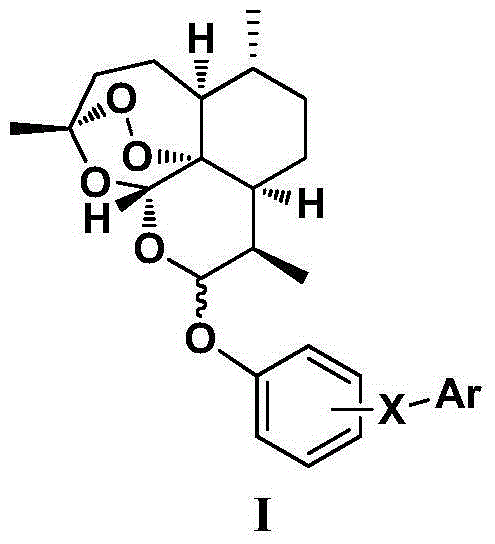
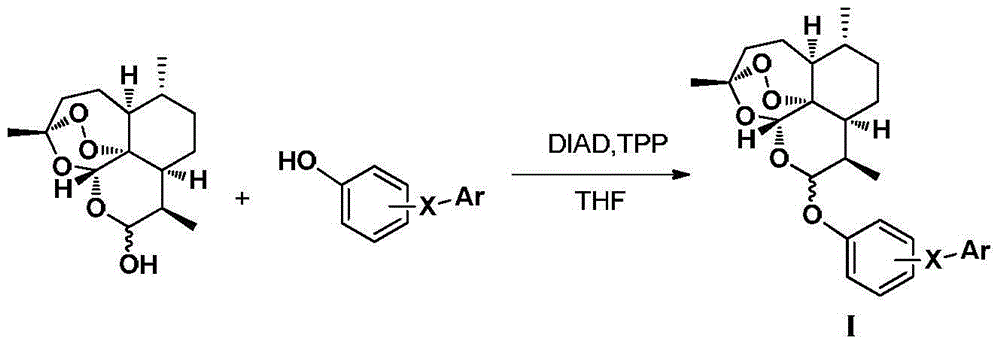
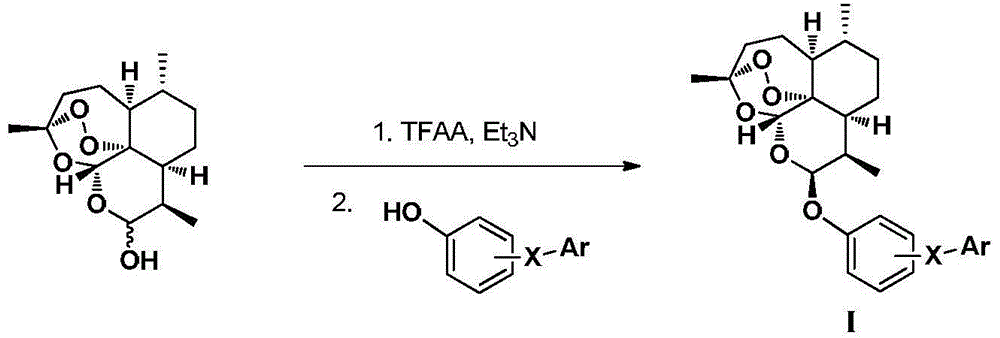
Patents
Literature
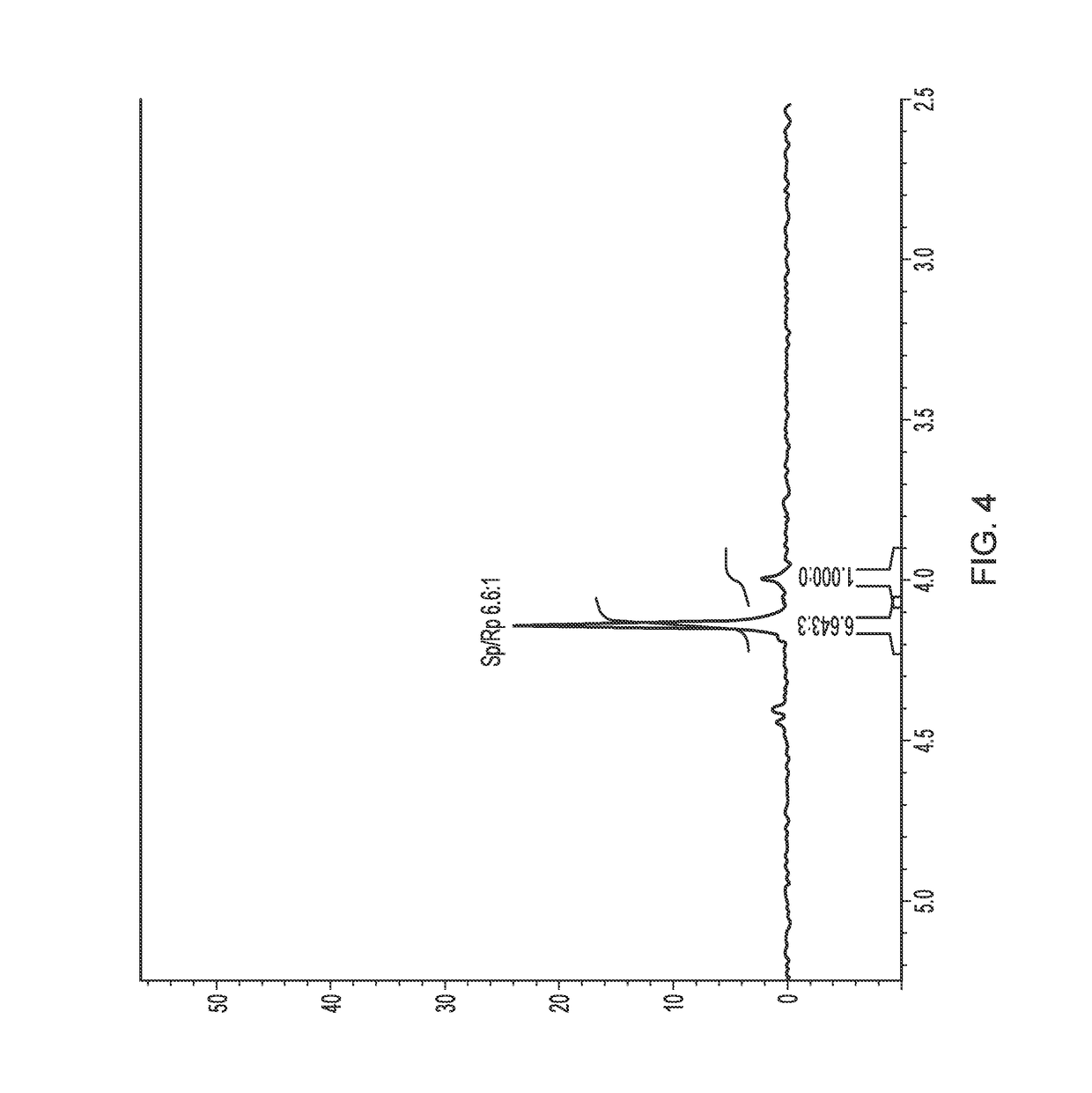
113 results about "Epimer" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In stereochemistry, an epimer is one of a pair of stereoisomers. The two isomers differ in configuration at only one stereogenic center. All other stereocenters in the molecules are the same in each. Doxorubicin and epirubicin are two epimers that are used as drugs.
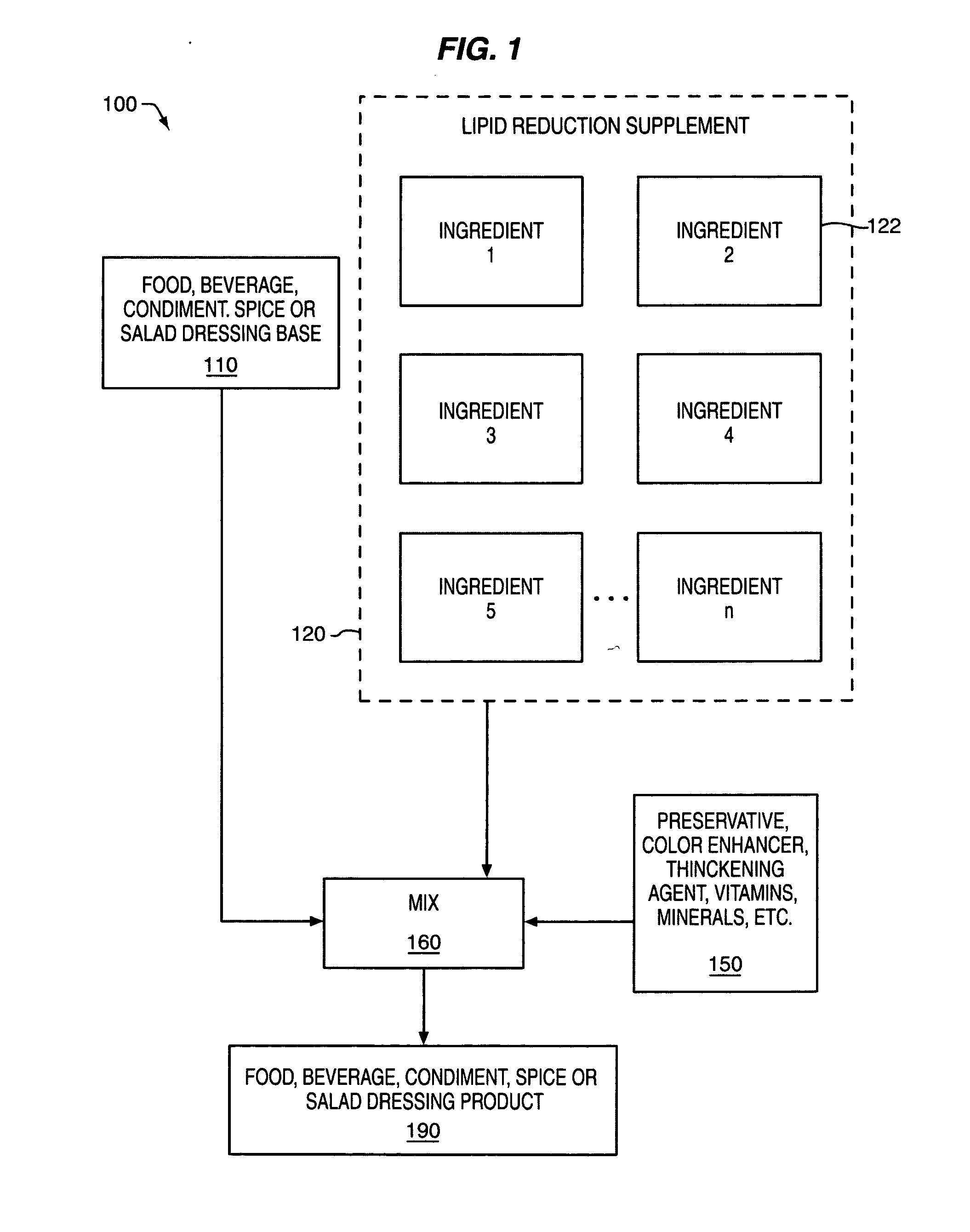
Composition and method for reducing lipid storage
InactiveUS20050095233A1Increasing fatty acid oxidationEnhancement of thermogenic futile cycleBiocidePeptide/protein ingredientsLipid storageAdditive ingredient
An orally or parenterally administered composition for reducing the storage of lipids in a human, the composition comprising: an effective amount of hydroxycitric acid; an effective amount of carnitine; an effective amount of biotin; an effective amount of one or more gluconeogenic substrates selected from the group consisting of: aspartate, lactate, glycerol, and a gluconeogenic amino acid or alphaketo analogue thereof; an effective amount of eicosapentanoic acid; and an effective amount of one or more ingredients selected from the group consisting of: medium chain triglycerides (MCT) with fatty acid backbones containing 6 to 14 carbon atoms or their individual fatty acid analogues or metabolic precursors, sesame seeds or derivative products, sesamin and / or its epimer episesamin, caffeine, forskolin, 7-keto dehydroepiandrosterone (7-keto DHEA), green tea extract containing epigallocatechingallate (EGCG), capsaicum, and 5-hydroxytryptophan (5-HTP). The supplement may also be combined with a food, beverage, condiment, spice or salad dressing base to provide a food, beverage, condiment, spice or salad dressing product designed to reduce lipid storage.
Owner:MCCLEARY EDWARD LARRY +2
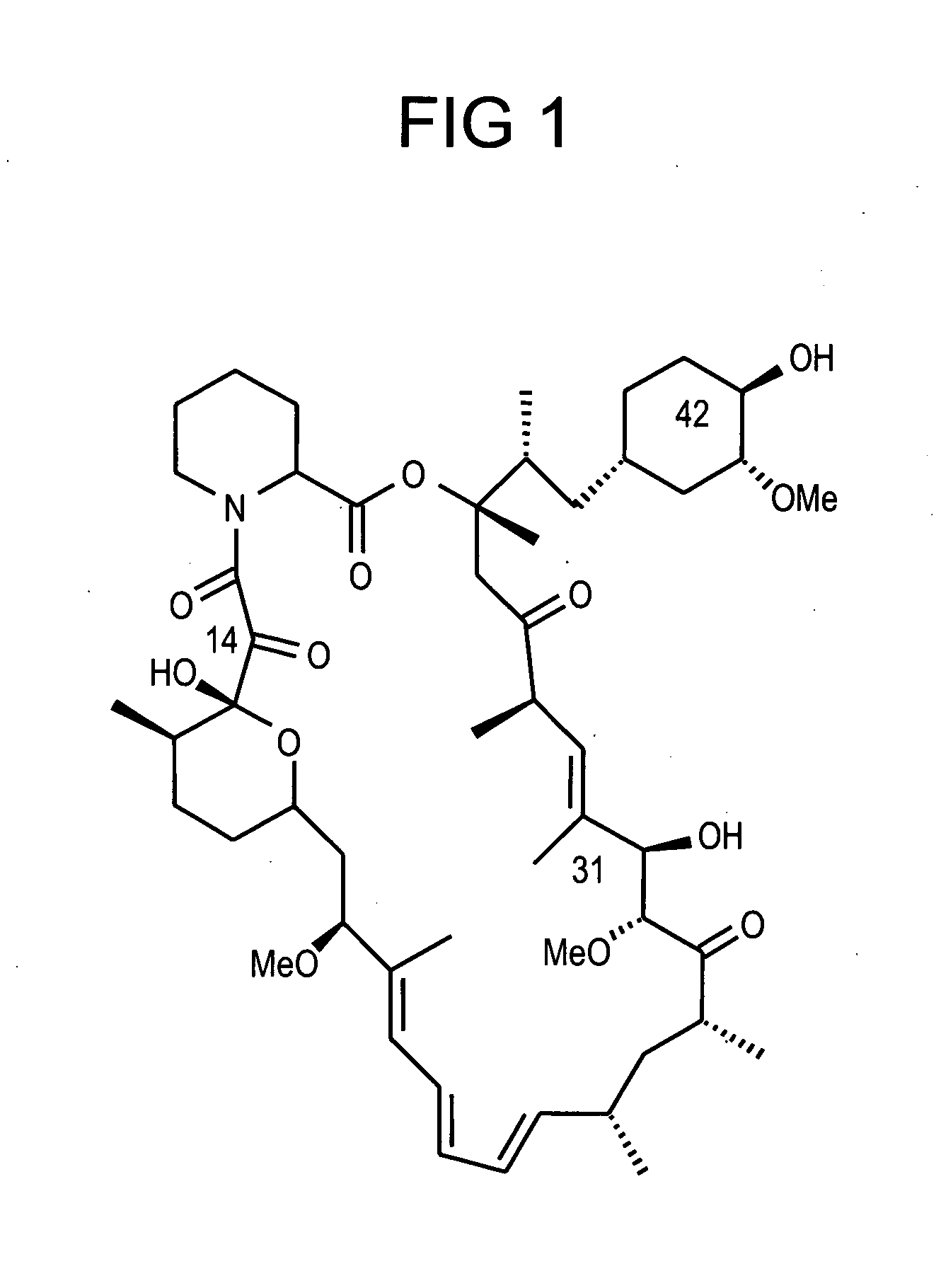
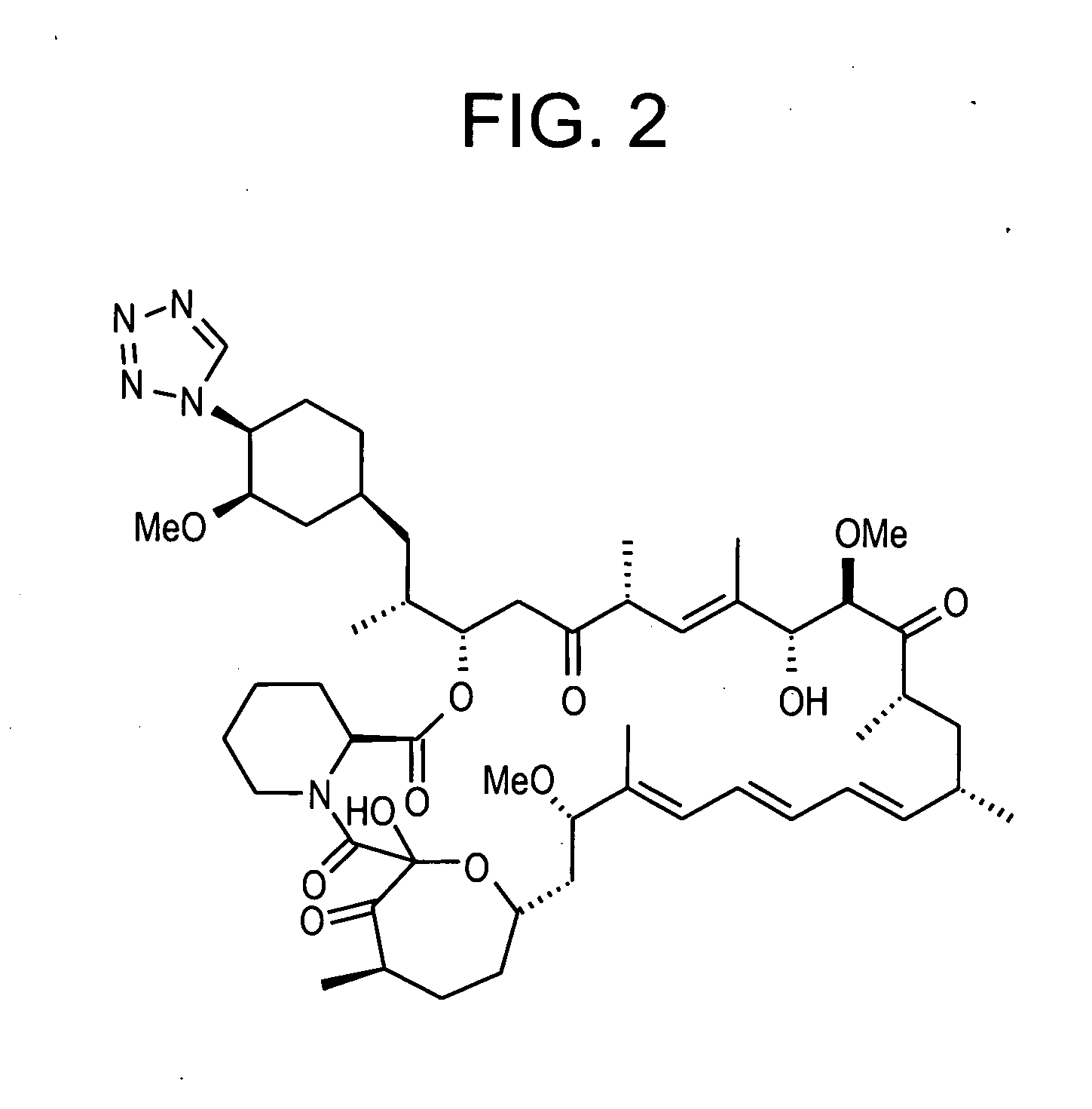
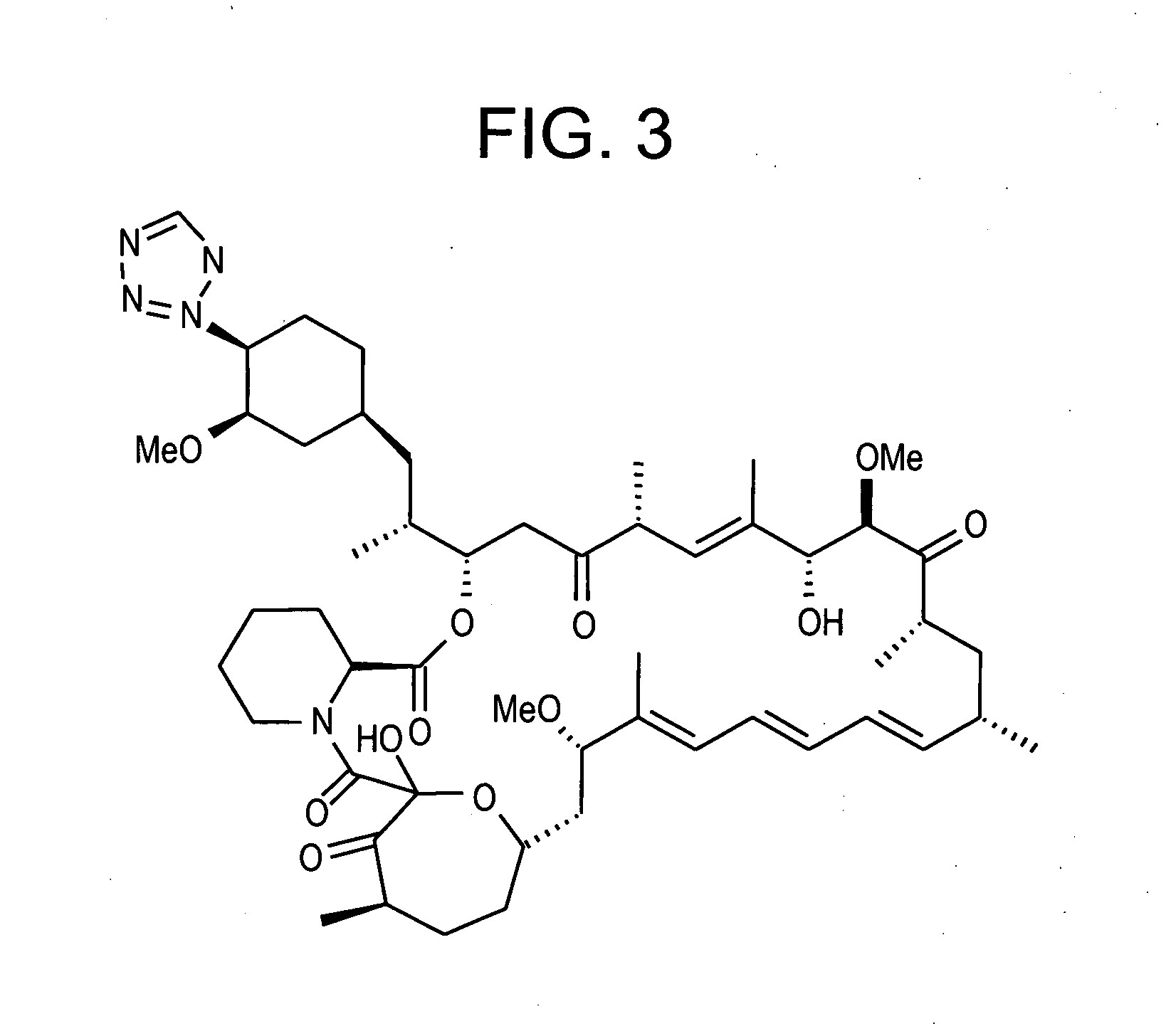
Combination of rapamycin and its tetrazole isomers and epimers, methods of making and using the same
Epimers and isomers of tetrazole-containing rapamycin analogs are immunomodulatory agents and are useful in the treatment of restenosis and immune and autoimmune diseases. Also disclosed are cancer-, fungal growth-, restenosis-, post-transplant tissue rejection- and immune- and autoimmune disease-inhibiting compositions and a method of inhibiting cancer, fungal growth, restenosis, post-transplant tissue rejection, and immune and autoimmune disease in a mammal. It is preferred to use a combination of native rapamycin and its tetrazole containing isomers and epimers. One particular preferred application of such a combination of rapamycin and its tetrazole containing isomers and epimers is in medicated devices and local vascular delivery wherein the stability and lipid solubility and subsequently diffusion through tissue and cell membranes of the tetrazole isomers and epimers are essential to the success of the combined rapamycin formulation.
Owner:WYETH LLC
Novel compounds from garcinia hanburyi, their use in treating cancer and method of separating epimers thereof
InactiveUS20070149610A1Little inhibitory effectEnhanced inhibitory effectBiocideOrganic chemistryCytochrome P450Xanthone
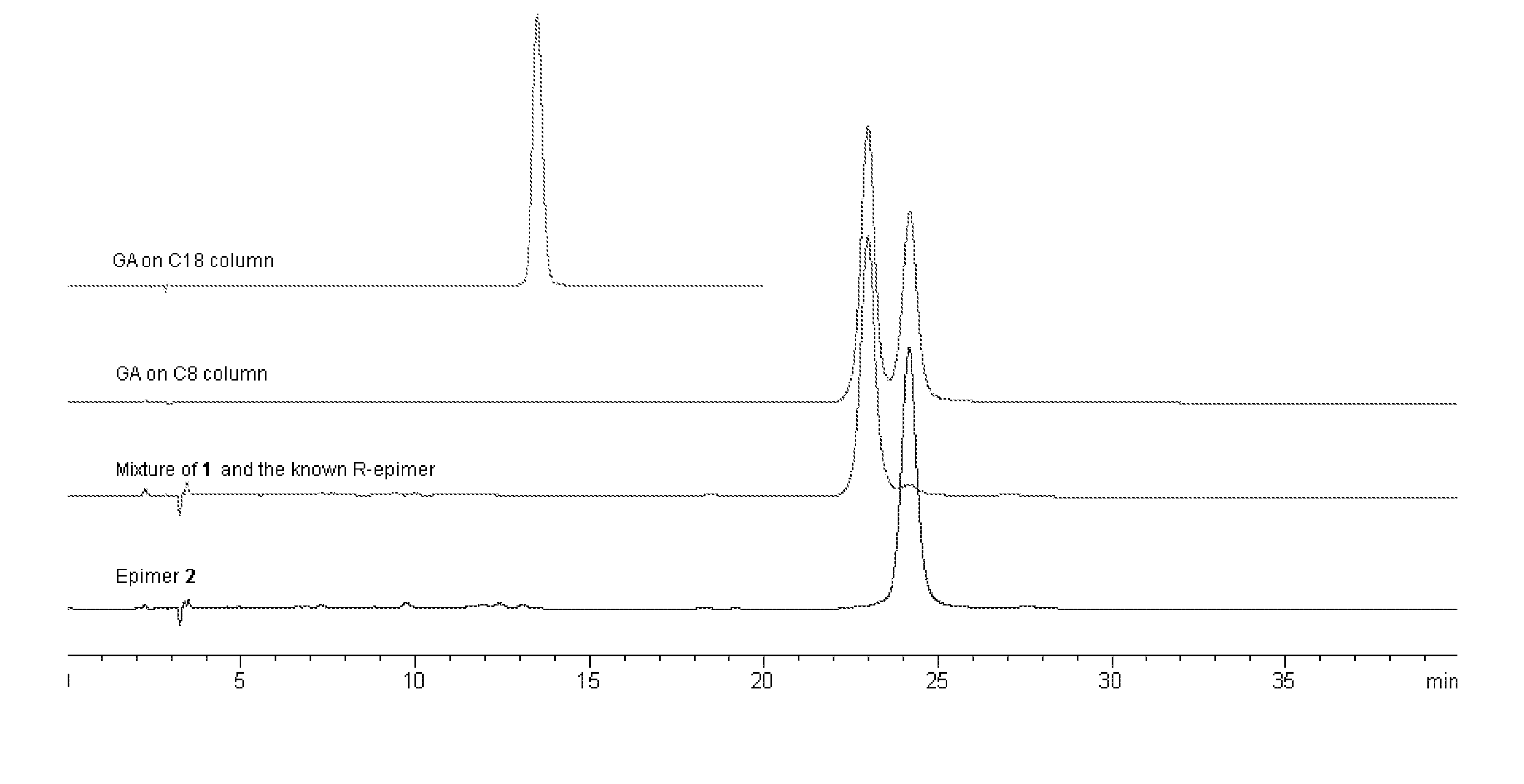
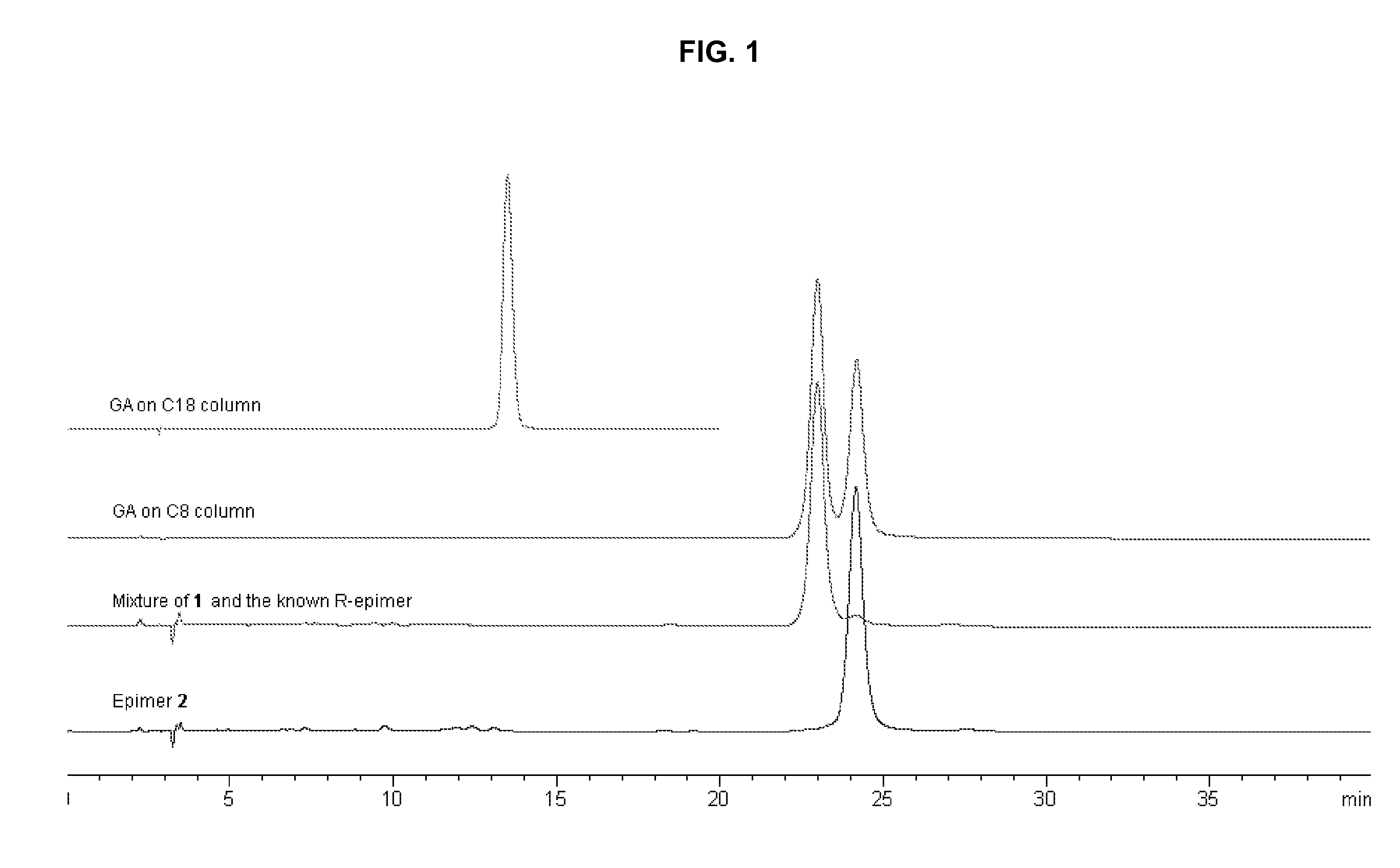
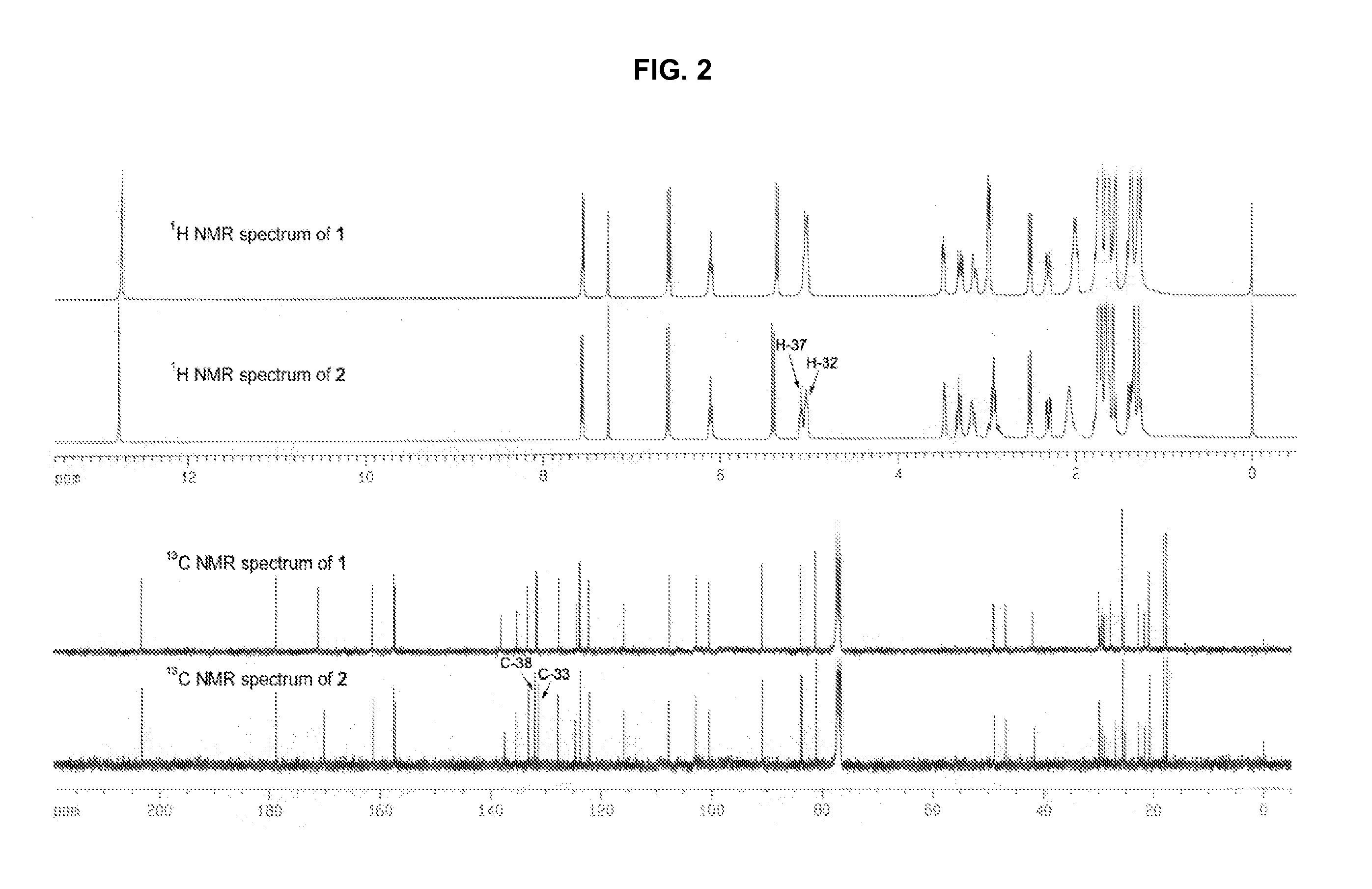
Three pairs of C-2 epimeric xanthones isolated from Garcinia hanburyi and method for efficiently separating the xanthone compounds into individual epimers, each of which possesses varying biological effects. The compounds are useful for their anticancer effects, particularly because they are shown to be non-substrates of the multidrug-resistance transporter. Some of the epimers have significant inhibitory effects on cytochrome P450 systems. The xanthone compounds of the present invention are gambogic acid, epigambogic acid, isogambogic acid, isoepigambogic acid, 30-hydroxygambogic acid and 30-hydroxyepigambogic acid.
Owner:HONG KONG JOCKEY CLUB INST OF CHINESE MEDICINE
Method of treatment of prostate cancer
InactiveUS7241753B2Heavy metal active ingredientsPhosphorous compound active ingredientsCancer preventionHormone dependence
The present invention relates to the field of cancer, and in particular hormone dependent cancers including, but not limited to prostate, breast, endometrial, ovarian, thyroid, bone, and testis. The present invention also relates to the use of steroid analogues, and in particular analogues of Δ5-androstene-3-β, 17α-diol, and its epimer Δ5-androstene-3-β, 17β-diol for the treatment and prevention of cancer.
Owner:HARBOR DIVERSIFIED
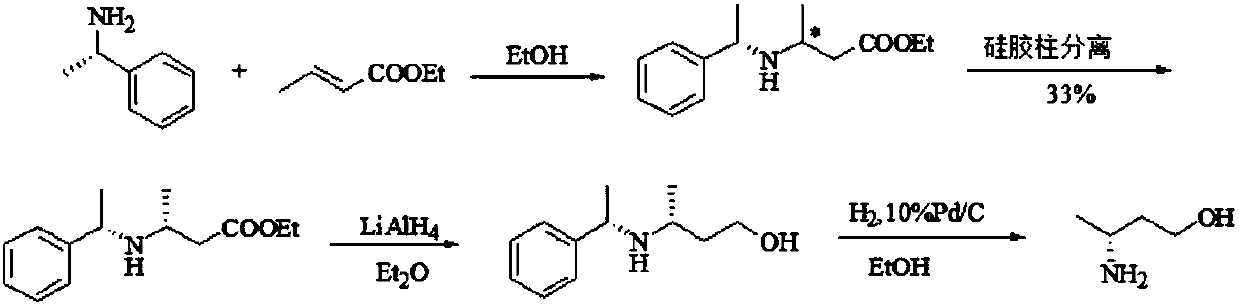
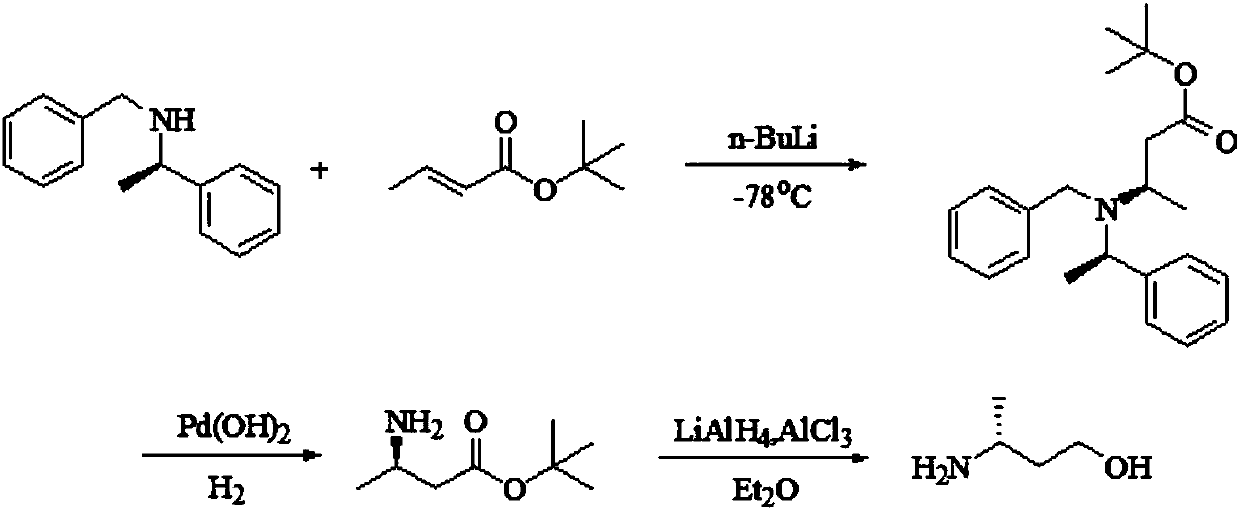
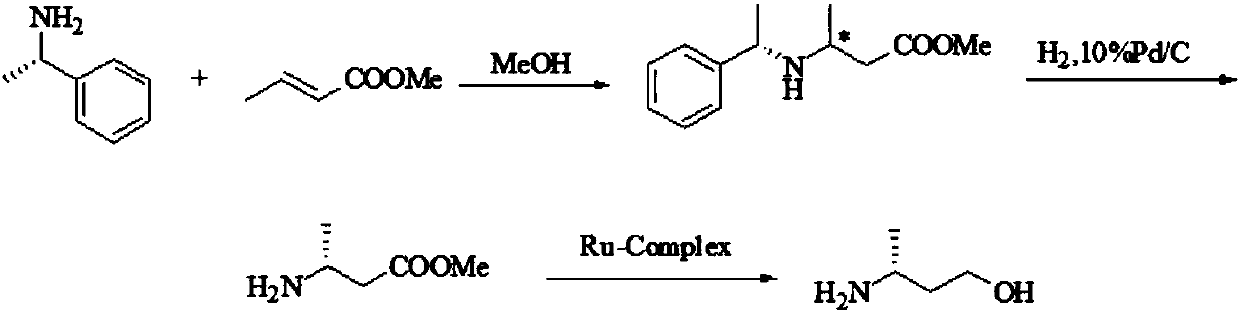
Preparation method of (R)-3-amino butanol
ActiveCN107805205ASimple process routeWide range of optionsOrganic compound preparationOrganic chemistry methodsAlcoholButanone
The invention discloses a preparation method of (R)-3-amino butanol. The preparation method includes following steps: (1), allowing (R)-1-methylbenzylamine and butanone alcohol to be in ammoniation reduction reaction to obtain a mixture of (R,R)-3-(1'-methylbenzylamine)-butanol and (R,S)-3-(1'methylbenzylamine)-butanol; (2), adopting an acidic chiral resolution reagent to split the mixture to obtain (R,R)-3-(1'-methylbenzylamine)-butanol; (3), allowing (R,R)-3-(1'-methylbenzylamine)-butanol to be in debenzylation reduction reaction to obtain (R)-3-amino butanol. According to the preparation method, a pair of epimers can be obtained by enabling butanone alcohol to react with a chiral reagent (R)-1-methylbenzylamine, the chiral reagent is added for further resolution, then subsequent reduction is performed, and obtained (R)-3-amino butanol has high purity and ee value.
Owner:ZHEJIANG NHU CO LTD
Process for synthesizing clindamycin hydrochloride
InactiveCN101891778ASimple processPractical applicationSugar derivativesSugar derivatives preparationChemical reactionHydrolysis
The invention relates to a process for synthesizing new clindamycin hydrochloride. The process comprises the following steps of: 1) finishing chlorination reaction by using lincomycin hydrochloride as a basic raw material and using low-C halogenated hydrocarbon as a solvent; 2) finishing hydrolysis reaction of sodium hydroxide in an aqueous phase by using a product obtained in the step 1), and demixing the solution to obtain clindamycin free alkali; and 3) in a solvent system of acetone, performing salt forming reaction on the clindamycin free alkali obtained in the step 2) and hydrochloric acid, and crystallizing the reaction product to obtain the clindamycin hydrochloride. The invention has the advantages that: 1, the process is simple; 2, the process reduces one-step chemical reaction on chemical unit reaction; 3, the process reduces one raw material and one intermediate on materials and intermediate links; and 4, the yield of the product is greatly improved, the epimer clint content of impurities is reduced by 80 percent, the process has high yield, and the yield is improved by over 5 percent compared with a four-step method.
Owner:ZHANGJIAGANG XINYI CHEM
Gemcitabine prodrugs
ActiveUS20170107246A1Intrinsic clearanceIncrease exposureOrganic active ingredientsSugar derivativesSolubilityPhosphate
This invention relates to a prodrug of the monophosphate nucleotide of the well-known oncology drug gemcitabine. Specifically, it relates to gemcitabine-[phenyl-benzoxy-L-alaninyl)]-phosphate when present as a single phosphate diastereoisomer and, in particular, it relates to the (S)-phosphate diastereoisomer which offers a remarkable and unexpected increase in solubility relative to the (R)-diastereoisomer. The (S)-phosphate epimer is also preferentially taken up into cyclodextrin solutions over the (R)-diastereoisomer.
Owner:NUCANA PLC
Synthetic method for high-purity tigecycline
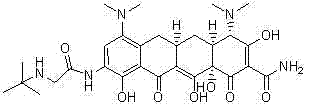
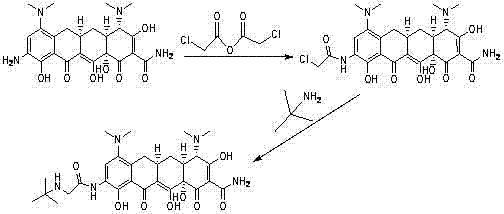
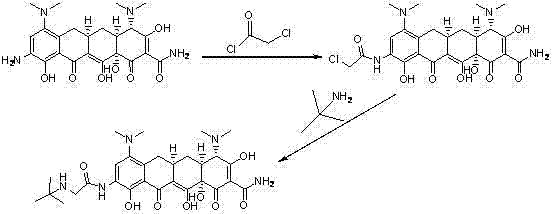
ActiveCN102391148AAvoid degradationShort synthetic routeOrganic compound preparationCarboxylic acid amides preparationGlycineTigecycline
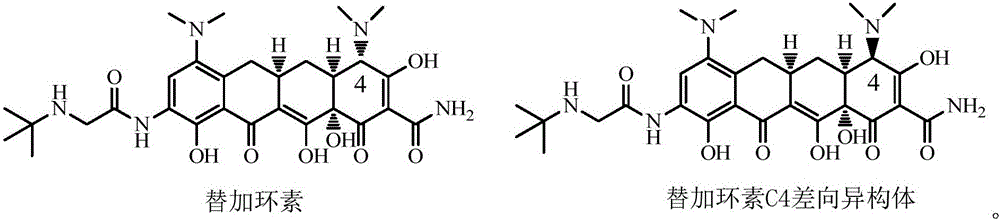
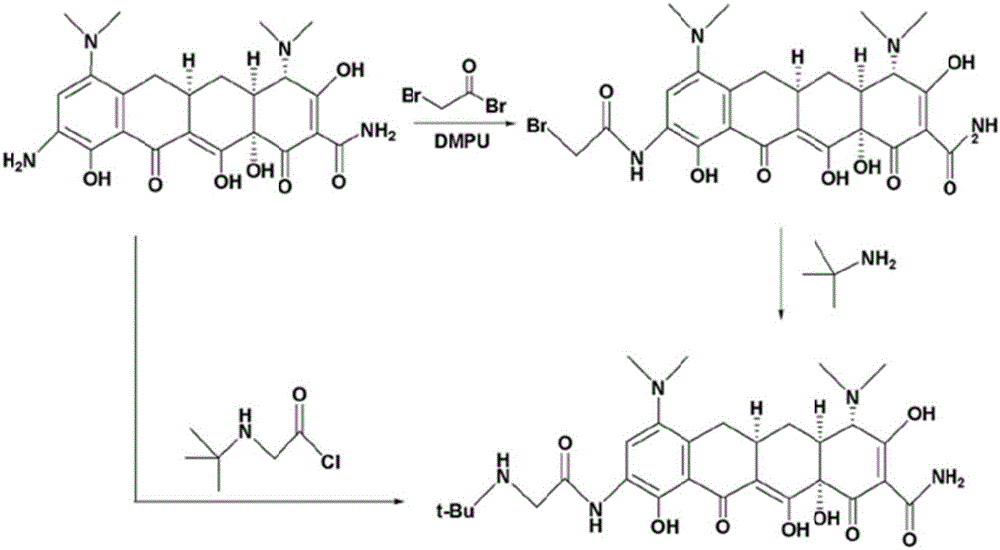
The invention relates to a novel preparation method for antibioticdrug tigecycline. 9-amino minocycline and N-tert-butyl group glycine are taken as starting materials. The novel preparation method is characterized in that the N-tert-butyl group glycine is dissolved in indifferent solvent, under the existence of an acidic acceptor and an amino acid condensating agent, reaction with 9-amino minocycline is carried out along the route of amino acid condensation for 3 hours to 10 hours, and then an indifferent solvent is cooled to a room temperature. Tigecycline is obtained through acidification, neutralization, extraction, drying, concentration and refining. In the preparation method disclosed by the invention, the operation is simplified, the purity of the obtained product reaches more than 99.5 percent, individual impurities are controlled to be lower than 0.1 percent, and an epimer is controlled to be lower than 0.5 percent; and the yield is high, the stability of the product is good, and the preparation method is suitable for industrial production.
Owner:NANJING HAIRUN PHARM CO LTD
Method for improving ribose purity
The invention discloses a method for improving the purity of ribose, which relates to a technical process for purifying ribose. The method improves the proportion of epimers of ribose produced by using gum sugar, uses molybdic acid as a catalyst, adds boric acid compounds into a reaction system, uses a spectrum separation technology to remove impurities such as xylose and lyxose in ribose liquid, and crystallizes the obtained high-purity ribose liquid to prepare ribose crystal. The process comprises: (1) material mixing and hot melting; (2) filtration and evaporation; (3) iron exchange; (4) separation; and (5) crystallization. The method improves the rate of ribose conversion from gum sugar by improving the proportion of the epimers and increases the purity of the ribose liquid to more than 90 percent by using the spectrum separation technology for separating azasugar and prepares ribose crystal with a purity of more than 99.8 percent. The method has the advantages that the purification process is simple, the production period is short and the obtained ribose liquid is high in purity, low in impurity content and easy to crystallize, and so on.
Owner:FUTASTE PHARM CO LTD
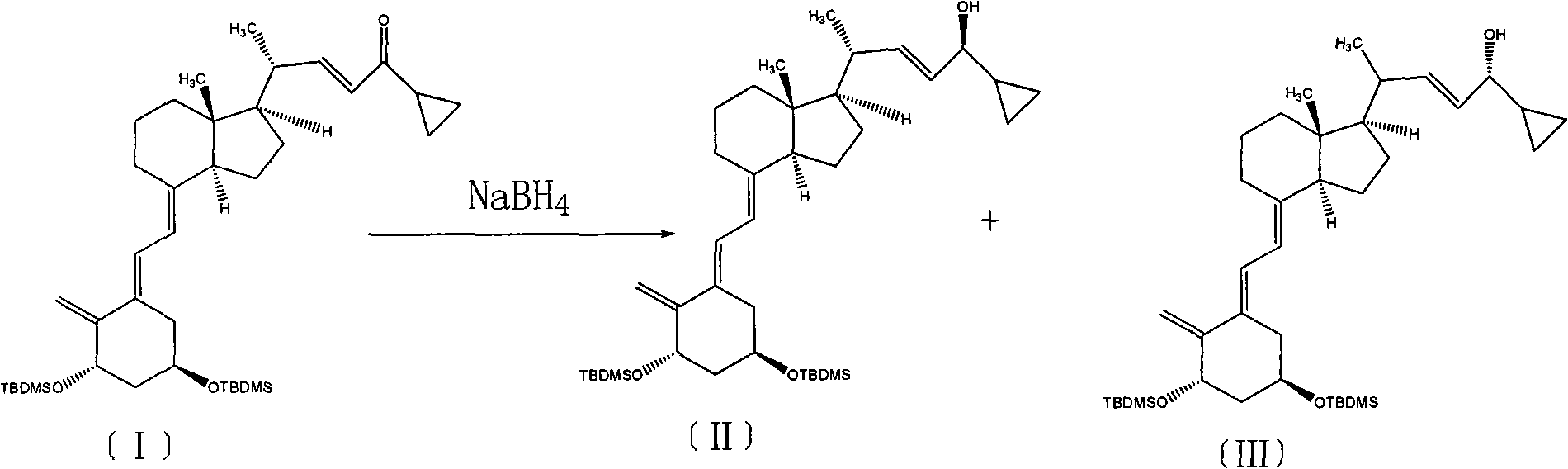
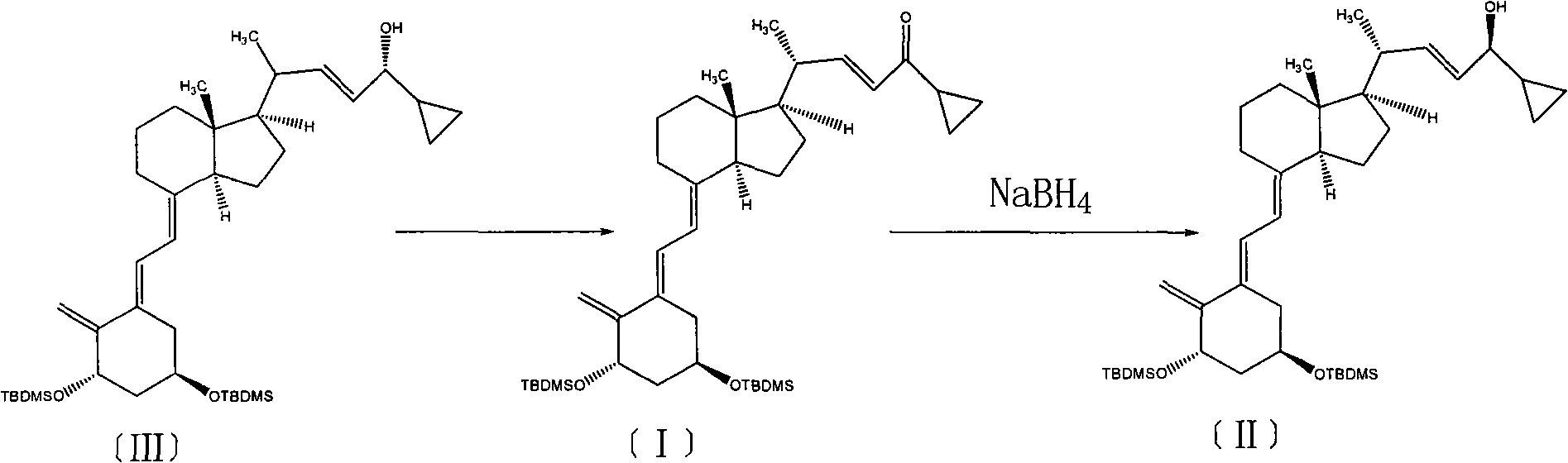
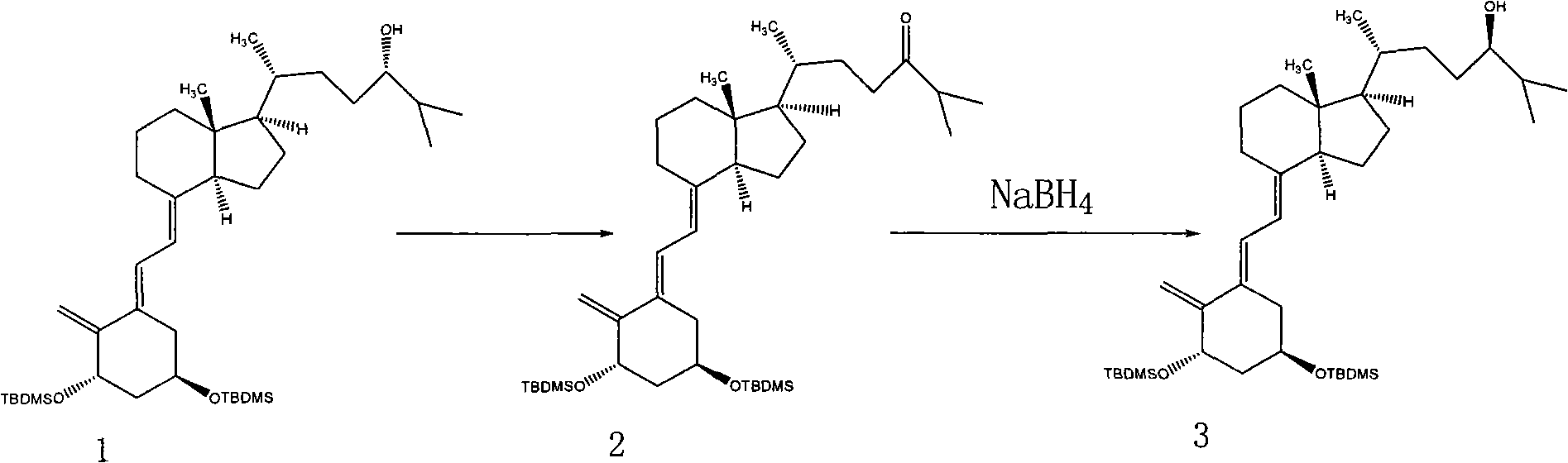
Method for preparing vitamin D2 derivative
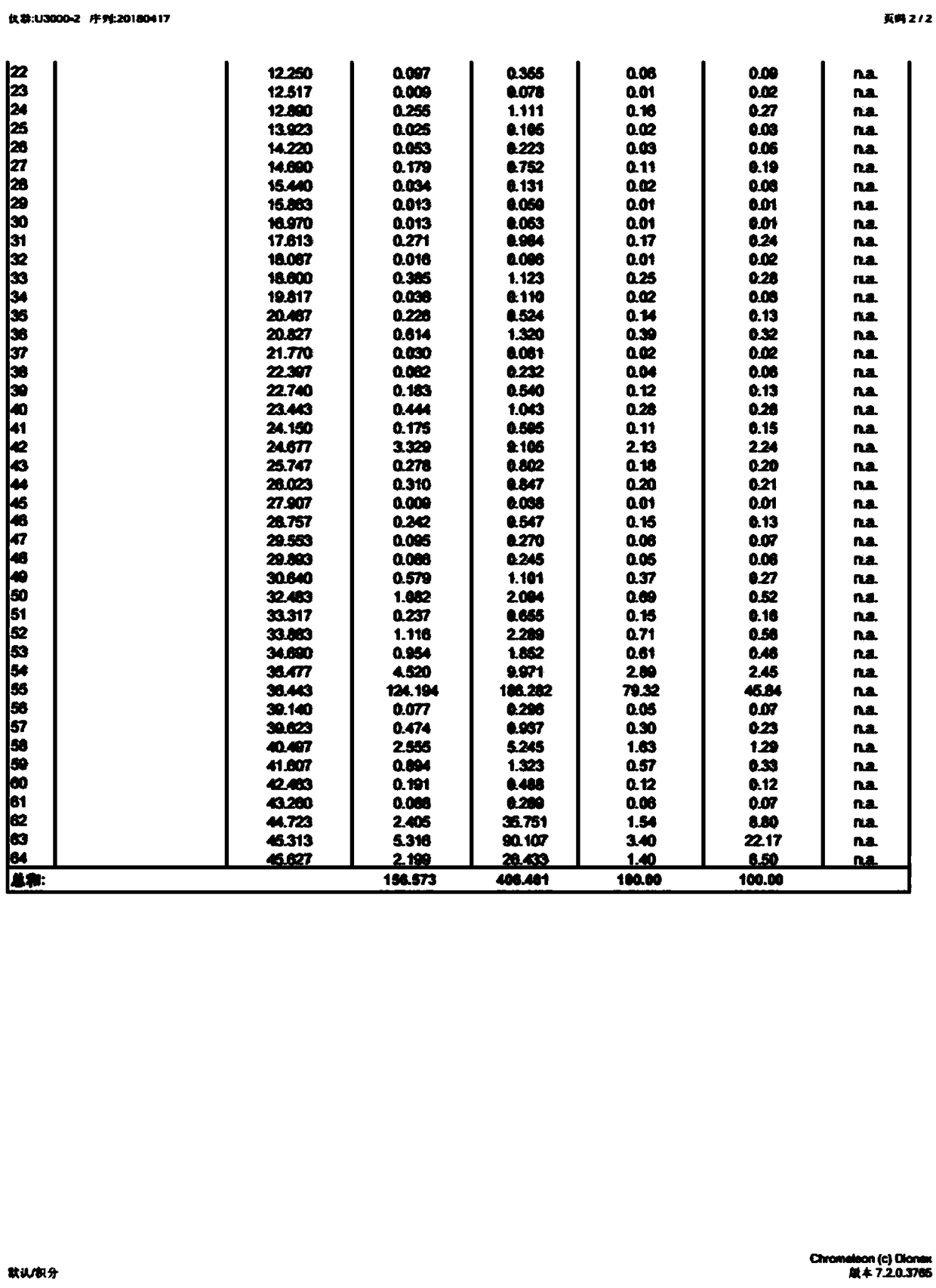
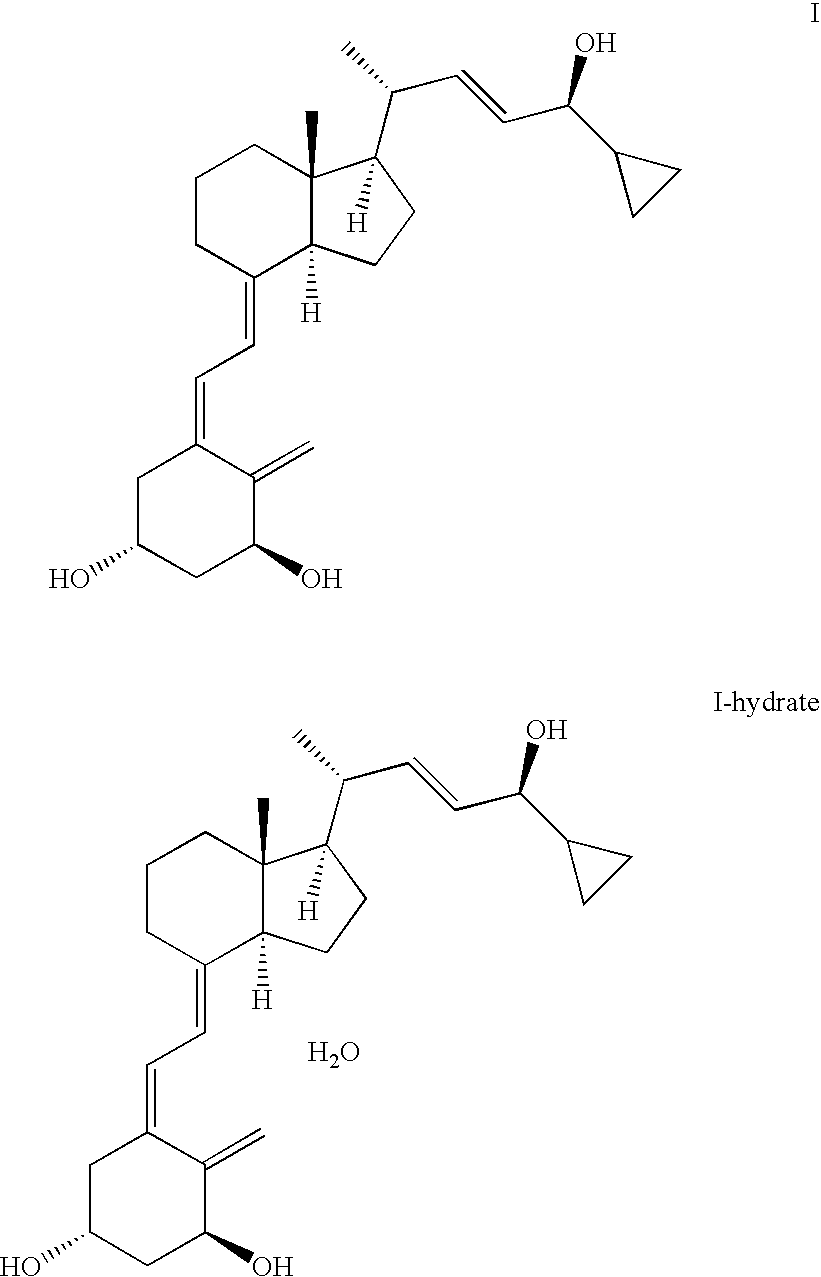
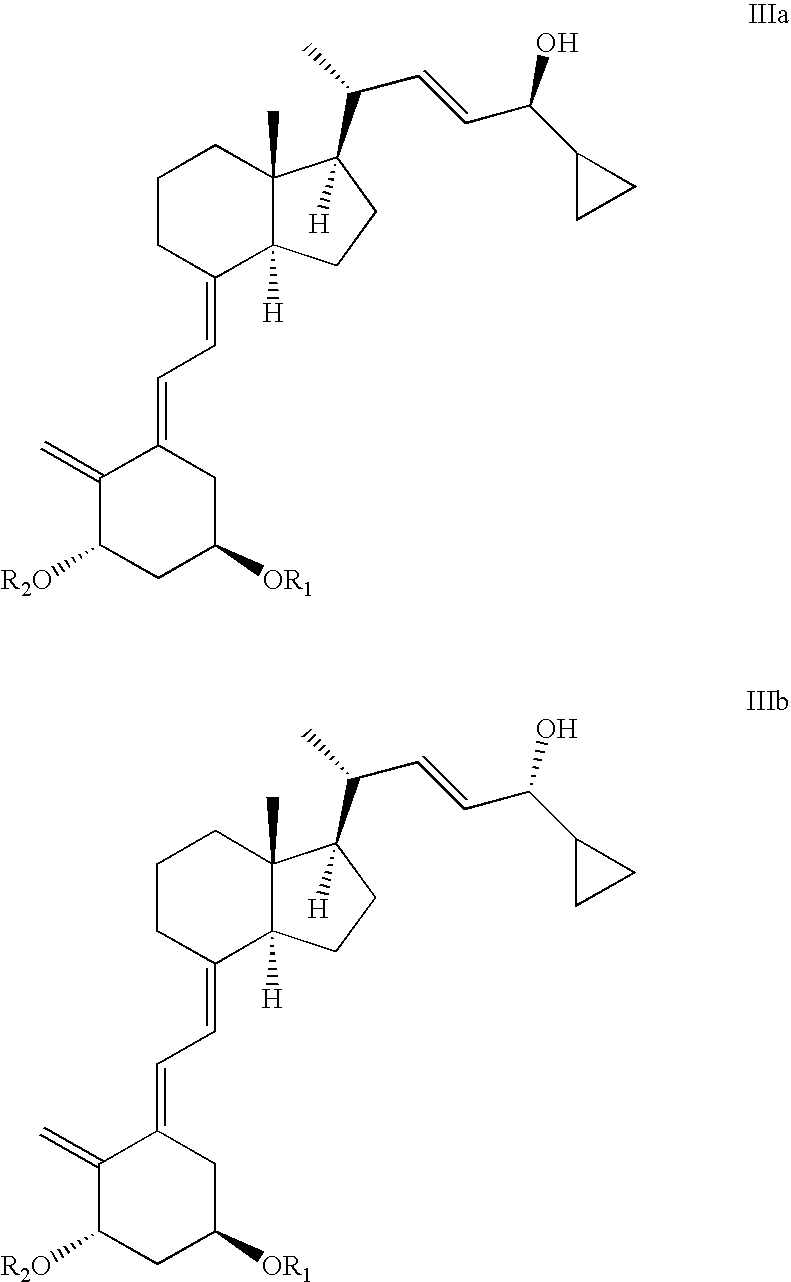
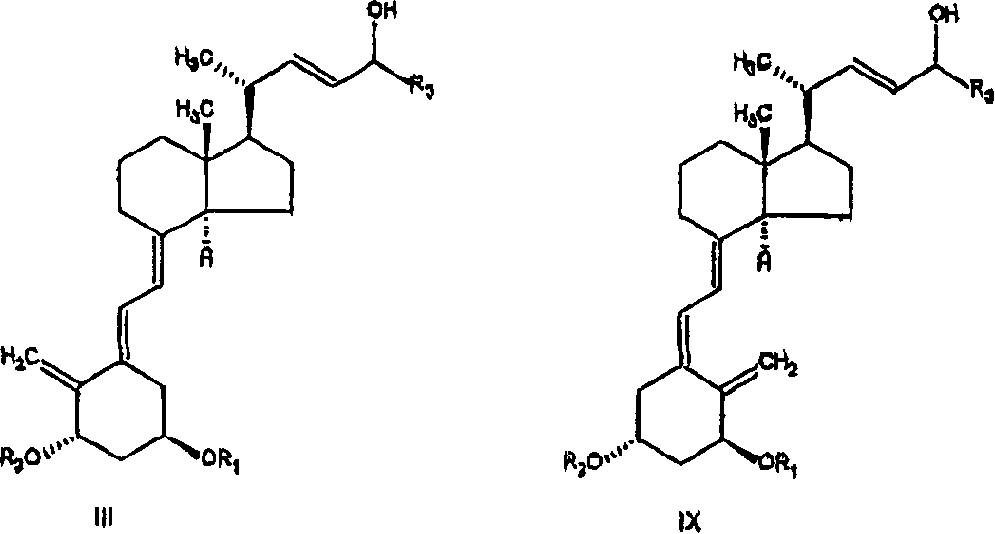
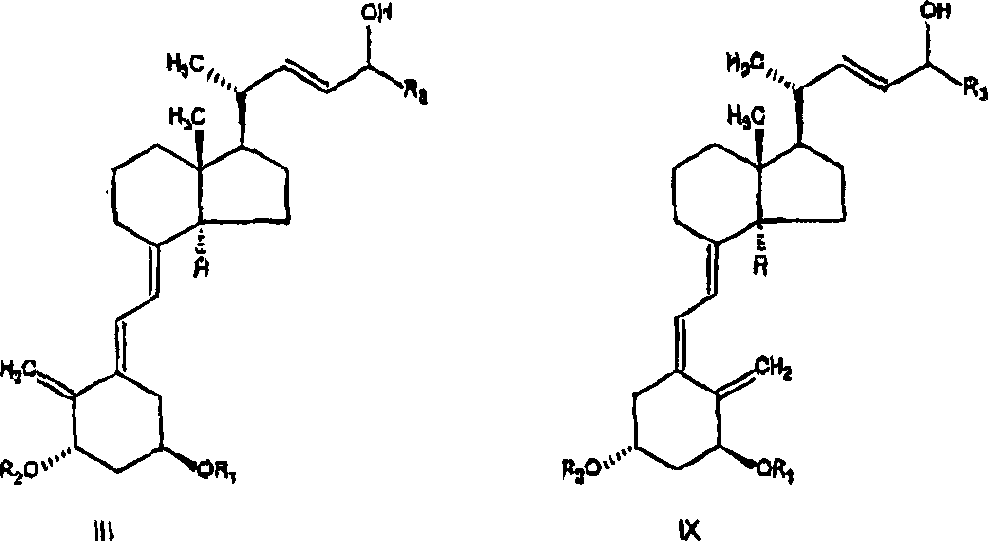
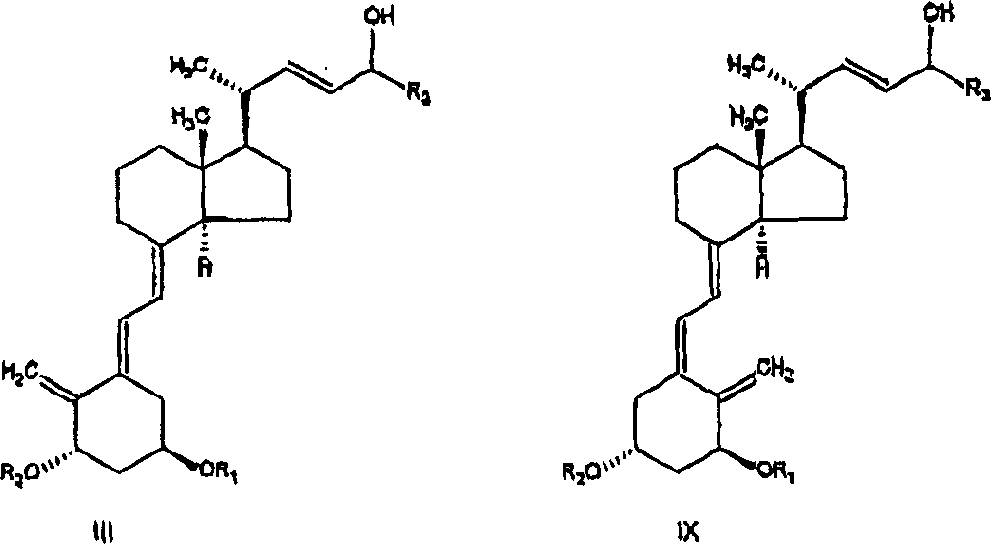
The invention discloses a method for preparing a vitamin D2 derivative. In the conventional method for preparing Calcipotriol, C-24 hydroxy epimeric alcohol (a compound III) is mainly obtained, the yield of the Calcipotriol is greatly reduced, and the C-24 hydroxy epimeric alcohol is a waste intermediate and does not have any utilization value so far. In the method of the invention, the waste intermediate compound III generated in the conventional process of preparing the Calcipotriol is taken as a starting material, and the method comprises the following steps of: in an organic solvent and in the presence of an oxidizer capable of oxidizing hydroxyl into carbonyl, performing heat preservation reaction at the temperature of between 20 and 120 DEG C, after complete reaction, cooling to room temperature, and treating to obtain a compound I; and performing reduction reaction on the compound I to obtain a compound II, namely the Calcipotriol. In the method, the waste intermediate compound III is recycled, the cost of raw materials is low, the utilization rate of the materials is improved, and the pollution is reduced; and by combining the conventional preparation process, the method can obviously improve the yield of the Calcipotriol in the conventional preparation process.
Owner:ZHEJIANG JINGXIN PHARMA
Liraglutide purifying method
ActiveCN108794618AEasy to separateSolve the problem of low purity and low yieldPeptide preparation methodsGlucagonsSolventLiraglutide
The invention discloses a liraglutide purifying method. The liraglutide purifying method comprises the following steps that 1, a liraglutide coarse product is pretreated to obtain a liraglutide crudepeptide water solution; 2, first-time HPLC purification for removing fragment impurities is performed; 3, a solvent is removed, and a first-step liraglutide sample solution is obtained; 4, second-timeHPLC purification for removing multi-Gly-sequence peptides, Gly-sequence-deleted peptides and epimers produced during synthesis is performed; 5, part of solvent is removed to obtain a second-step liraglutide sample solution, and a PH value is regulated to 6.5 so as to obtain a final liraglutide sample solution. The multi-Gly-sequence peptides, Gly-sequence-deleted peptides, epimers and other impurities produced during synthesis can be removed, and the purity and yield of liraglutide samples are improved.
Owner:HANGZHOU SINOPEP ALLSINO PHARMA TECH DEV CO LTD
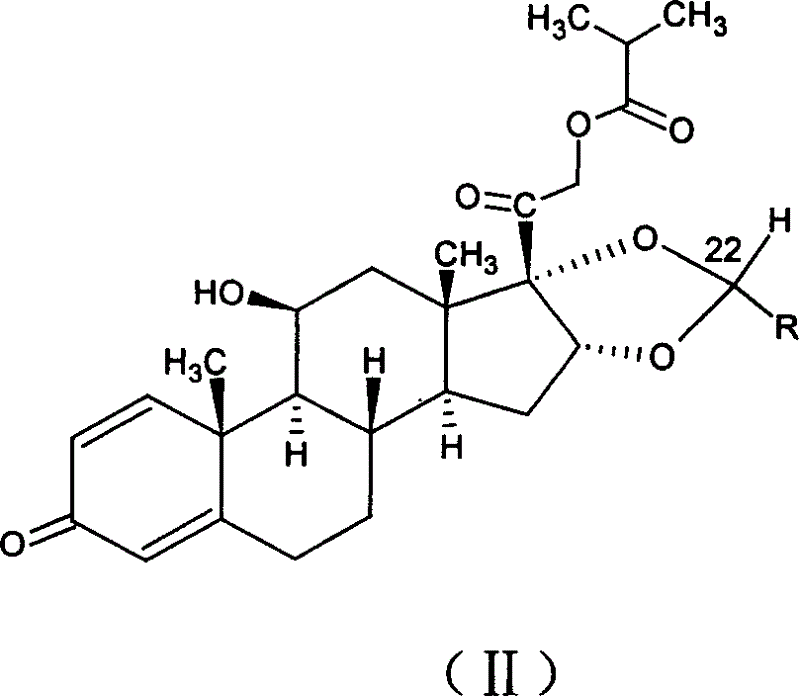
Selective enzymatic esterification and solvolysis of epimeric vitamin D analog and separation of the epimers
Provided is a method of selectively enzymatically esterifying or selectively enzymatically solvolyzing epimers of analogs of vitamin D having a stereogenic center at C-24 that has a free or esterified OH group. The metod can be used, for example, for separating mixed epimers of the vitamin D analog.
Owner:TEVA PHARM USA INC
Method for preparing taxanes by epimerization biological transformation 7-epimer taxanes
The present invention relates to a method of forming taxane diterpene by difference transformation 7- taxane using microorganisms. The present invention comprised the following steps: 1. Completely dissolve the 7- taxane compound which has a content of over 1% into the organic solvent that can be mixed with water at any ratio; 2. Add the solution of step 1 into the microbes biological culture, or abstraction enzyme from themicro-organism. An alternative is that after the extractenzyme immobilization, the solution of step 1 is added and the conversion time is 3-100 hours; 3. Extract taxane diterpene using conventional methods from the convert product of step 2. The technical problem solved in this invention is that the no valueble 7- taxane is converted into a valuble enantiomer, so as to save the resources of Yew.
Owner:YUNNAN UNIV
Application of eudesmane sesquiterpene lactone epimers in preparation of anti-breast cancer medicine
The invention discloses a pair of eudesmane sesquiterpene lactone epimers (1S,4S,5S,6S,7S,8S,9R,10S)-1,9-diacetoxy-4-hydroxy-6-isobutyryloxyprostatolide and (1S,4S,5R,6S,7S,8S,9R,10S)-1,9-diacetoxy-4-hydroxyl-6-isobutyryloxyprostatolide, pharmaceutically acceptable salts or solvates of the epimers, and pharmacodynamic activity of a pharmaceutical composition composed of the compounds, and mainly relates to a medical application of an anti-breast cancer medicine in inducing breast cancer cell MCF-7 cell morphology to retract and become round and the level of intracellular reactive oxygen species (ROS) to rise to show tumor cytotoxicity.
Owner:HAINAN NORMAL UNIV
Preparation method of amygdalin
ActiveCN105461765AImprove efficiencyHigh puritySugar derivativesSugar derivatives preparationNMR - Nuclear magnetic resonanceOrganic solvent
The invention discloses a preparation method of D-amygdalin and / or neoamygdalin; the amygdalin is extracted from peach kernels or bitter apricot kernels, an organic solvent is used for extraction, chromatography is used for separating D-amygdalin and neoamygdalin isomers and for detecting the purity of the two isomers, and nuclear magnetic resonance is used for detecting the structures of the two isomers. By adoption of the reverse chromatography technique and combination of the NMR technology, high-precision separation preparation of arbitrary one epimer of D-amygdalin and / or neoamygdalin of the amygdalin is realized. The separation operation process is simple, easy to operate and easy to repeat, the separation cycle is short, and the efficiency is high; moreover, the method is low in cost, friendly to the environment, and suitable for large-scale industrialized production; and in addition, the purity and the yield of the prepared D-amygdalin and neoamygdalin are higher.
Owner:JIANGSU KANION PHARMA CO LTD
Catalytic conversion method of aldose into ketose
ActiveCN106032386ARich varietyHigh isomerization rateSugar derivativesSugar derivatives preparationKetoneNanotube
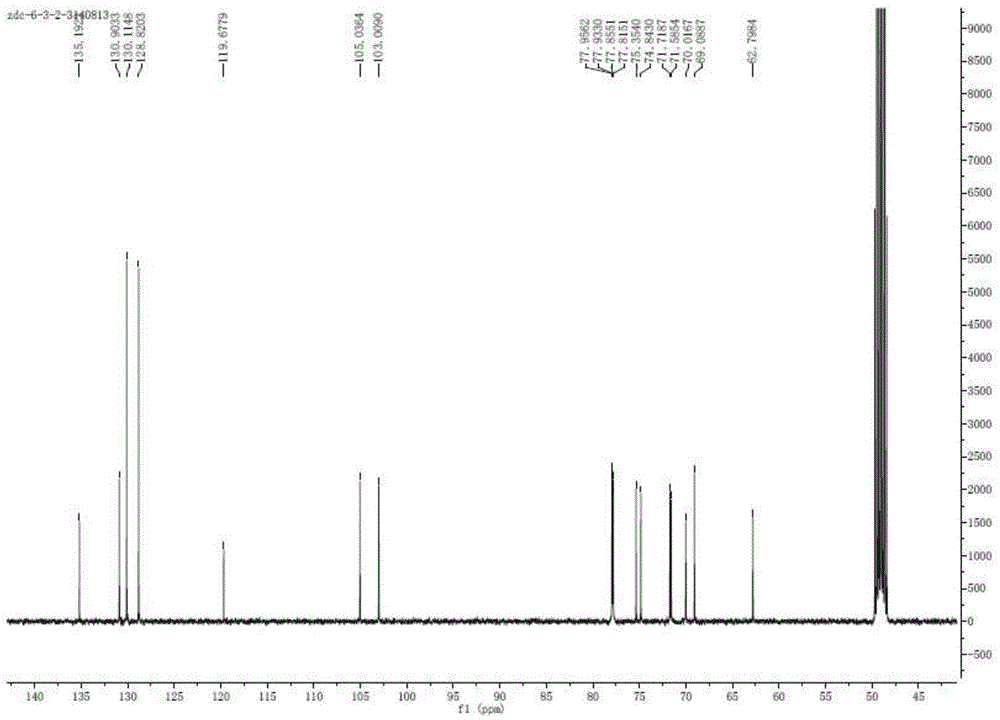
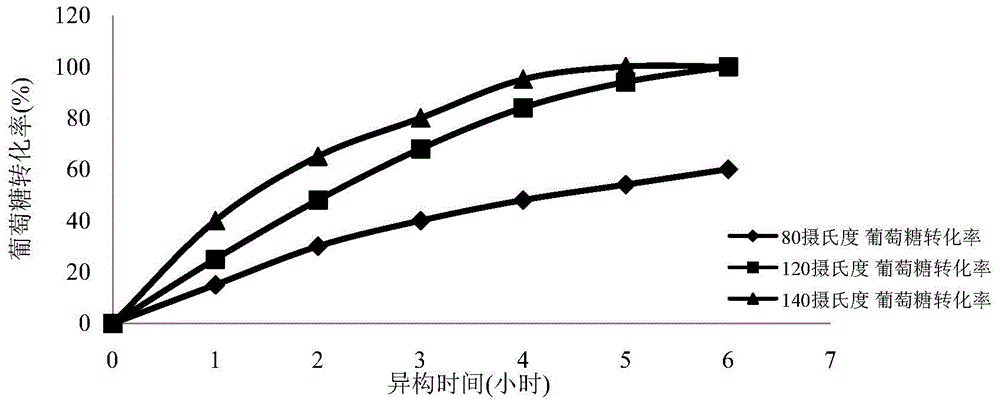
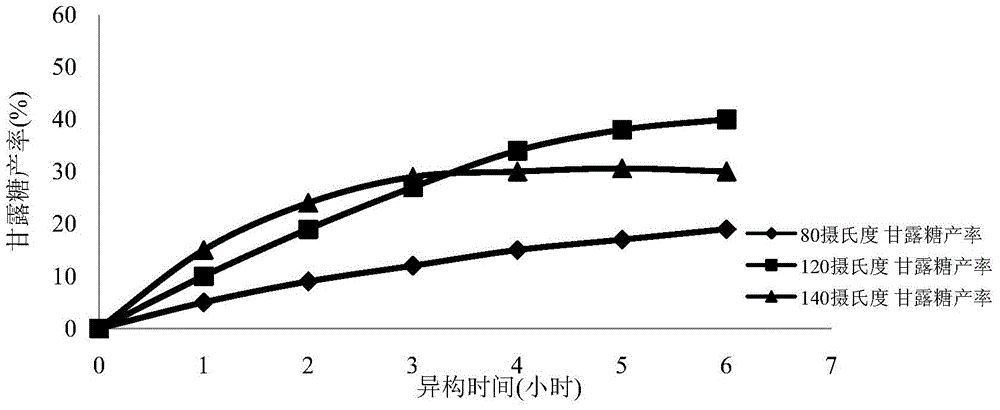
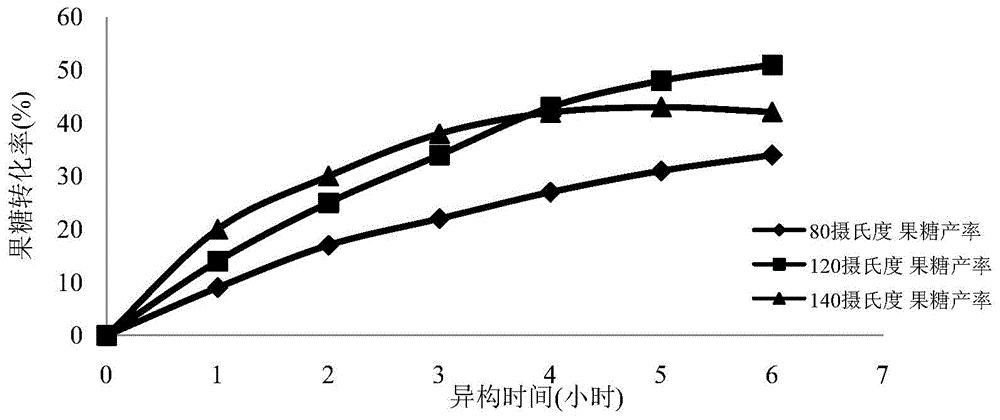
The invention provides a method for simultaneous isomerism of aldose into corresponding ketose and epimer aldose. The method uses the aldose as a substrate, and selects oxidation modified carbon nanotube supported with iron, aluminum, calcium, magnesium, molybdenum, chromium, and manganese elements of carbon nanotubes, or graphene as a catalyst. When the catalyst is mixed with the substrate aldose to not only catalyze aldehyde ketone isomerism to obtain corresponding ketose but also catalyze isomerism of C2 to obtain a corresponding C2 isomerism aldose catalyst, so as to realize simultaneous isomerism by one catalyst to obtain two rare functional sugars. The method has the advantages of high isomerism efficiency, simple operation conditions, reutilization of catalyst, small energy consumption, green, low cost, and potential in industrial mass production.
Owner:NINGBO INST OF MATERIALS TECH & ENG CHINESE ACADEMY OF SCI
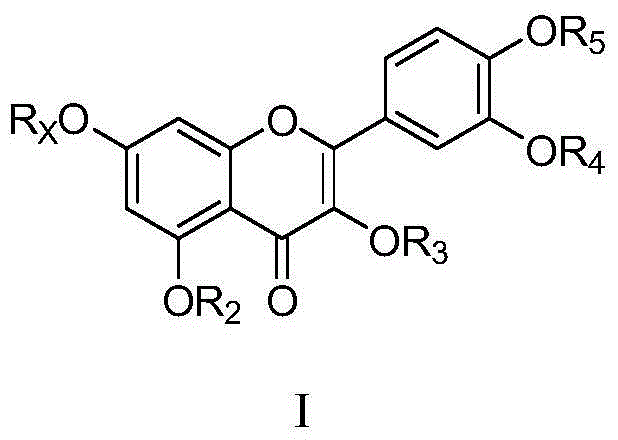
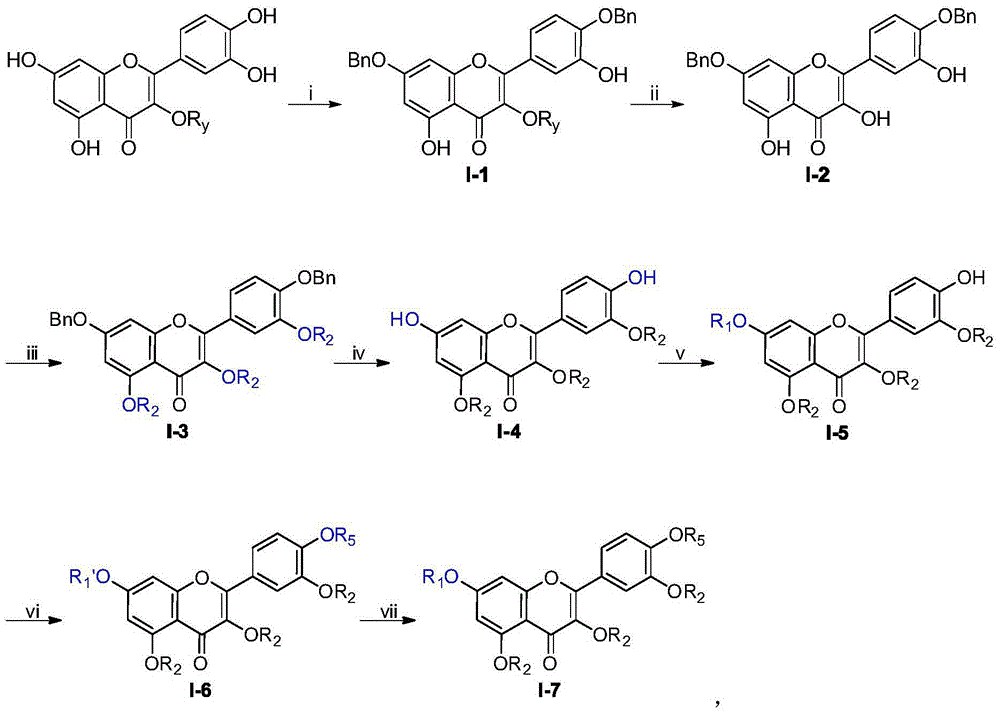
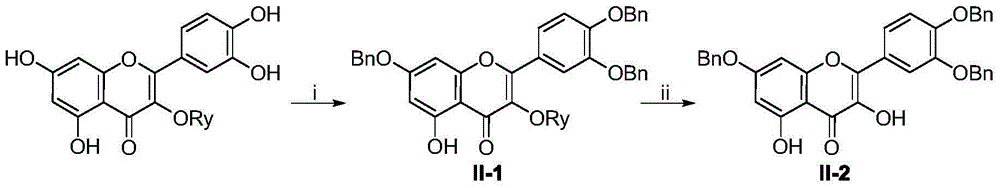
Flavone glycoside derivatives, and preparation method and application thereof
InactiveCN105481919AGood curative effectImprove securityOrganic active ingredientsSugar derivativesAcute hyperglycaemiaDiabrezide
The invention discloses flavone glycoside derivatives which are compounds with a general structural formula I shown in the description, and pharmaceutically acceptable salts or hydrates thereof, including racemates, optical isomers and epimers of the derivatives. The invention also discloses a preparation method of the flavone glycoside derivatives, and an application of the derivatives in preparing medicines used for treating diabetes. When the flavone glycoside derivatives provided by the invention are used in preparing medicines used for treating diabetes, the derivatives have good inhibition effects on alpha-glucosidase, such that the speed that starch breaks down into glucose is reduced, intestinal absorption of glucose is retarded, and the phenomenon of postprandial hyperglycemia is reduced. Therefore, the activity of the compounds in treating diabetes is improved, the toxicity is lower, and the physiochemical properties are more suitable for pharmaceutical preparations used for treating diabetes. The derivatives can be used for preparing diabetes-treating medicines with better efficacy and higher safety. The preparation method has the advantages of simple steps, easily available raw materials, high product yield, and high product purity. The flavone glycoside derivatives can be prepared into various pharmaceutical preparations used for treating diabetes.
Owner:GUIZHOU BAILING GRP PARMACEUTIAL CO LTD
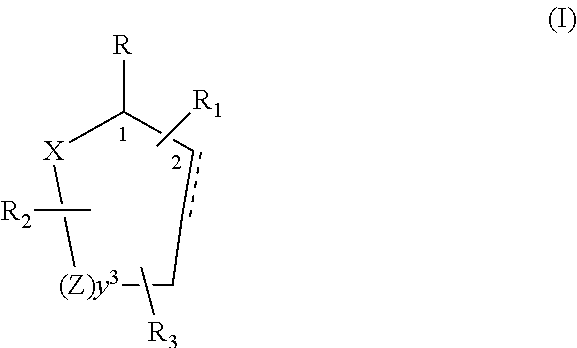
Control and repellency of mosquitoes
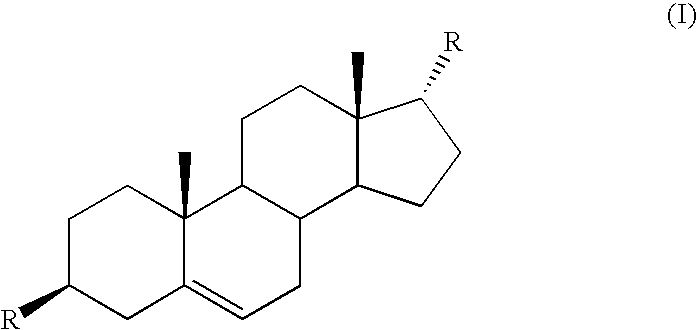
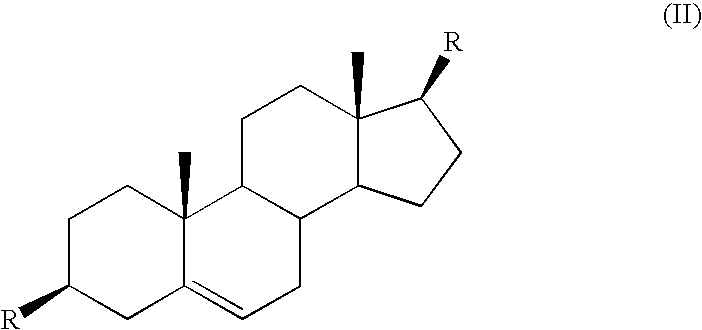
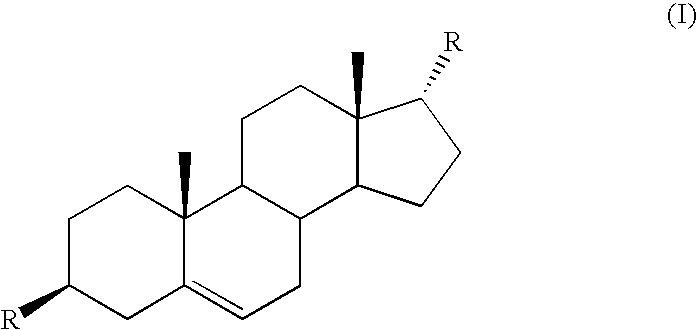
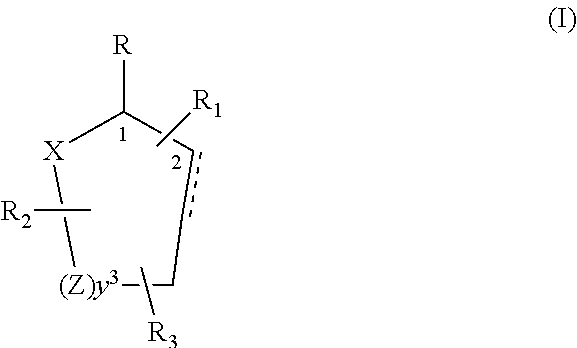
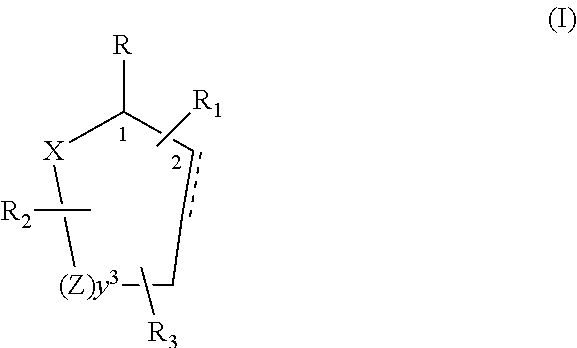
Control or repellency of mosquitoes is accomplished by bringing the insects into contact with at least one of the compounds of the structure (I)whereinR is selected from —OH, ═O, —OC(O)R4, —OR6, —(OR6)2, wherein each R6 is independently selected from an alkyl group containing from 1 to 4 carbon atoms and R4 is a branched or straight chain, saturated or unsaturated hydrocarbyl group with zero to two double bonds and from 1 to 15 carbon atoms;X is O or CH2, with the proviso that when X is O R can only be ═O;each Z is independently selected from (CH) and (CH2);y is a numeral selected from 1 and 2;R1 is selected from H or a branched or straight chain, saturated or unsaturated hydrocarbyl group with zero to two double bonds and from 1 to 15 carbon atoms;R2 is selected from H and a branched or straight chain, saturated or unsaturated hydrocarbyl group with zero to three double bonds and from 1 to 15 carbon atoms;R3 is selected from the group consisting of H and a branched or straight chain, saturated or unsaturated hydrocarbyl group with zero to three double bonds and from 1 to 15 carbon atoms, —(CH2)nOH, —C(O)OR5, —CH2C(O)OR7, —CH2C(O)R8, —C(O)NR9R10, —CH2C(O)NR11R12 where each of R5, R7, R8, R9, R10, R11 and R12 is independently selected from H and a branched or straight chain, saturated or unsaturated hydrocarbyl group with zero to three double bonds and from 1 to 15 carbon atoms and n is an integer of from 1 to 12;the bond between the 2 and 3 positions in the ring structure may be a single or a double bond; andwherein the compounds of structure (I) contain from 11 to 20 total carbon atoms in the compounds, with the proviso that when R is ═O, X is CH2, Z is CH2, y is 1 R2 is H, and R3 is CH2C(O)OR7 then the total number of carbon atoms in the compounds of structure (I) is from 15 to 20 carbon atoms, and when X is O and R is ═O the total number of carbon atoms in the compounds of structure (I) is from 11 to 17 carbon atoms. The invention also includes optical isomers, diastereomers and enantiomers of the named structures. Thus, at all stereocenters where stereochemistry is not explicitly defined, all possible epimers are envisioned.
Owner:BEDOUKIAN RES
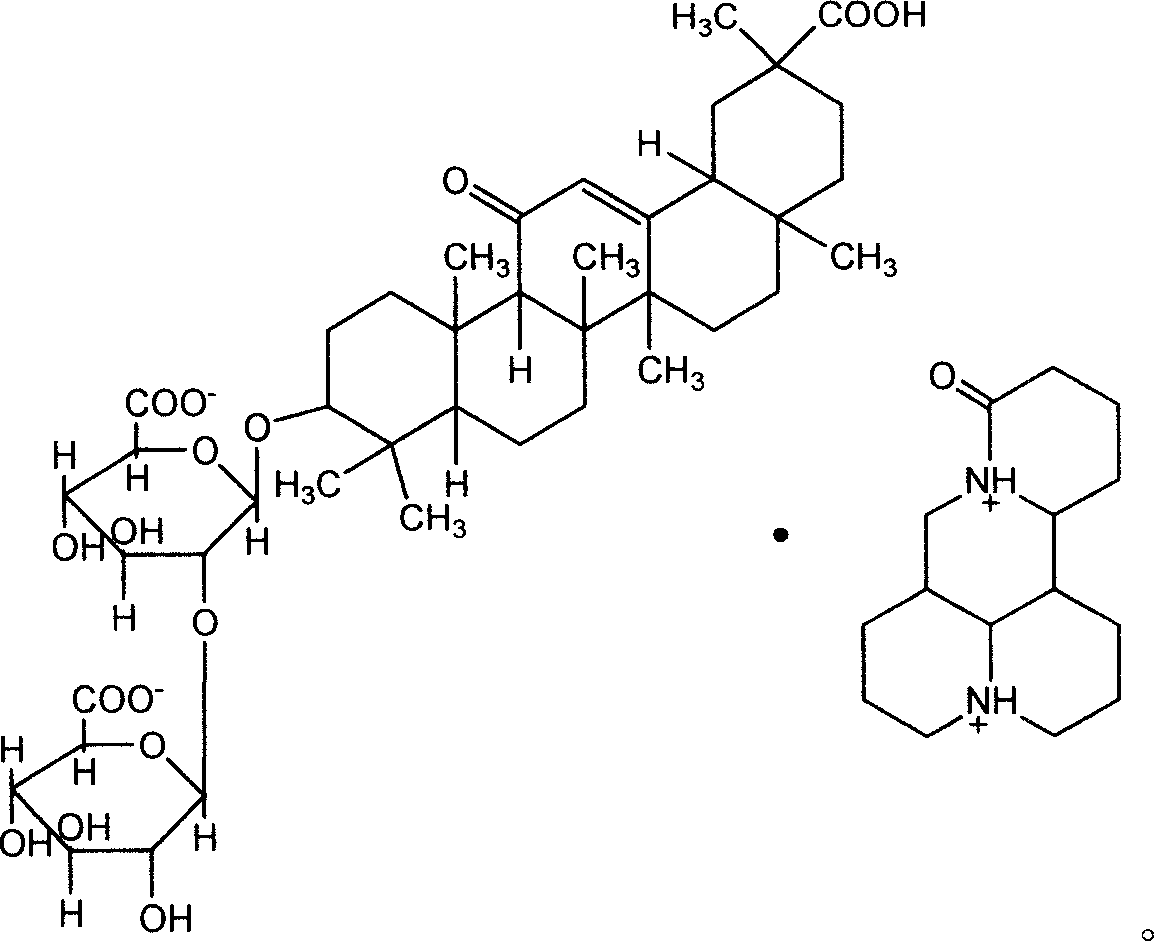
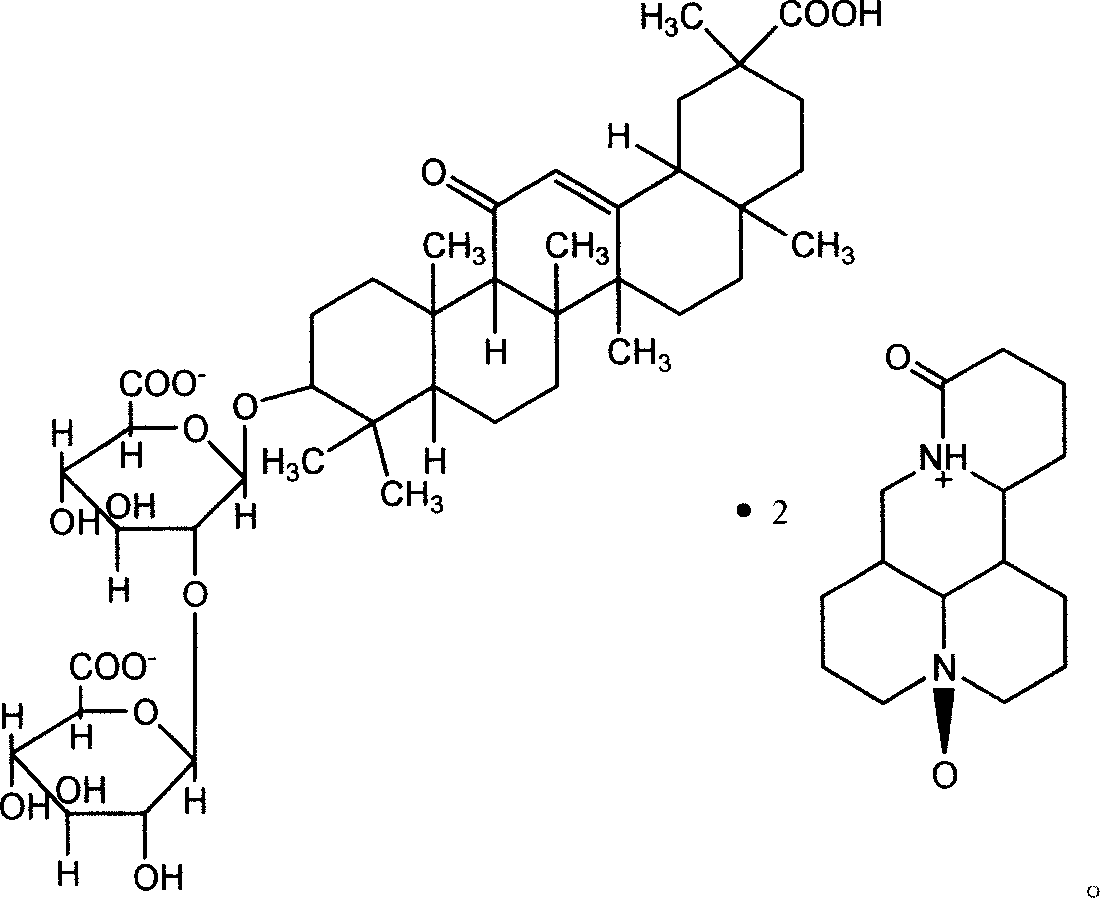
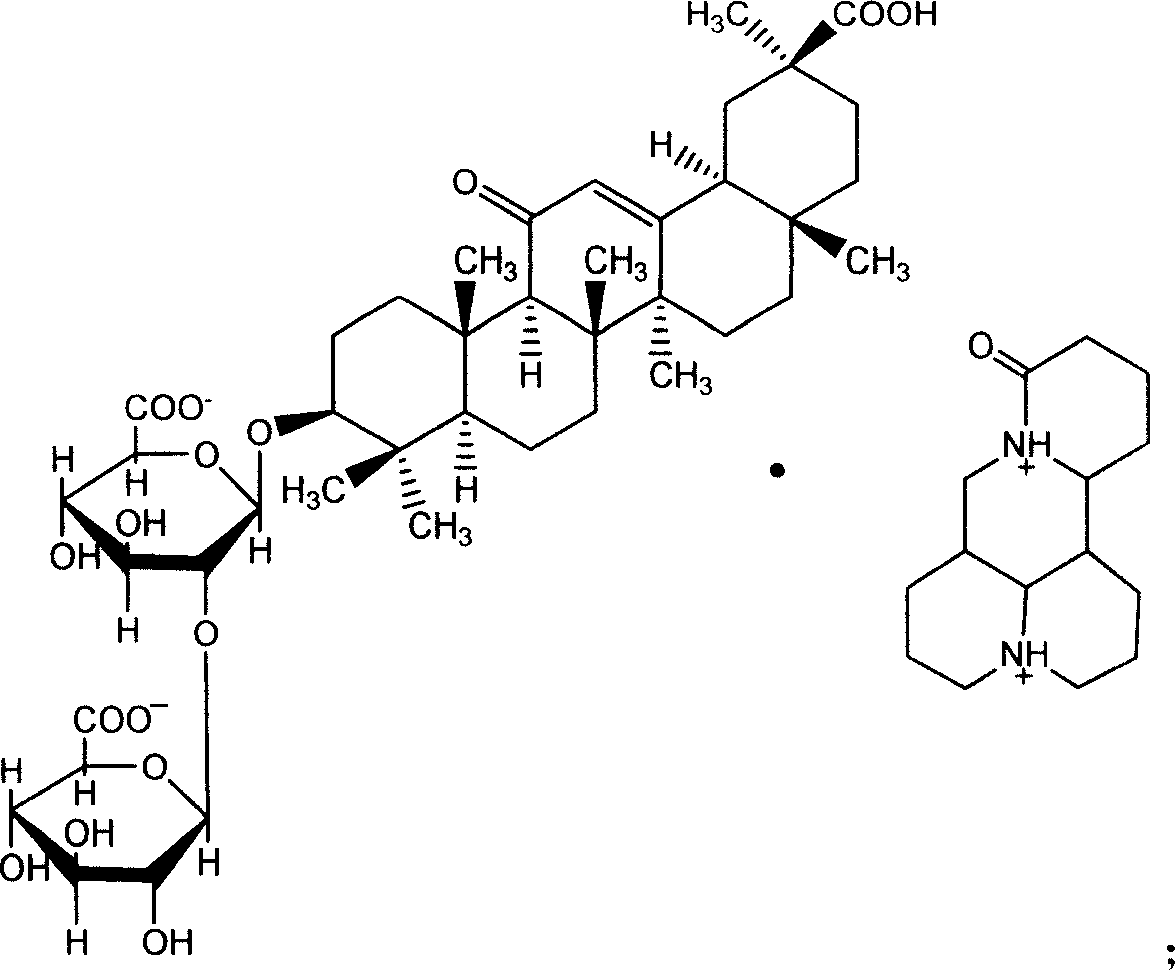
Glycyrrhizic acid matrine salt and glycyrrhizic acid marine salt, its preparing method and use
ActiveCN101012267AReasonable salt ratioReasonable positionOrganic active ingredientsDigestive systemHepatic DiseasesMatrine
The invention discloses a new synthesizing method of glycyrrhizin matrine salt and glycyrrhizin sophora flavescent salt, which also provides epimer 18-beta or 18-alpha glycyrrhizin sophora flavescent salt and their manufacturing method and application to make liver disease drug.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
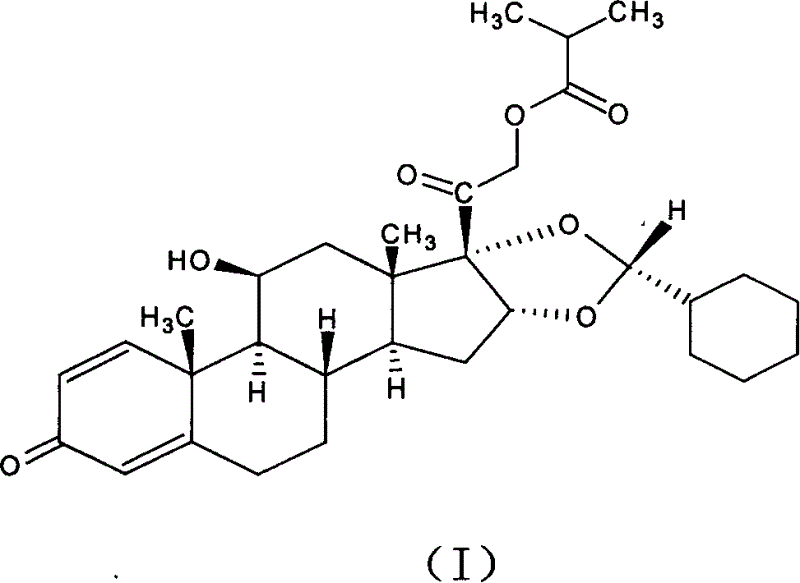
New method for preparing Ciclesonide medication for treating asthma disease
InactiveCN1626546AMeet pharmaceutical standardsOrganic active ingredientsSteroidsDiseaseMedicinal chemistry
A process for preparing the medical Huansuonaide features the aldehyde exchange reaction between the compound shown by formula (B) and cyclohexyl formaldehyde to obtain particular epimer, that is Huansuonaide.
Owner:CHONGQING PHARMA RES INST
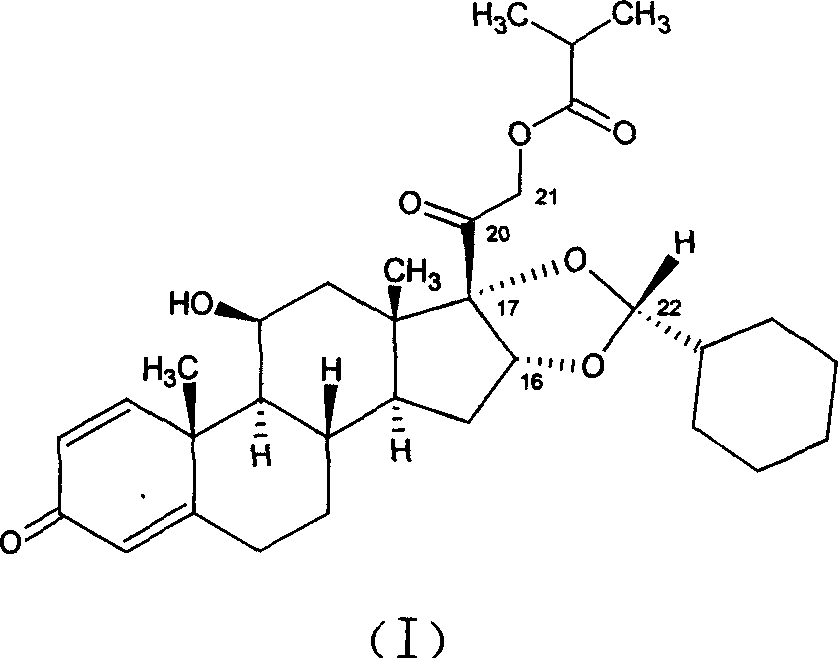
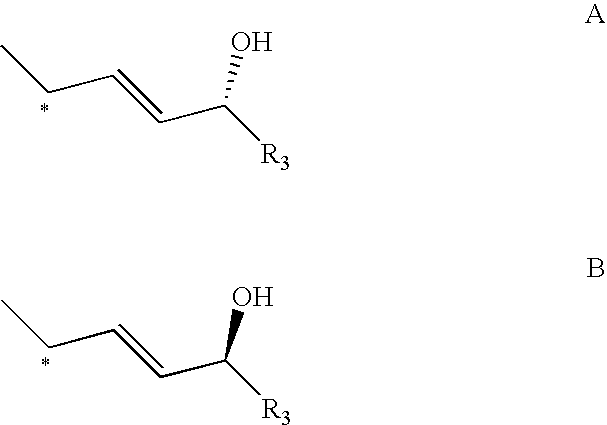
Epimerisation of Allylic Alcohols
InactiveUS20070255066A1Easy to operateImprove processing productivityOrganic compound preparationSteroidsAlcoholAllylic alcohol
The present invention relates to processes for epimerising alcohols of compounds having a hydroxyl substituent on an asymmetric allylic carbon, such as compounds useful for the synthesis of vitamin D analogues where the epimeric hydroxyl substituent is at the 24 position. The Invention further relates to methods of producing intermediates useful for the synthesis of calcipotriol by said epimerisation processes.
Owner:LEO PHARMA AS
Selective enzymatic esterification and solvolysis of epimeric vitamin D analog and separation of the epimers
Provided is a method of selectively enzymatically esterifying or selectively enzymatically solvolyzing epimers of analogs of vitamin D having a stereogenic center at C-24 that has a free or esterified OH group. The metod can be used, for example, for separating mixed epimers of the vitamin D analog.
Owner:TEVA PHARMA IND LTD
Hydrogel with multiple stimulus response as well as preparation method and application thereof
ActiveCN108250459AResponsive to multiple stimuliHas multiple applicationsAerosol deliveryWater contaminantsPolyolRoom temperature
The invention discloses hydrogel with multiple stimulus response as well as a preparation method and application thereof. The preparation method comprises the following steps: adding a polyol double acetal compound into water, heating to dissolve the polyol double acetal compound, and cooling to room temperature to obtain hydrogel, wherein the polyol double acetal compound is generated by performing condensation reaction on a polyol compound containing 4 to 6 hydroxyl groups and an aromatic aldehyde compound. In addition, the hydrogel has various kinds of applicability of chirally identifyingchiral amino acid and an epimer medicine, adsorbing dye in waste water, serving as a medicine carrier, proliferating cells and the like.
Owner:TAISHAN MEDICAL UNIV
18beta-glycyrrhetinic acid molecularly imprinted polymer with metal ions as bridging agent and monolithic column
InactiveCN105504162AEasy to operateEasy to makeIon-exchange process apparatusOther chemical processesSolid phase extractionMolecularly imprinted polymer
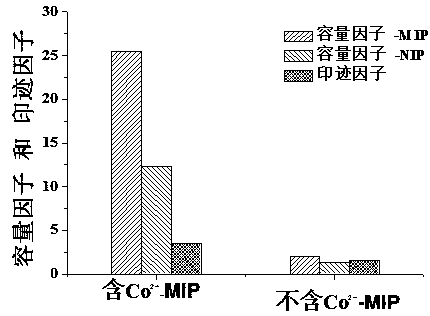
The invention relates to an 18beta-glycyrrhetinic acid molecularly imprinted polymer with metal ions as a bridging agent and a monolithic column. The polymer is used as an adsorbing agent for solid phase extraction and separation of glycyrrhetinic acid epimers. The polymer is prepared from 1.55-4.12% of 18beta-glycyrrhetinic acid, 0.82-2.18% of cobaltous acetate, 2.73-2.80% of 4-vinyl pyridine, 0.26-0.32% of azodiisobutyronitrile, 15.63-31.25% of ethylene glycol dimethacrylate, 43.28-43.80% of 1-butyl-3-methylimidazolium tetrafluoroborate, 3.18-3.59% of N,N-dimethylformamide and 18.47-20.01% of dimethyl sulfoxide. Compared with an imprinted polymer with no metal ion, the imprinting factor of the polymer synthesized through the method is improved by about 3 times, and the polymer is dried, ground and screened (74 micrometers) to be used as an adsorbing agent to be placed into an SPE column for carrying out separation and enrichment on 18beta-glycyrrhetinic acid in glycyrrhetinic acid crude extract. The recovery rate of the obtained sample is 91.1%, and the purity of the obtained sample is 93.8%. Accordingly, the polymer can be used for separation, purification and enrichment of the glycyrrhetinic acid crude extract.
Owner:TIANJIN MEDICAL UNIV
Epimerisation of allylic alcohols
The present invention relates to processes for epimerising alcohols of compounds having a hydroxyl substituent on an asymmetric allylic carbon, such as compounds useful for the synthesis of vitamin D analogues where the epimeric hydroxyl substituent is at the 24 position. The invention further relates to methods of producing intermediates useful for the synthesis of calcipotriol by said epimerisation processes.
Owner:LEO PHARMA AS
Method for preparing tigecycline intermediate
ActiveCN106831469ASuitable for industrial mass productionEasy to operateOrganic compound preparationCarboxylic acid amides preparationTigecyclineEpimer
The invention relates to a method for preparing a tigecycline intermediate. The tigecycline intermediate can be obtained at a high yield and high purity. The method has the advantages of convenience in operation, safety and environment protection. The obtained product has the characteristics of high purity and low epimer contents and is favorable for industrial amplification.
Owner:JIANGSU HANSOH PHARMA CO LTD
Dihydroartemisinin phenyl ether derivatives and applications thereof
InactiveCN104974171AAchieve separationStrong growth inhibitory effectOrganic active ingredientsOrganic chemistryCancer cellPhenyl Ethers
The invention relates to the technical field of medicine, and specifically relates to nitrogen-substituted dihydroartemisinin phenyl ether, optical isomers thereof and a preparation method thereof; pharmaceutical compositions with the derivatives as active components; and applications thereof in preparing medicines used for treating and / or preventing various cancers. The compound or pharmaceutically acceptable salts thereof have a structure as the following. The variables are as described in the claims and in the specifications. According to the invention, a pair of epimers with dihydroartemisinin C-10 site of R or S configuration can be prepared at a same time and with an equal amount, and separation can be realized. The prepared compounds have a significant effect in inhibiting cancer cell growth, and a selective killing effect against drug-resistant cells. The compounds have a potential of overcoming multidrug resistance.
Owner:SHENYANG PHARMA UNIVERSITY
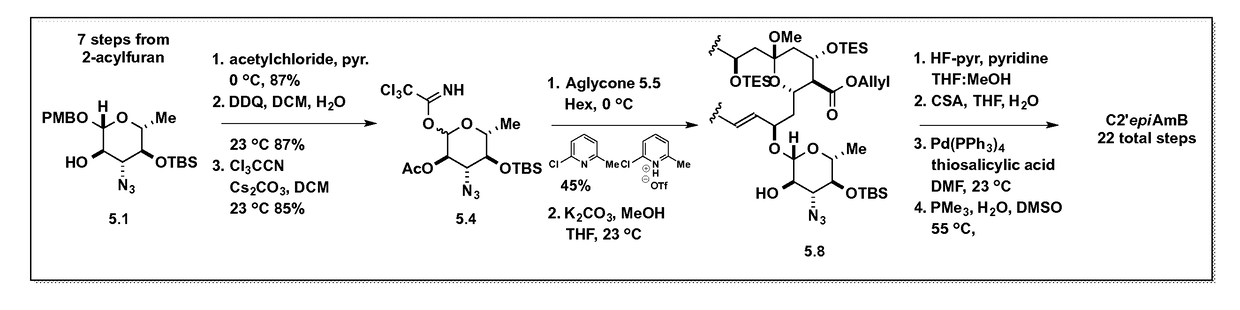
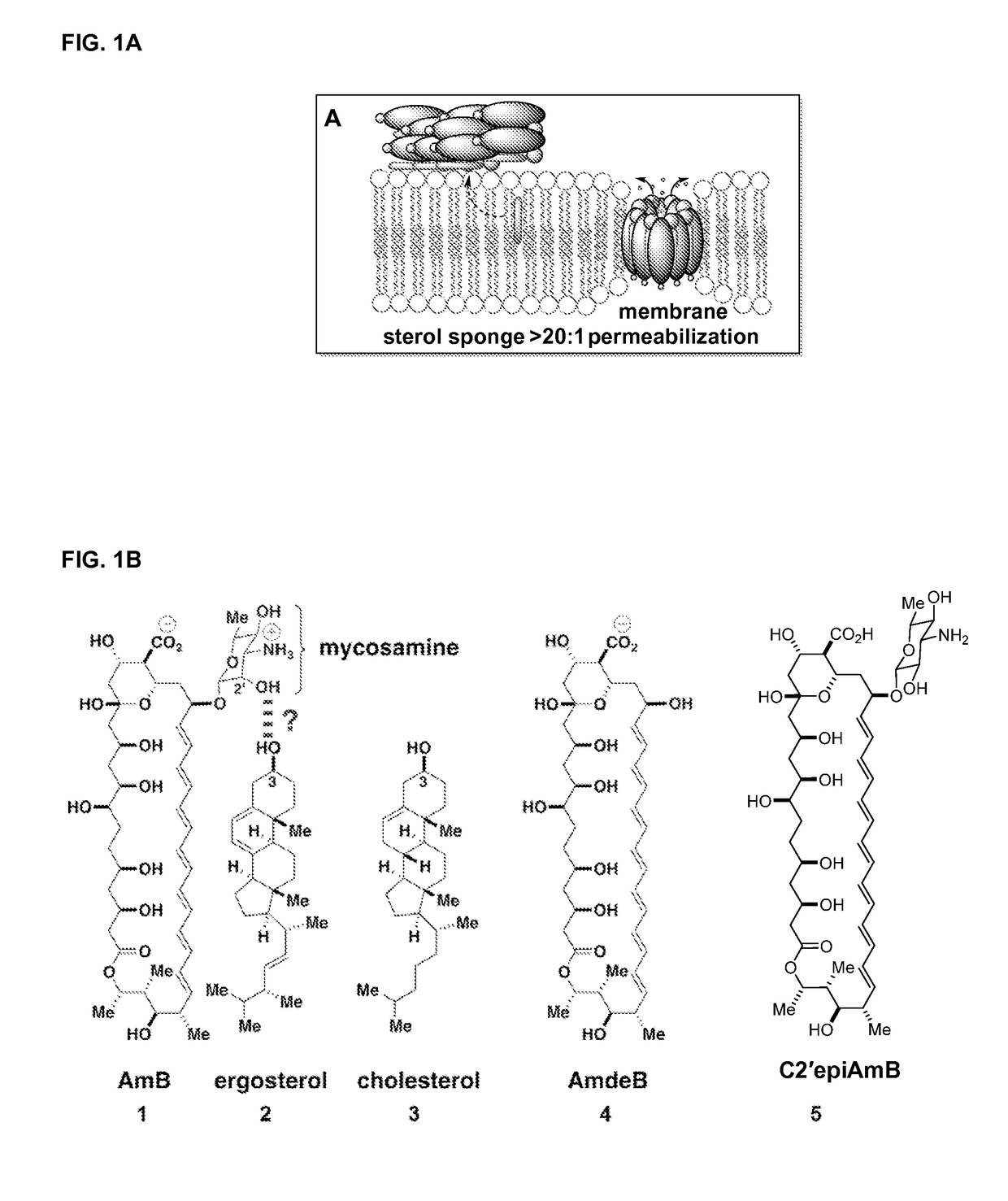
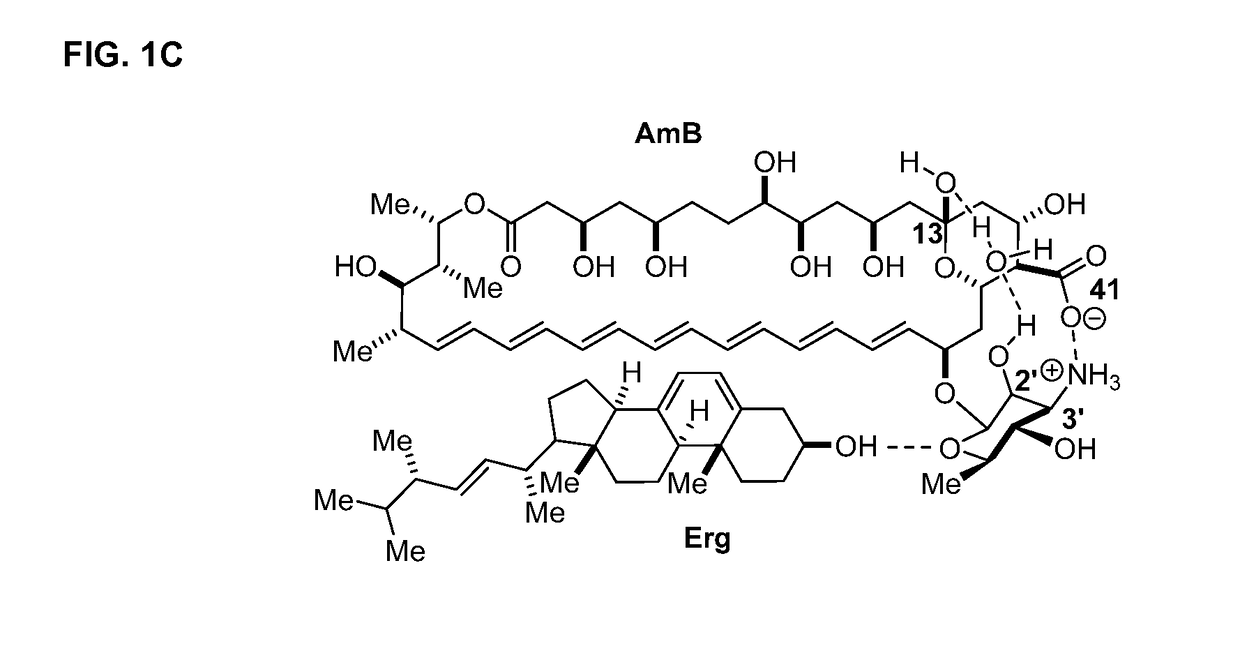
Amphotericin b derivative with reduced toxicity
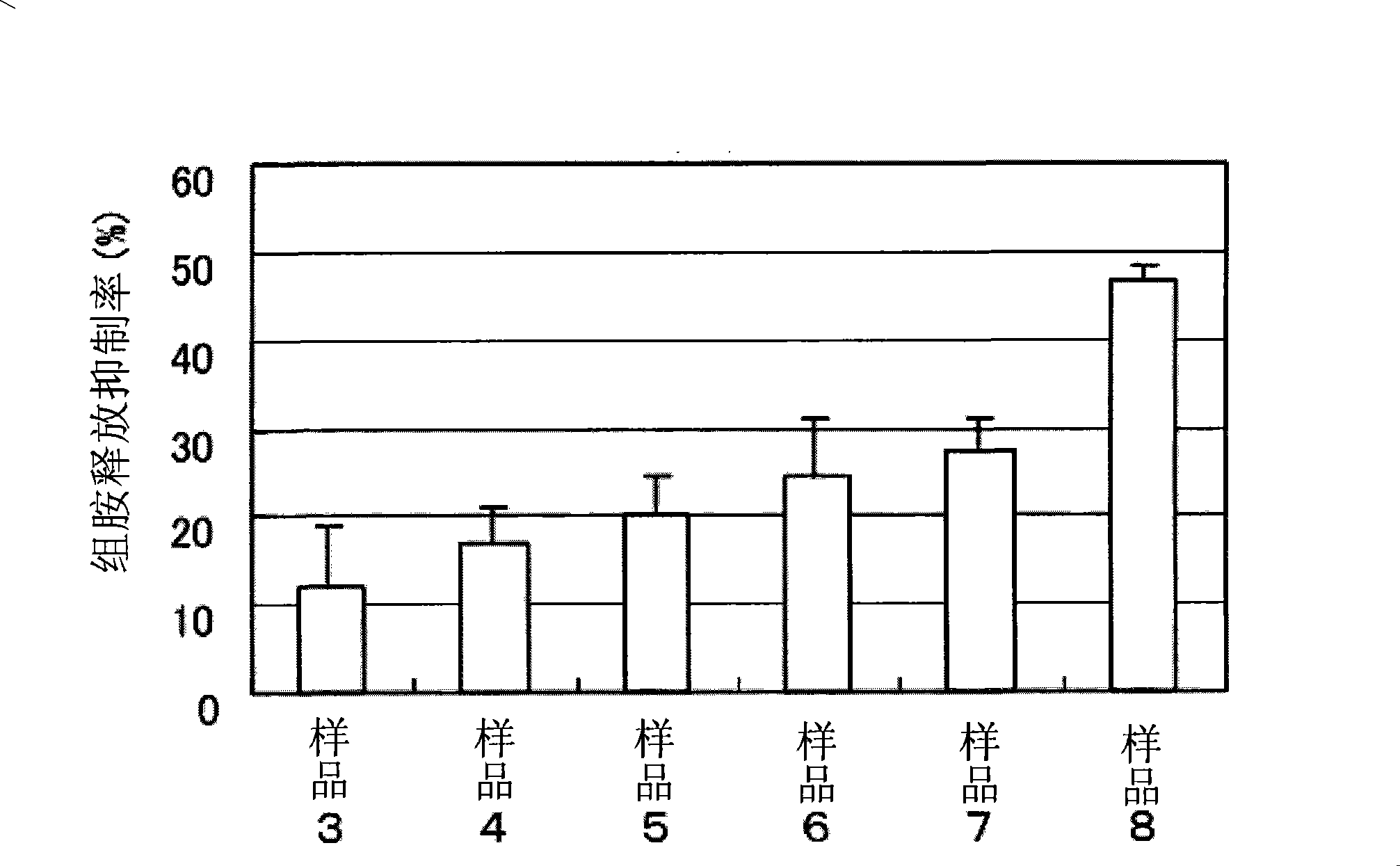
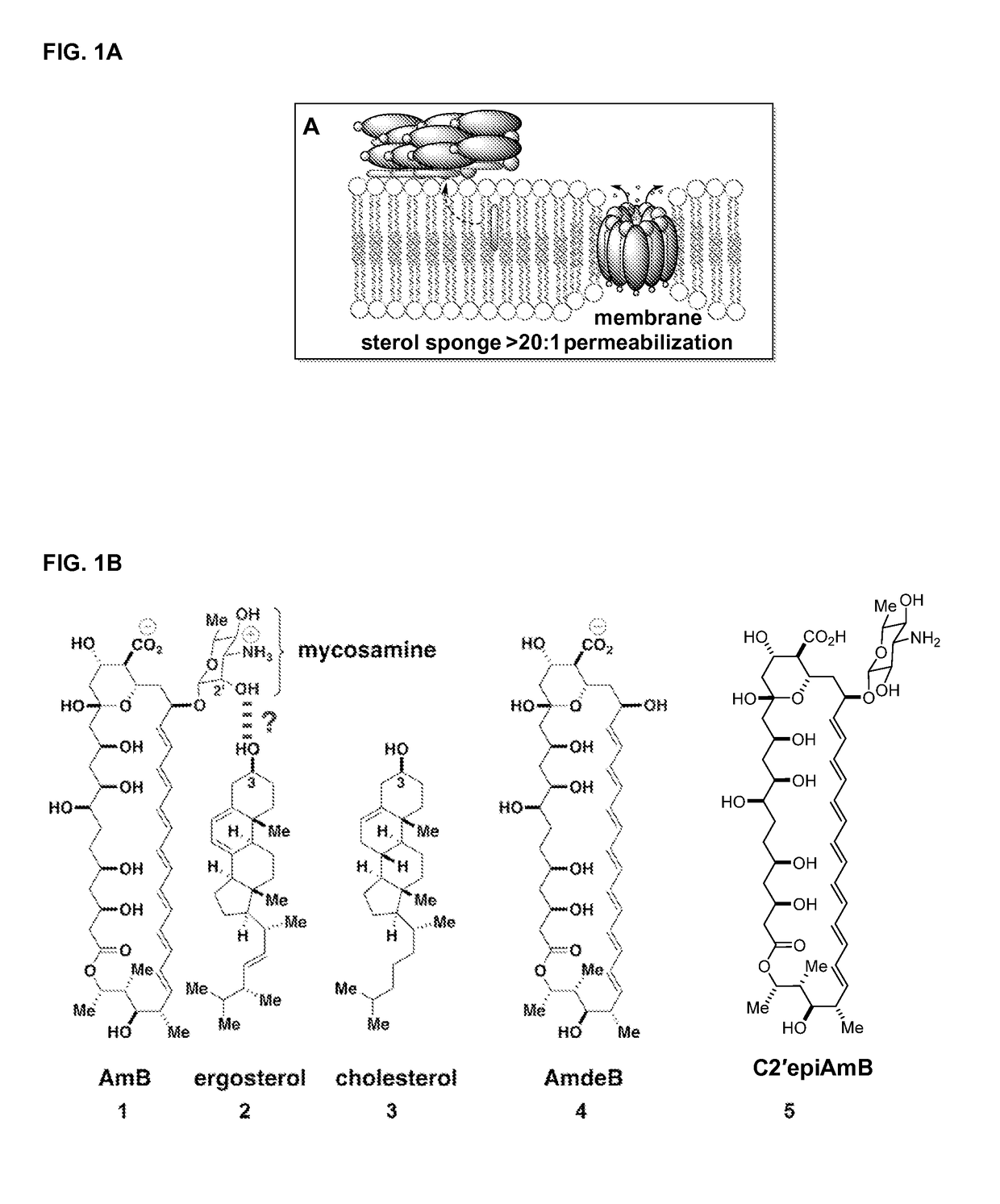
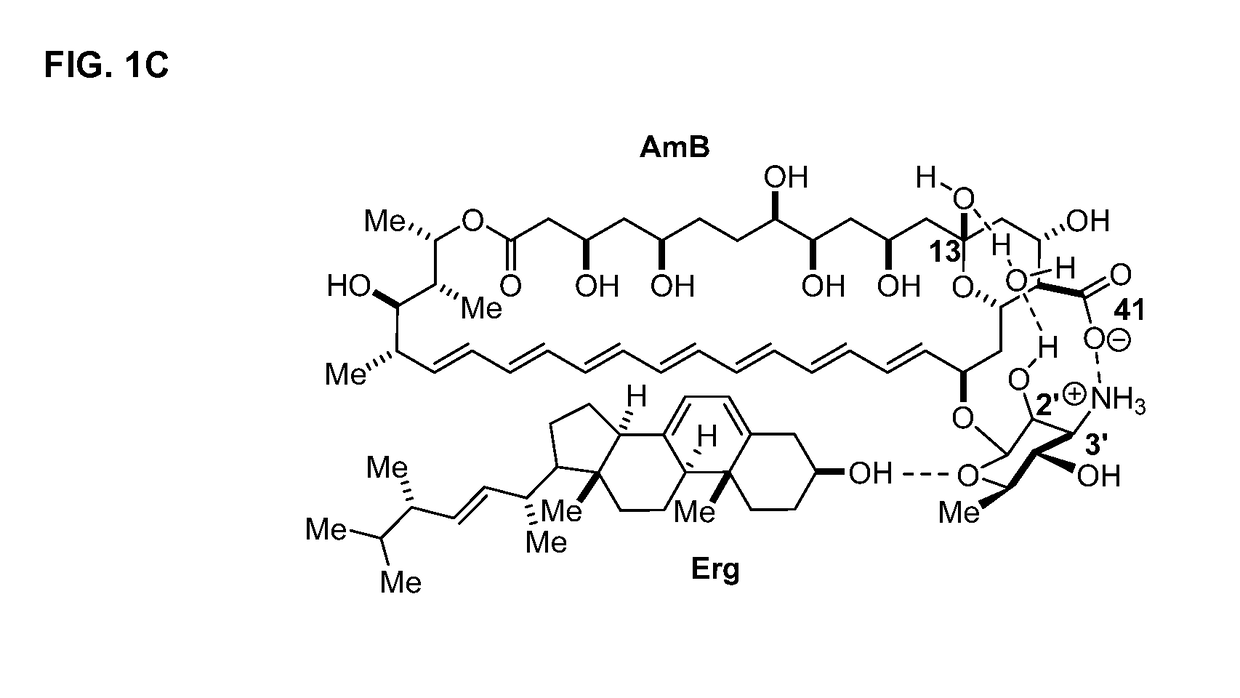
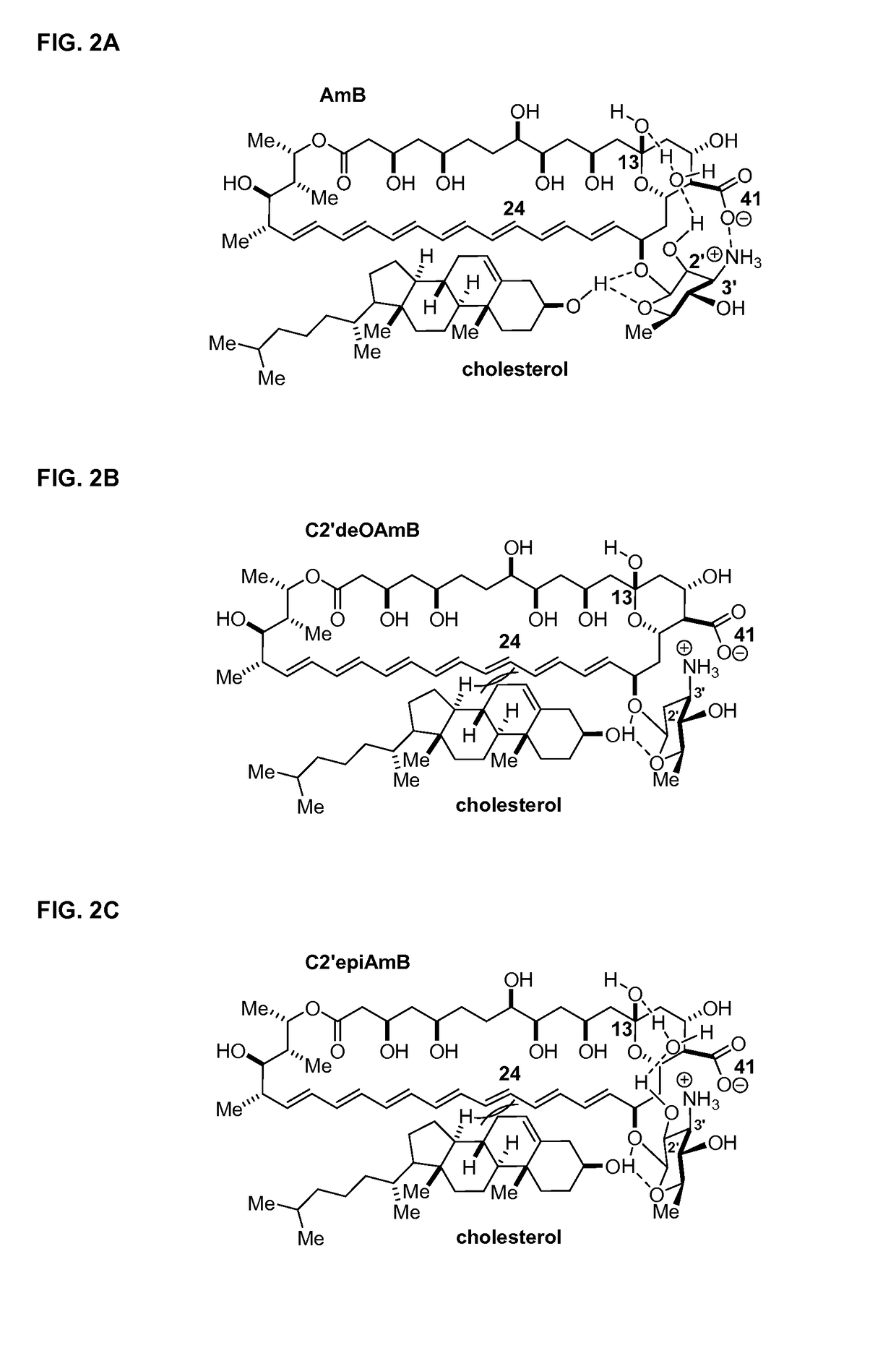
Disclosed is a derivative of amphotericin B (AmB), denoted C2′epiAmB, with an improved therapeutic index over amphotericin B, pharmaceutical compositions comprising the AmB derivative, methods of making the AmB derivative and the pharmaceutical composition, and their use in methods of inhibiting growth of a yeast or fungus and treating a yeast or fungal infection. C2′epiAmB is an epimer of the parent compound. Specifically, C2′epiAmB differs from the parent compound at the CT stereogenic center on mycosamine. This difference in structure results in (i) retained capacity to bind ergosterol and inhibit growth of yeast, (ii) greatly reduced capacity to bind cholesterol, and (iii) essentially no toxicity to human cells.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
Antialergic agent, and food and drink, external preparation and cosmetic containing it
InactiveCN101365443AImprove quicknessReduce worriesOrganic active ingredientsOrganic chemistryAnti-Allergic AgentsHydrogen atom
The object is to isolate a substance having a potent anti-allergic effect inherent in a tea leaf of an Assam hybrid line. Thus, disclosed is an anti-allergic agent comprising, as an active ingredient, a catechin yielded by the isolation separation, i.e., epicatechin-3-O-(3-O-methyl)gallate, epicatechin-3-O-(4-O- methyl)gallate, epicatechin-3-O-(3,4-O-dimethyl)gallate, epicatechin-3-O-(3,5-O-dimethyl)gallate, epicatechin-3-O- (3,4,5-O- tromethyl)gallate, an epimer thereof or the like. Also disclosed is a beverage / food, a preparation for external application or a cosmetic comprising the anti-allergic agent. An anti-allergic composition can be prepared using a compound represented by the general formula (1): (1) wherein R1, R2 and R3 independently represent a hydrogen atom or a methyl group.
Owner:NAT AGRI & FOOD RES ORG +1
Amphotericin B derivative with reduced toxicity
Disclosed is a derivative of amphotericin B (AmB), denoted C2′epiAmB, with an improved therapeutic index over amphotericin B, pharmaceutical compositions comprising the AmB derivative, methods of making the AmB derivative and the pharmaceutical composition, and their use in methods of inhibiting growth of a yeast or fungus and treating a yeast or fungal infection. C2′epiAmB is an epimer of the parent compound. Specifically, C2′epiAmB differs from the parent compound at the C2′ stereogenic center on mycosamine. This difference in structure results in (i) retained capacity to bind ergosterol and inhibit growth of yeast, (ii) greatly reduced capacity to bind cholesterol, and (iii) essentially no toxicity to human cells.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com