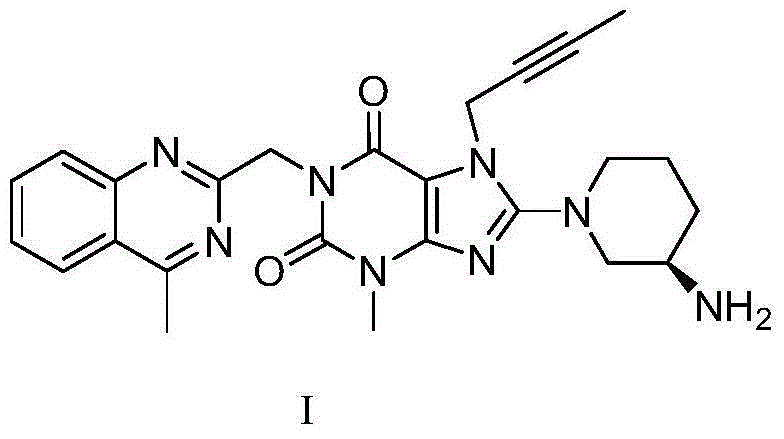
Linagliptin purification method
A purification method, an organic technology, applied in the direction of organic chemistry, etc., can solve the problem of high impurity content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Preparation of crude linagliptin:
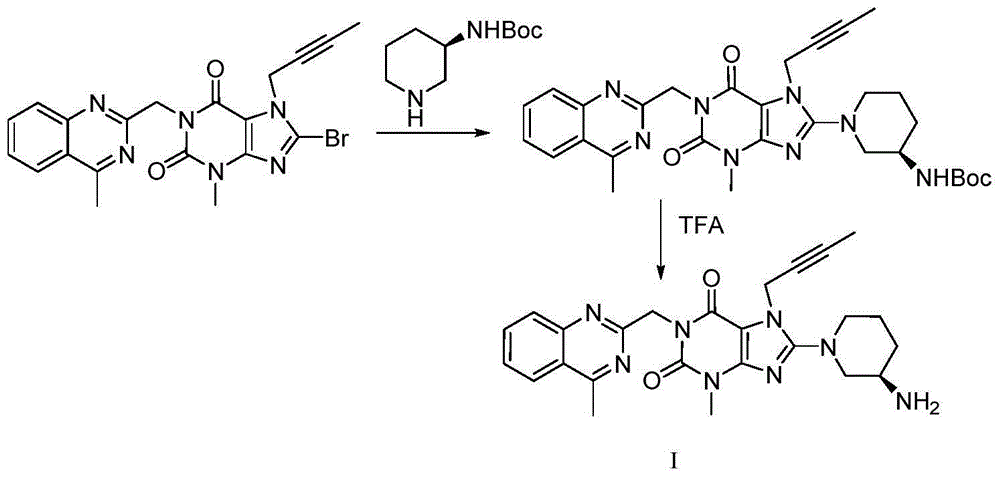
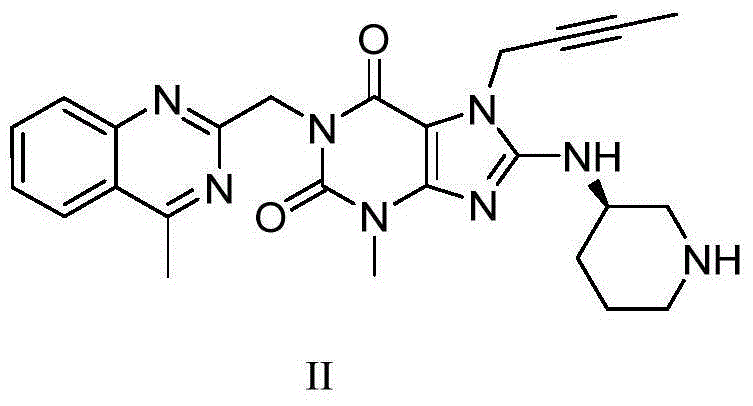
[0031] In a 500ml reaction flask, add 10g of intermediate IV (dissolved in 100ml of N,N-dimethylformamide), add 9.4g of potassium carbonate and 5.72g of aminopiperidine dihydrochloride, and heat the reaction to 90°C for reaction 6 hour, TLC monitors that the reaction ends, naturally cools to room temperature, adds 100ml of dichloromethane, removes potassium carbonate by filtration, and the filtrate is extracted with 100ml of water to separate liquids, and 100ml of dichloromethane is extracted twice again, the organic phases are combined, concentrated, and Liglie Tin crude product 8.2 g, HPCL=98.7%, isomer impurity formula II impurity HPLC=0.72%.
Embodiment 2
[0033] Preparation of crude linagliptin:
[0034]
[0035] In a 500ml reaction flask, add 4.53g of intermediate IV (dissolved in 50ml of N,N-dimethylformamide), add 2.76g of potassium carbonate and 2.0g of Boc-protected aminopiperidine, and heat the reaction to 80°C for 10 hours , TLC monitors the end of the reaction, naturally cools to room temperature, adds 100ml of dichloromethane, removes potassium carbonate by filtration, extracts the filtrate with 100ml of water, and extracts twice with 100l of dichloromethane, combines the organic phases, concentrates, and dissolves the crude product in 100ml of dichloromethane Add 3.52 grams of trifluoroacetic acid to methyl chloride, deprotect under stirring at room temperature, 5 hours, concentrate excess trifluoroacetic acid and solvent at room temperature, dissolve the crude product in 50 ml of dichloromethane, extract and separate the liquids with 50 ml of 10% sodium bicarbonate water Three times, the organic phase was concentr...
Embodiment 3
[0037] Purification of Linagliptin
[0038] 4.72 grams of linagliptin crude product (prepared in implementation one) was dissolved in 50 milliliters of 95% ethanol, and 2.0 grams of N-acetyl-L glutamic acid was added under reflux and dissolved in 20 milliliters of 95 percent ethanol solution. After adding, the temperature was naturally cooled to After stirring at room temperature for 2 hours, filter, wash with 10 ml of ethanol, and dry under reduced pressure at 50°C to obtain 6.35 grams of white linagliptin N-acetyl-L-glutamate, HPLC=99.83%, isomer impurity formula IIHPLC= 0.06%.
[0039] Put the above salt in 50 ml of dichloromethane, add 50 ml of 30% potassium carbonate aqueous solution, stir at room temperature for 2 hours, free and clear, separate liquid extraction, and concentrate the organic phase to obtain pure linagliptin free base. HPLC=99.86%, isomer impurity formula II HPLC=0.06%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com