A chemical-enzymatic method for preparing ursodeoxycholic acid
A technology for the preparation of ursodeoxycholic acid by enzymatic method, which is applied in the field of chemical-enzymatic preparation of ursodeoxycholic acid, which can solve the problems of low yield and high pollution, and achieve the effect of high purity, good activity and wide application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment 1 A method for preparing ursodeoxycholic acid by chemical-enzymatic method, comprising:
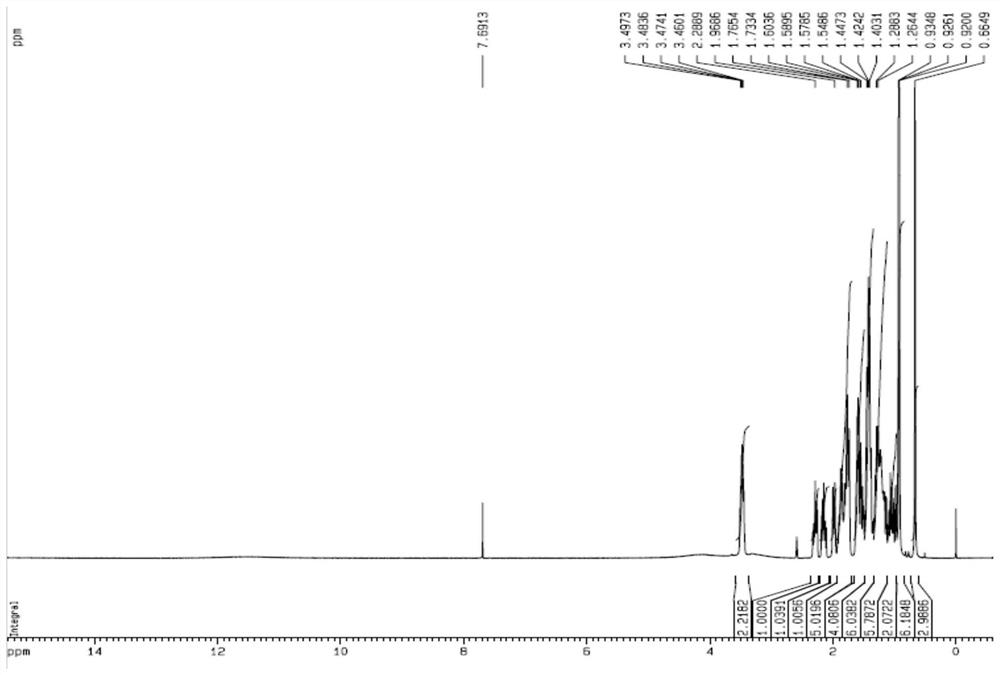
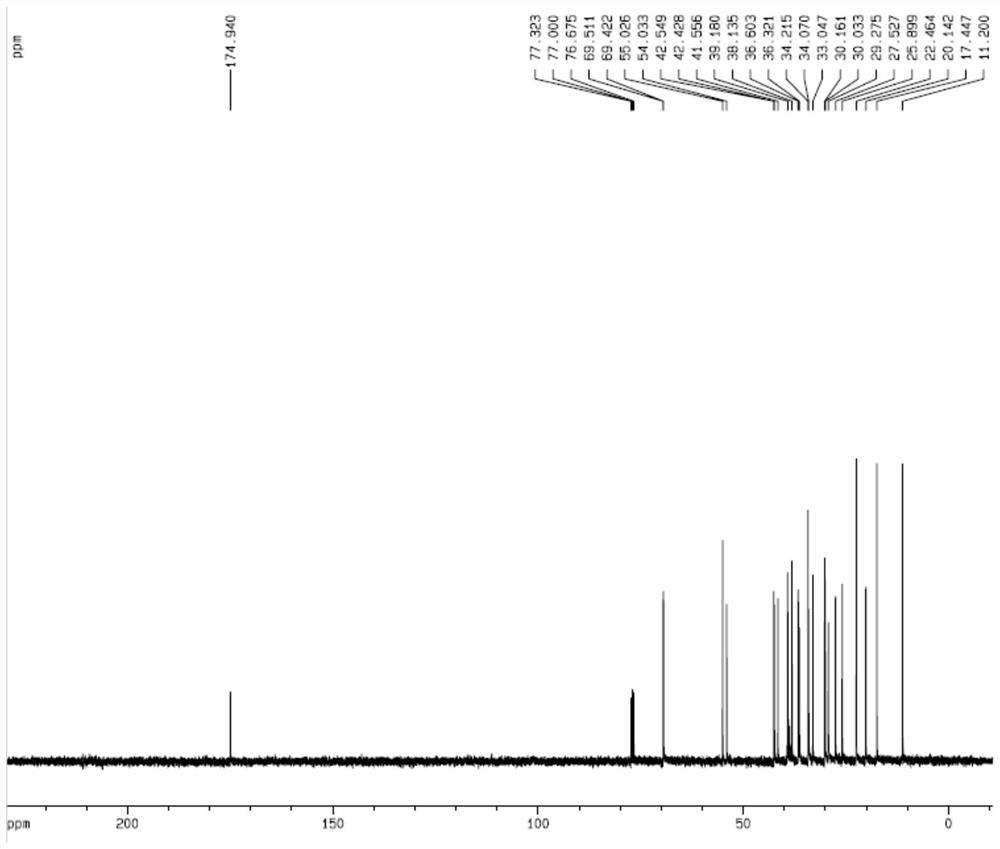
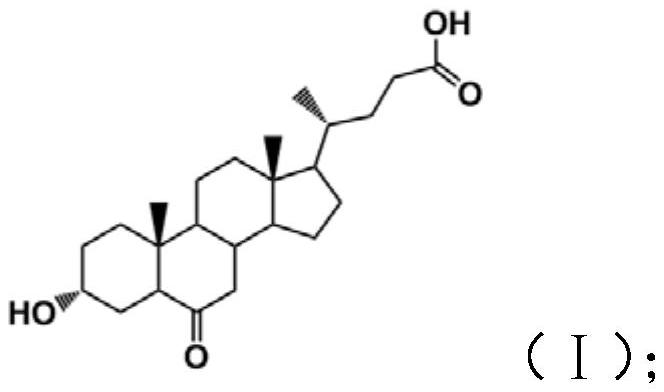
[0050] (1) Dissolve 10 g of hyodeoxycholic acid in 200 mL of dichloromethane, continue to add 60 mL of DMSO, stir until dissolved, add 7.50 g of 2-iodoxybenzoic acid and stir for 4 hours at room temperature, then filter, and concentrate the filtrate to about 40 mL under reduced pressure. Slowly add 200 mL of deionized water dropwise to obtain 6-oxo-lithocholic acid, which is then dried. The above product was further recrystallized, including dissolving the 6-oxo-lithocholic acid obtained in the above process in 50 mL of methanol and filtering, slowly dripping 100 mL of deionized water into the filtrate, filtering, and drying to obtain 9.18 g of the product 6-oxo-lithocholic acid after recrystallization.
[0051] (2) Dissolve the above 9.18g 6-oxo-lithocholic acid and 8.50g p-toluenesulfonyl hydrazide in a mixed solution of 200mL methanol and 2mL acetic acid, stir at roo...
Embodiment 2
[0055] Embodiment 2 A chemical-enzymatic method for preparing ursodeoxycholic acid, comprising:
[0056] (1) Dissolve 10g of hyodeoxycholic acid in 200mL of dichloromethane, continue to add 40mL of DMSO, stir until dissolved, add 8.2g of 2-iodoxybenzoic acid and stir for 3 hours at room temperature, then filter, and concentrate the filtrate to about 40mL under reduced pressure. Slowly add 200 mL of deionized water dropwise to obtain 6-oxo-lithocholic acid, which is then dried. The above product was further recrystallized, including dissolving the 6-oxo-lithocholic acid obtained in the above process in 50 mL of methanol and filtering, slowly dripping 100 mL of deionized water into the filtrate, filtering, and drying to obtain 9.05 g of the product 6-oxo-lithocholic acid after recrystallization.
[0057] (2) Dissolve the above-mentioned 9.05g 6-oxo-lithocholic acid and 8.2g p-toluenesulfonylhydrazide in 200mL ethanol and 2mL acetic acid solution, stir at room temperature, then ...
Embodiment 3
[0061] Embodiment three A chemical-enzymatic method for preparing ursodeoxycholic acid, comprising:
[0062] (1) Dissolve 10g of hyodeoxycholic acid in 150mL of acetone, stir with 50mL of DMSO until dissolved, add 7.50g of 2-iodoxybenzoic acid and stir for 3 hours at room temperature, then filter, concentrate the filtrate under reduced pressure to about 40mL, slowly drop into 150mL of deionized Water, precipitated to obtain 6-oxo-lithocholic acid, and dried. The above product was further recrystallized, including dissolving the 6-oxo-lithocholic acid obtained in the above process in 50 mL of methanol and filtering, slowly dripping 100 mL of deionized water into the filtrate, filtering, and drying to obtain 9.25 g of the product 6-oxo-lithocholic acid after recrystallization.
[0063] (2) Dissolve the above 9.25g 6-oxo-lithocholic acid and 10.0g p-toluenesulfonylhydrazide in a mixed solution of 200mL ethanol and 4mL acetic acid, stir at room temperature, then add 100mL 80% sod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com