Levodropropizine-guaifenesin capsule and preparation method thereof
A technology of levoropropazine and guaiacol glyceryl ether, which is applied in the field of levorfeetol capsules and preparation thereof, can solve the problems of many auxiliary materials, complicated operation steps, unstable mixing and the like, and achieves production cost saving and good quality Dissolution, the effect of simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
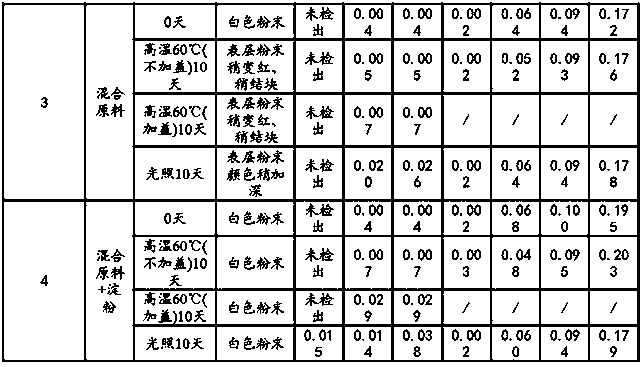
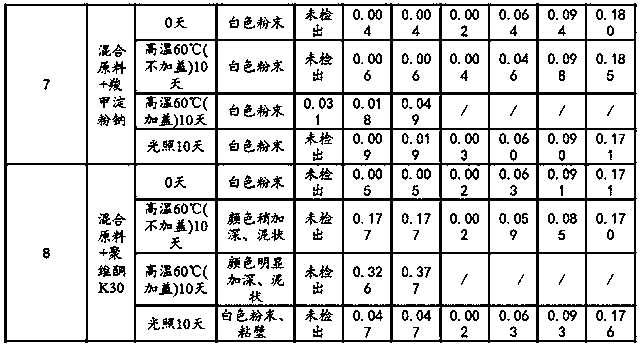
[0028] Embodiment 1: experimental research on the compatibility of raw materials and auxiliary materials
[0029] Referring to the requirements of the "Basic Technical Guidelines for the Research of Chemical Drug Preparations", separate raw materials, excipients, and mixed powders of raw materials and auxiliary materials were taken, and the influencing factors (light, high temperature, etc.) test.
[0030] Illumination test: Expose the raw materials, excipients, and mixed powders of raw and excipient materials in various ratios to the light of 4500±500Lx for 10 days, take samples, and detect the appearance properties and related substances.
[0031] High temperature test: the raw materials, excipients, and mixed powders of raw and excipient materials in different proportions are set out in two forms of weighing bottles (without lids) and lids respectively, and placed at a high temperature of 60°C for 10 days, sampling, and inspection of appearance properties and related subst...
Embodiment 2-21
[0044] Example 2-21 Prescription and preparation method of levoguaicol capsules
Embodiment 2
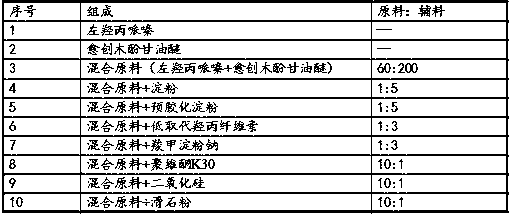
[0045] Table 1 Example 2-6 Levoguaiacol Capsules Prescription
[0046]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com