoxycholic acid.
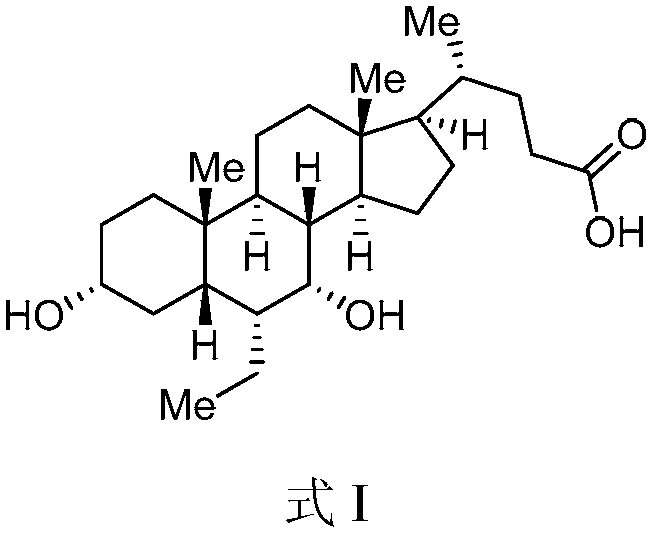
[0067] Preparation of obeticholic acid shown in the embodiment formula I
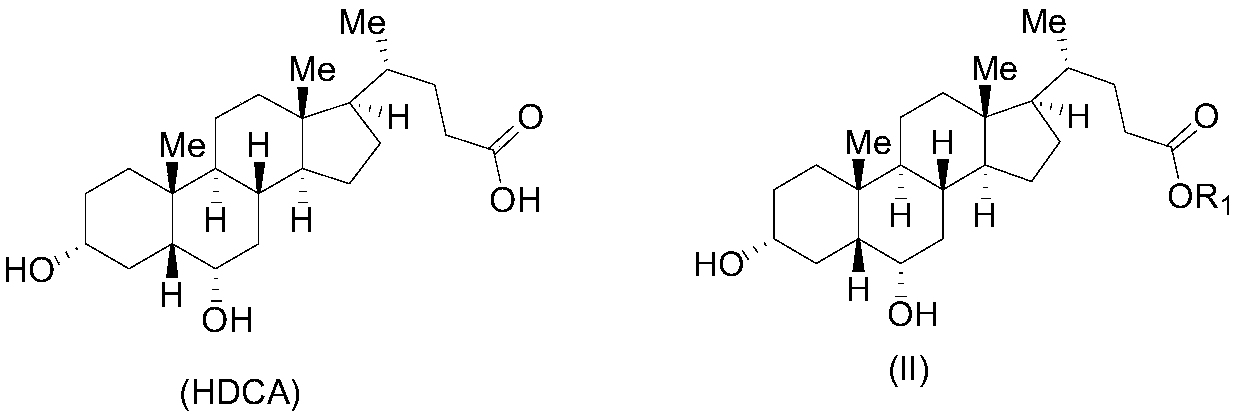
[0068] 1. The compound represented by formula II (R 1 Is ethyl) preparation
[0069] 50g of the starting material hyodeoxycholic acid (HDCA) was dissolved in 500mL of absolute ethanol, 2mL of concentrated sulfuric acid was added dropwise, stirred at room temperature for 24 hours, concentrated under reduced pressure to dryness, to obtain 52g of the compound of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com