New method for preparing Ciclesonide medication for treating asthma disease
A ciclesonide, pharmaceutical-grade technology, applied in the field of preparing ciclesonide, can solve the problems of cumbersome process, high cost of ciclesonide R type, high product price and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
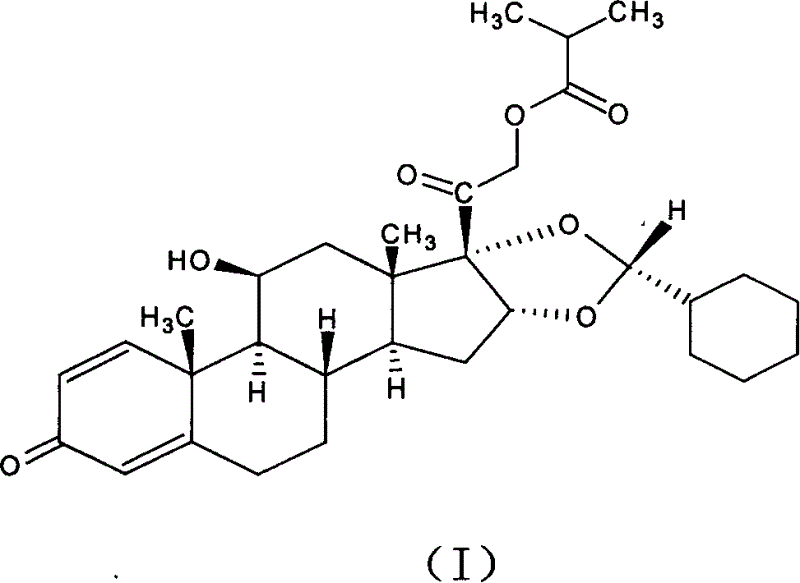
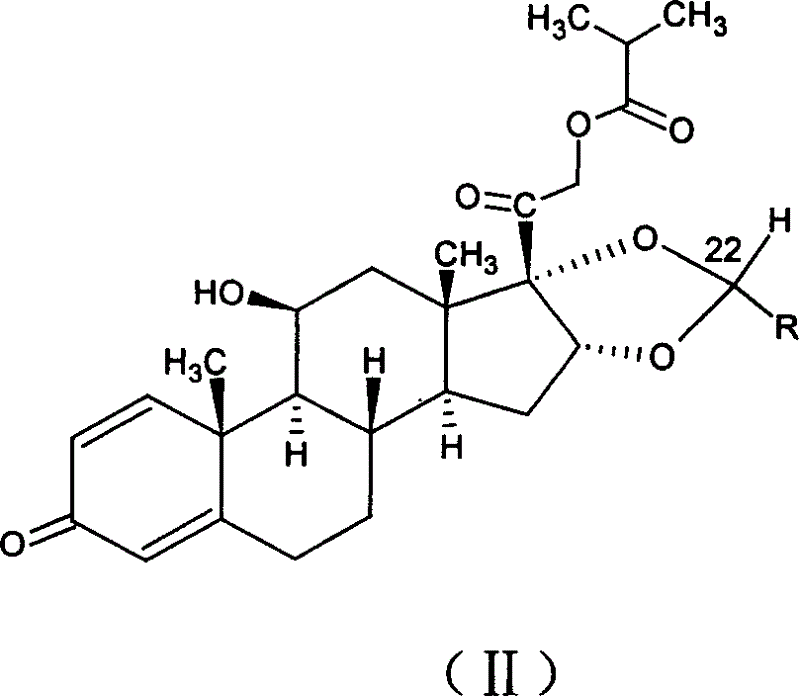
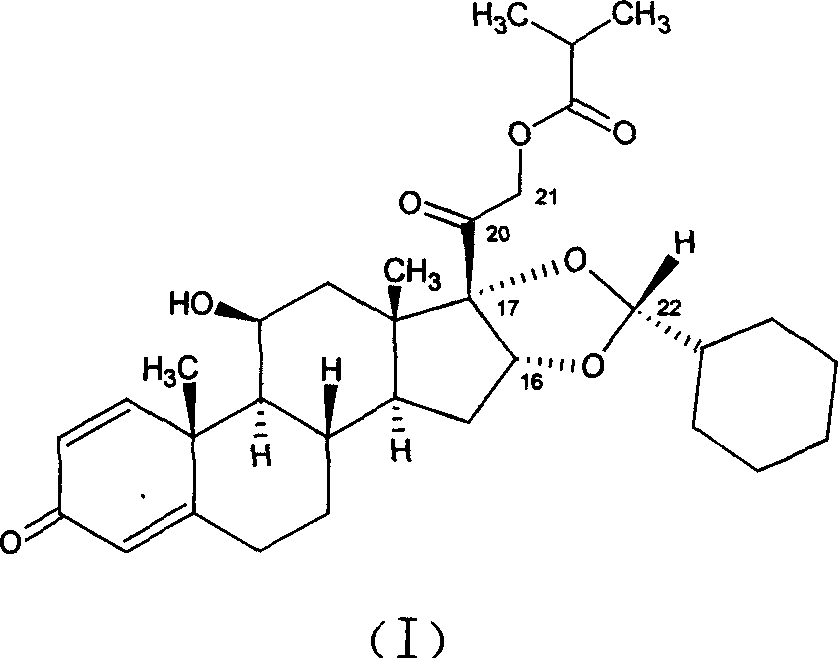
[0035] In a 250ml three-necked flask equipped with agitator, put pregnant-1,4-diene-3,20-dione, 16,17-[(ethylmethylene)bis(oxo)]-11-hydroxyl-21 -(2-Methyl-1-carbonylpropoxy)-, [11β, 16α], that is, 10g (0.021mol) of the raw material of formula (II) and 50ml of nitromethane, form a suspension under stirring, and add 70% dropwise 5.3ml (0.0616mol) of perchloric acid, followed by dropwise addition of 15ml (0.126mol) of cyclohexyl formaldehyde, reacted at room temperature for 2-10 hours, dripped 200ml of 5% potassium carbonate aqueous solution, and caused the solution to be neutral, and an oily solid was obtained. The filtrate was poured off, and about 30 ml of ethanol was added to the obtained oily solid to dissolve it, then spun to dryness, and the solid was collected. About 8.8 g of the ciclesonide product (I) was obtained, and its epimer ratio R / S=98.5 / 1.5. The total yield is about 80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com