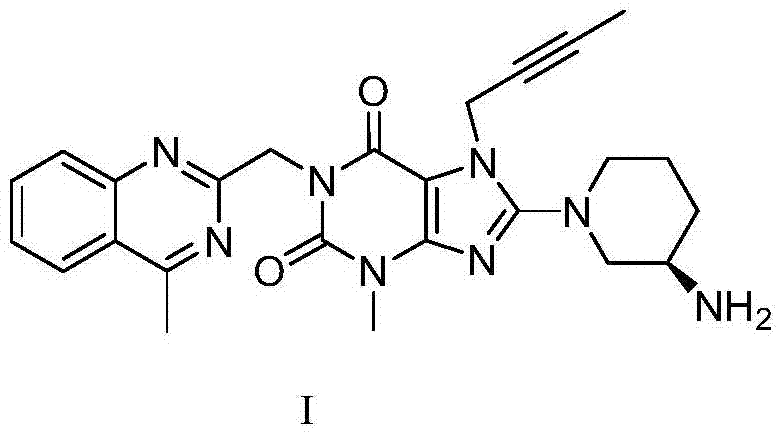
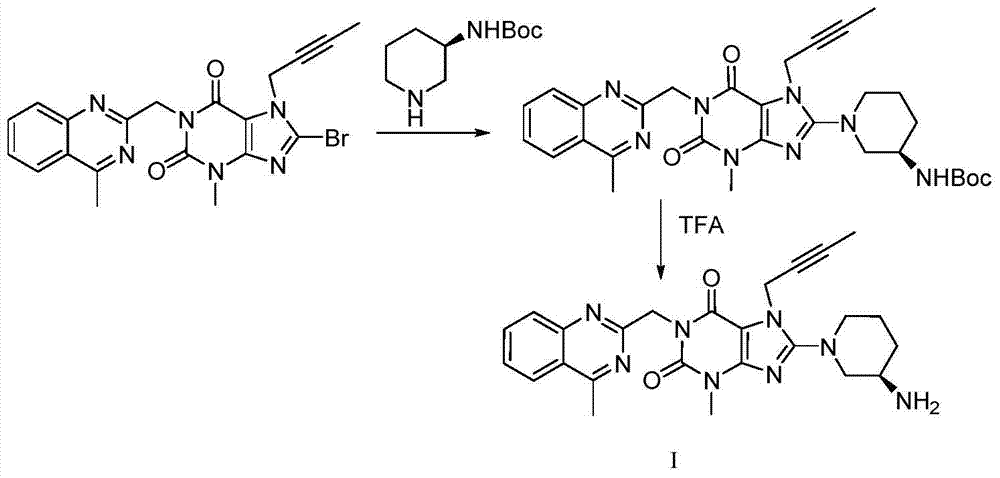
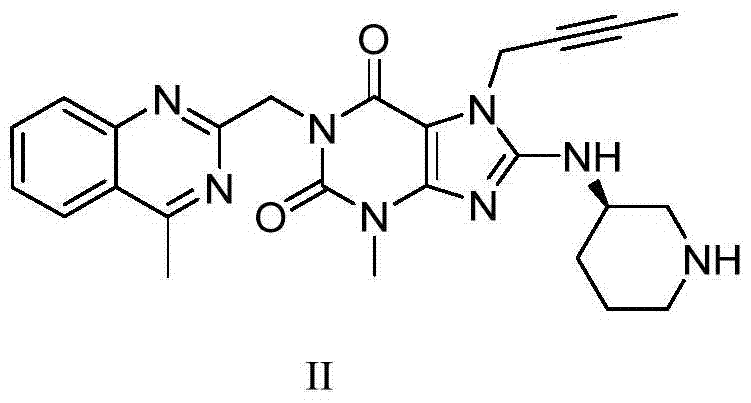
A kind of purification method of linagliptin
A purification method, amino technology, applied in organic chemistry, etc., can solve the problem of high impurity content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Preparation of crude linagliptin:
[0031] In a 500ml reaction flask, add 10g of intermediate IV (dissolved in 100ml of N,N-dimethylformamide), add 9.4g of potassium carbonate and 5.72g of aminopiperidine dihydrochloride, and heat the reaction to 90°C. After 6 hours, the TLC monitoring reaction was completed, cooled to room temperature naturally, added 100ml of dichloromethane, filtered to remove potassium carbonate, and the filtrate was extracted with 100ml of water to separate the liquids, extracted twice with 100ml of dichloromethane, combined the organic phases, concentrated, Delig Gliptin crude product 8.2 g, HPCL=98.7%, isomer impurity formula II impurity HPLC=0.72%.
Embodiment 2
[0033] Preparation of crude linagliptin:
[0034]
[0035] In a 500ml reaction flask, add 4.53g of intermediate IV (dissolved in 50ml of N,N-dimethylformamide), add 2.76g of potassium carbonate and 2.0g of Boc-protected aminopiperidine, and heat the reaction to 80°C for 10 Hours, TLC monitors the end of the reaction, naturally cools to room temperature, adds 100ml of dichloromethane, removes potassium carbonate by filtration, extracts the filtrate with 100ml of water, and extracts twice with 100l of dichloromethane, combines the organic phases, concentrates, and dissolves the crude product in 100ml In dichloromethane, add 3.52 g of trifluoroacetic acid, deprotect under stirring at room temperature, 5 hours, concentrate excess trifluoroacetic acid and solvent at room temperature, dissolve the crude product in 50 ml of dichloromethane, and extract fractions with 50 ml of 10% sodium bicarbonate water liquid for three times, and the organic phase was concentrated to obtain 9.44...
Embodiment 3
[0037] Purification of Linagliptin
[0038] 4.72 grams of linagliptin crude product (prepared in implementation one) was dissolved in 50 milliliters of 95% ethanol, and 2.0 grams of N-acetyl-L glutamic acid was added under reflux and dissolved in 20 milliliters of 95 percent ethanol solution. After adding, the temperature was naturally cooled to After stirring at room temperature for 2 hours, filter, wash with 10 ml of ethanol, and dry under reduced pressure at 50°C to obtain 6.35 g of white linagliptin N-acetyl-L-glutamate, HPLC=99.83%, isomer impurity formula II HPLC = 0.06%.
[0039] Put the above salt in 50 ml of dichloromethane, add 50 ml of 30% potassium carbonate aqueous solution, stir at room temperature for 2 hours, free and clear, separate liquid extraction, and concentrate the organic phase to obtain pure linagliptin free base. HPLC=99.86%, isomer impurity formula II HPLC=0.06%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com