Method for preparing vitamin D2 derivative
A technology of derivatives and vitamins, applied in the field of preparation of vitamin D2 derivatives, can solve the problems of reducing the yield of calcipotriol and having no utilization value, and achieves the effects of low cost of raw materials, improved utilization, and reduced pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
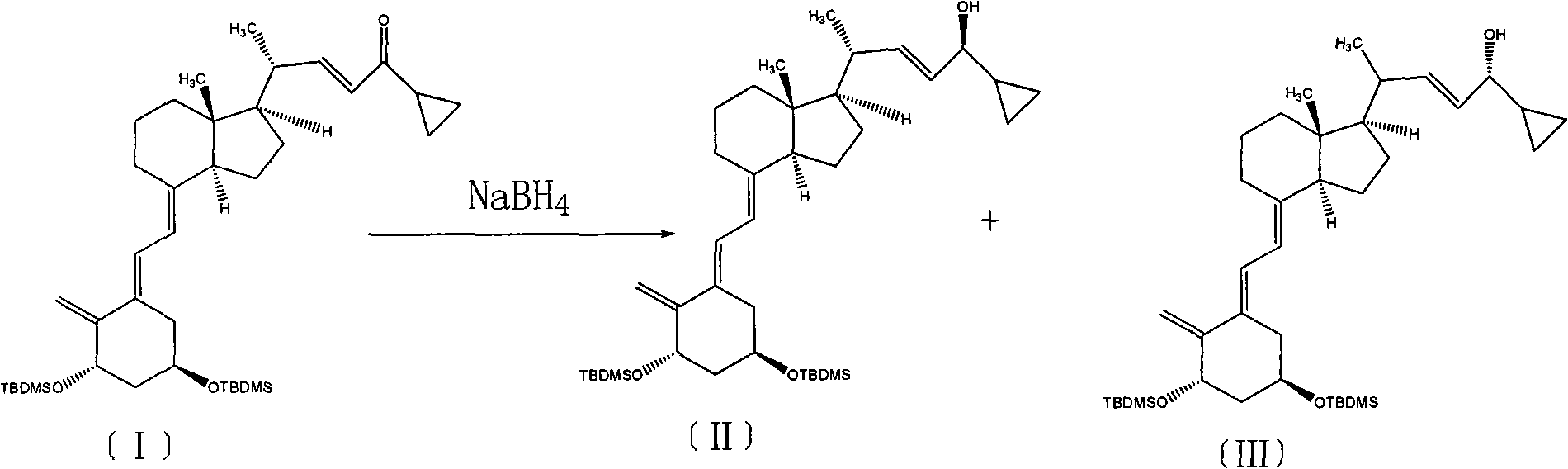
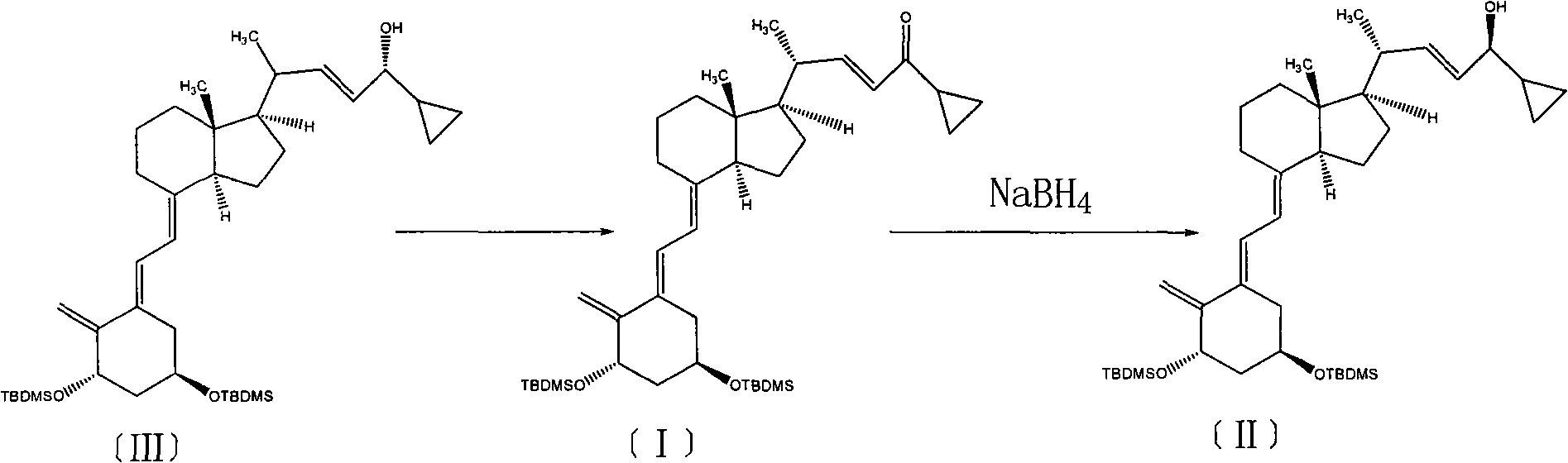
[0021] Add 5.0 g (0.0078 mol) of compound (III) and 100 ml of toluene, stir to dissolve, add 4.4 g (0.0117 mol) of PDC, heat to reflux at 110 ° C, keep warm for 6 h, TLC test confirms that the reaction is complete, and the developer is petroleum ether: Ethyl acetate = 9:1, cool down to room temperature to stop the reaction, add an appropriate amount of column chromatography silica gel to the floxacin funnel, filter the reaction solution, evaporate to dryness to obtain an oil, and separate by column chromatography to obtain 4.3 g of compound (I), with a yield of 86.3 %.
Embodiment 2
[0023] Put in 5.0g (0.0078mol) of compound (III) and 100ml of N,N'-dimethylformamide, stir to dissolve, add 4.4g (0.0117mol) of PDC, heat to reflux at 120°C, keep the reaction for 4h, and cool down to room temperature Stop the reaction, add an appropriate amount of silica gel for column chromatography into the floxacin funnel, filter the reaction solution, and evaporate to dryness to obtain an oily substance, which is separated by column chromatography to obtain 4.1 g of compound (I), with a yield of 82.3%.
Embodiment 3
[0025] Put in 5.0g (0.0078mol) of compound (III) and 100ml of chloroform, stir to dissolve, add 20g of manganese dioxide, heat to 70°C for reflux reaction for 24h, cool down to stop the reaction, add an appropriate amount of column chromatography silica gel to the Shaxing funnel, and the reaction solution After filtering and evaporating to dryness to obtain an oil, 4.0 g of compound (I) was obtained by column chromatography with a yield of 80.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com