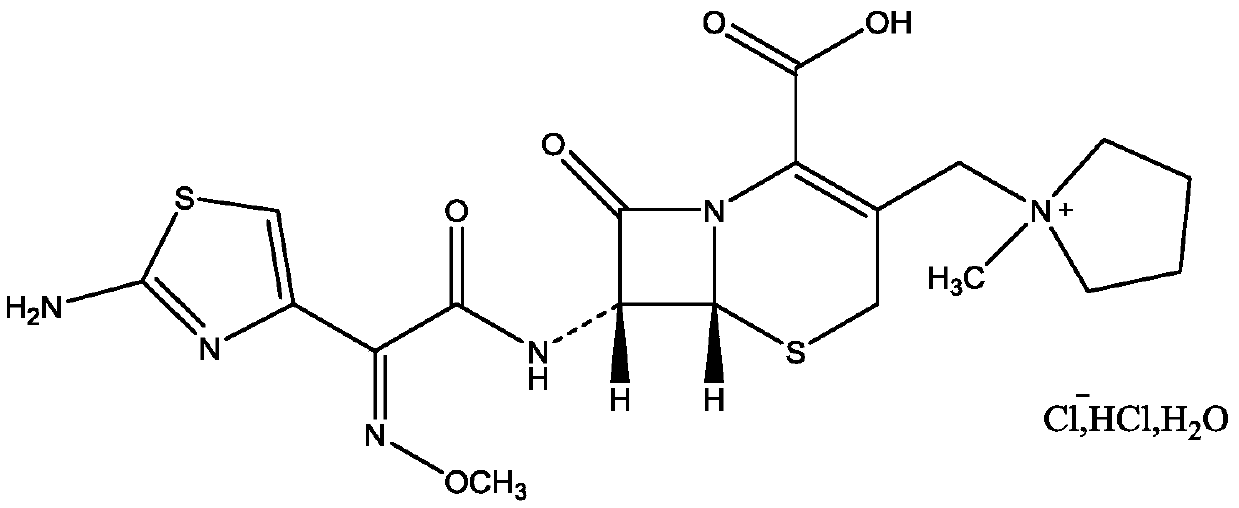
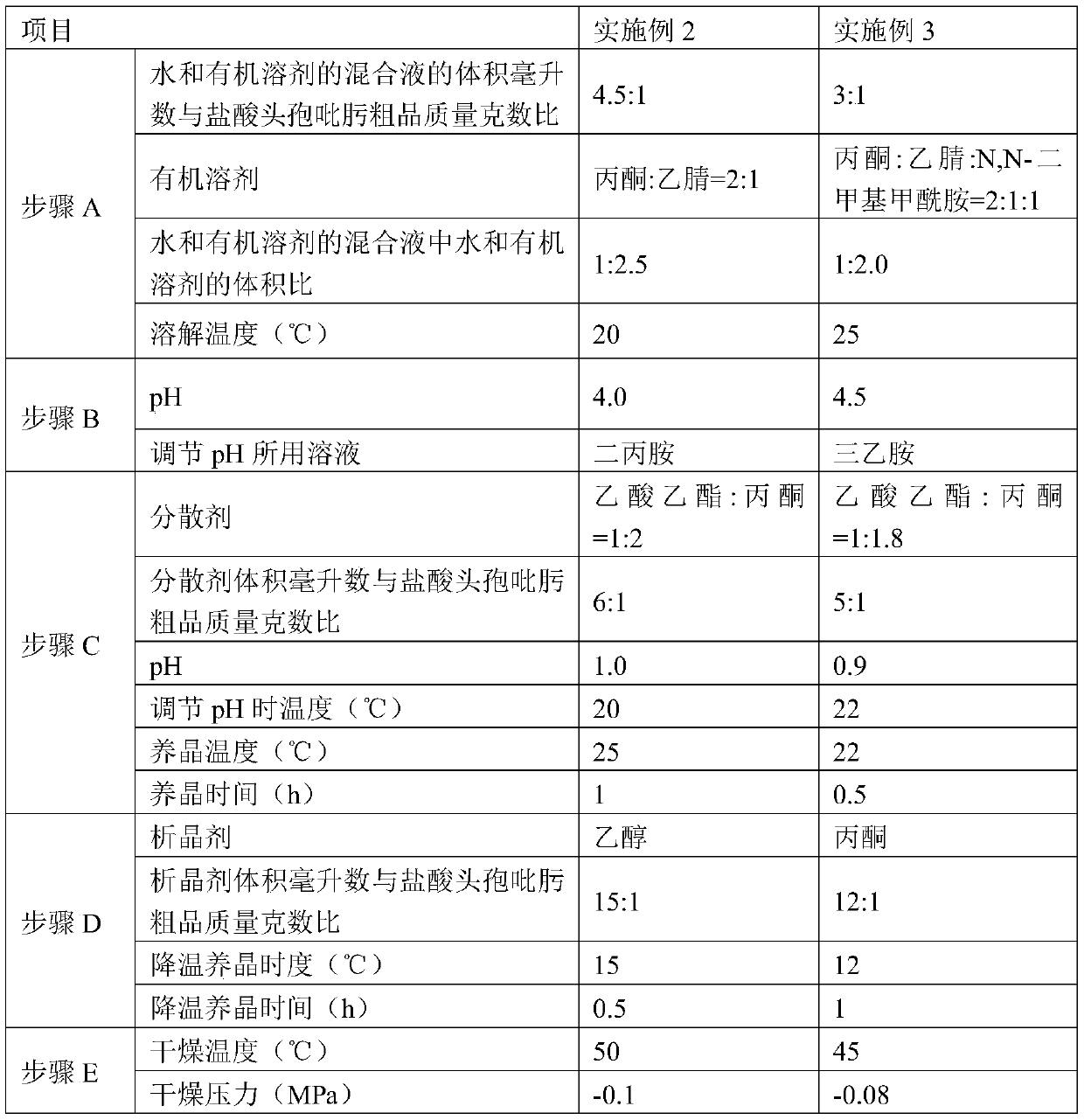
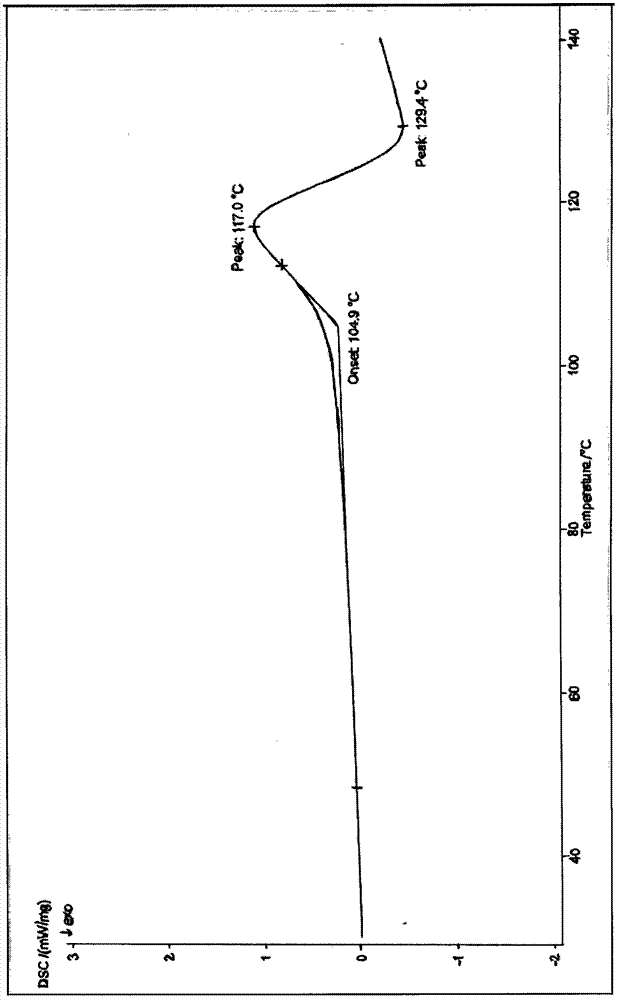
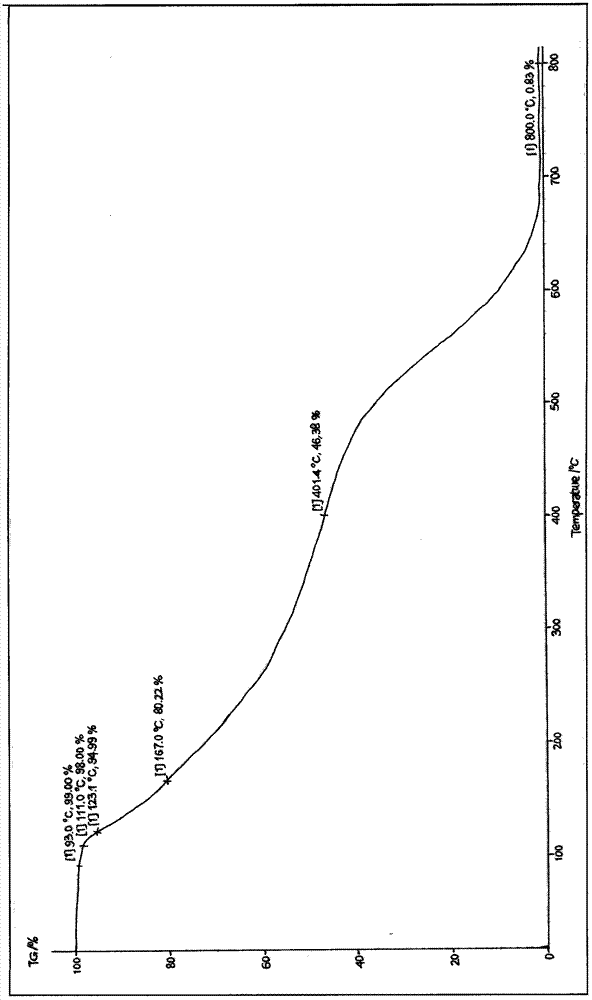
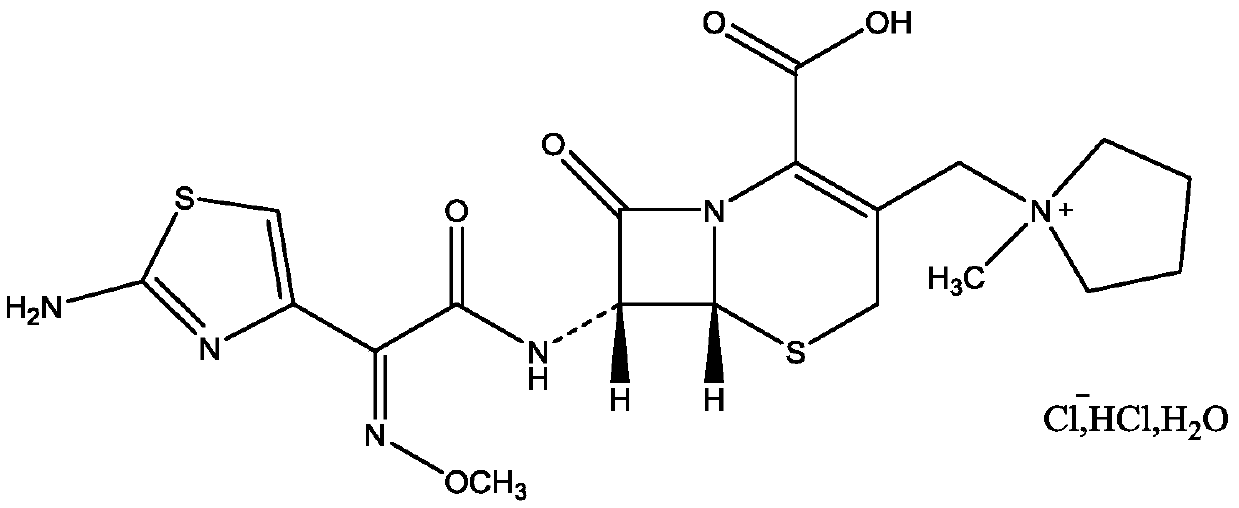
Patents
Literature
35results about How to "Low color level" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
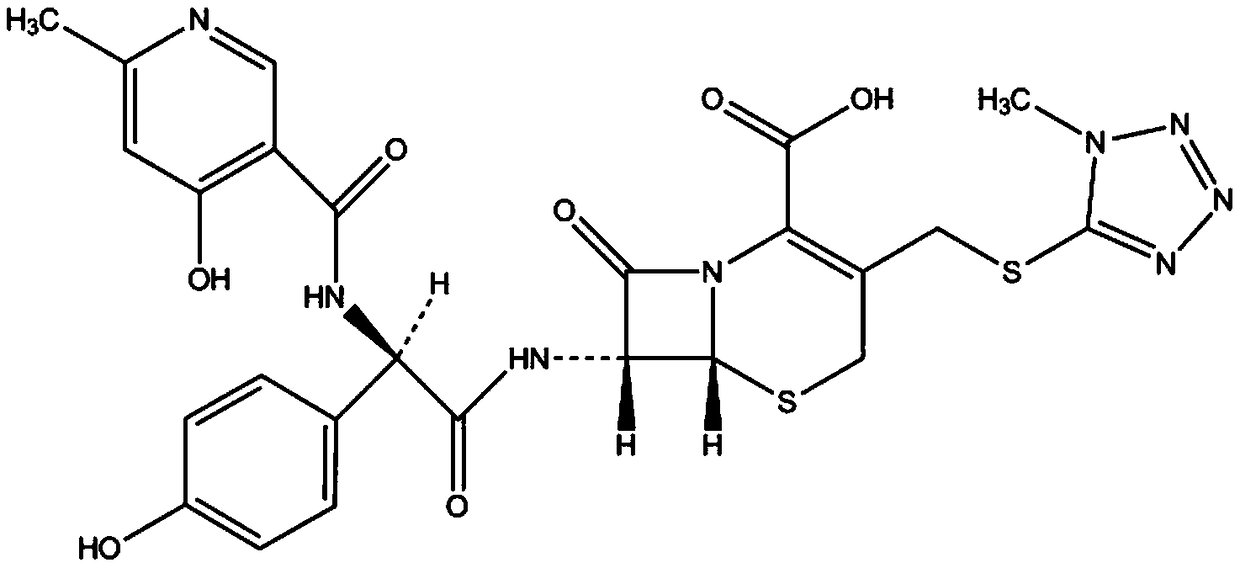
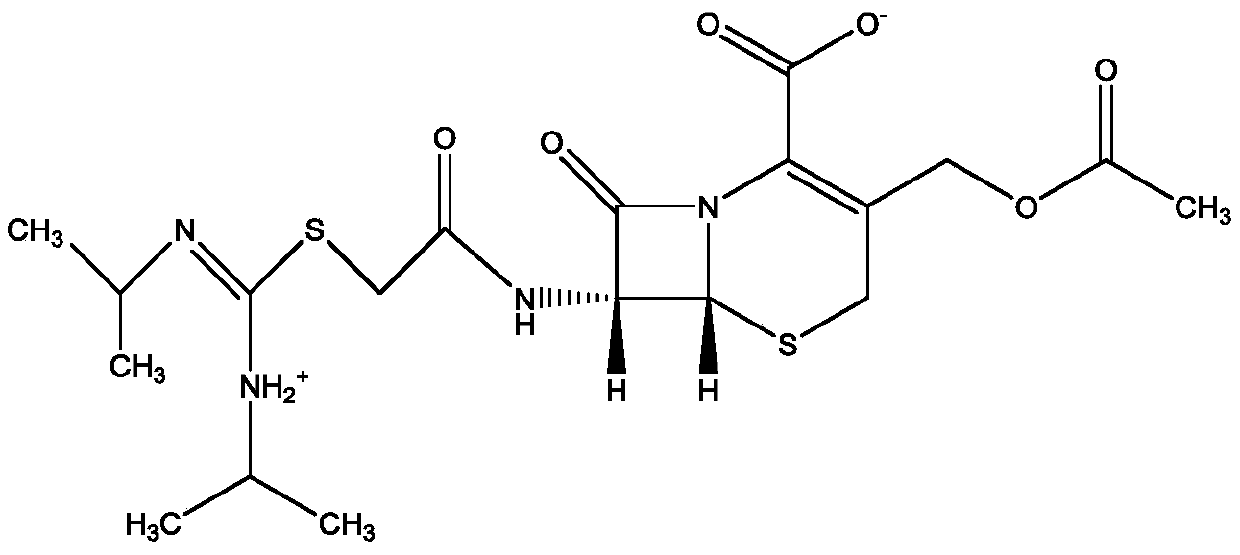
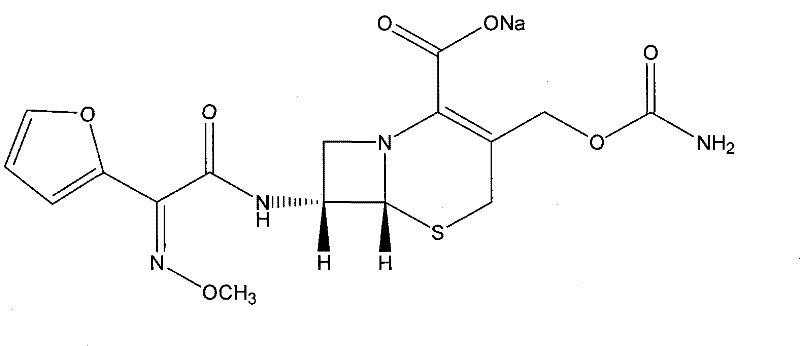
Method for preparing ceftezole sodium compound
ActiveCN102617606AReduce pollutionHigh yieldAntibacterial agentsOrganic chemistryCeftezole SodiumMethyl carbonate
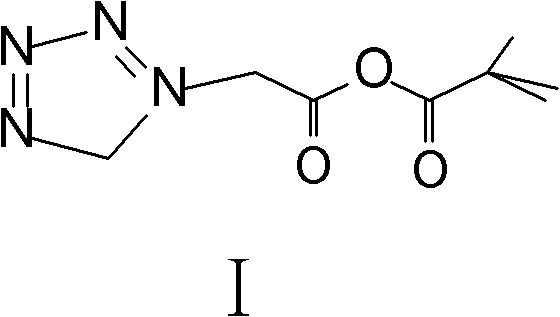
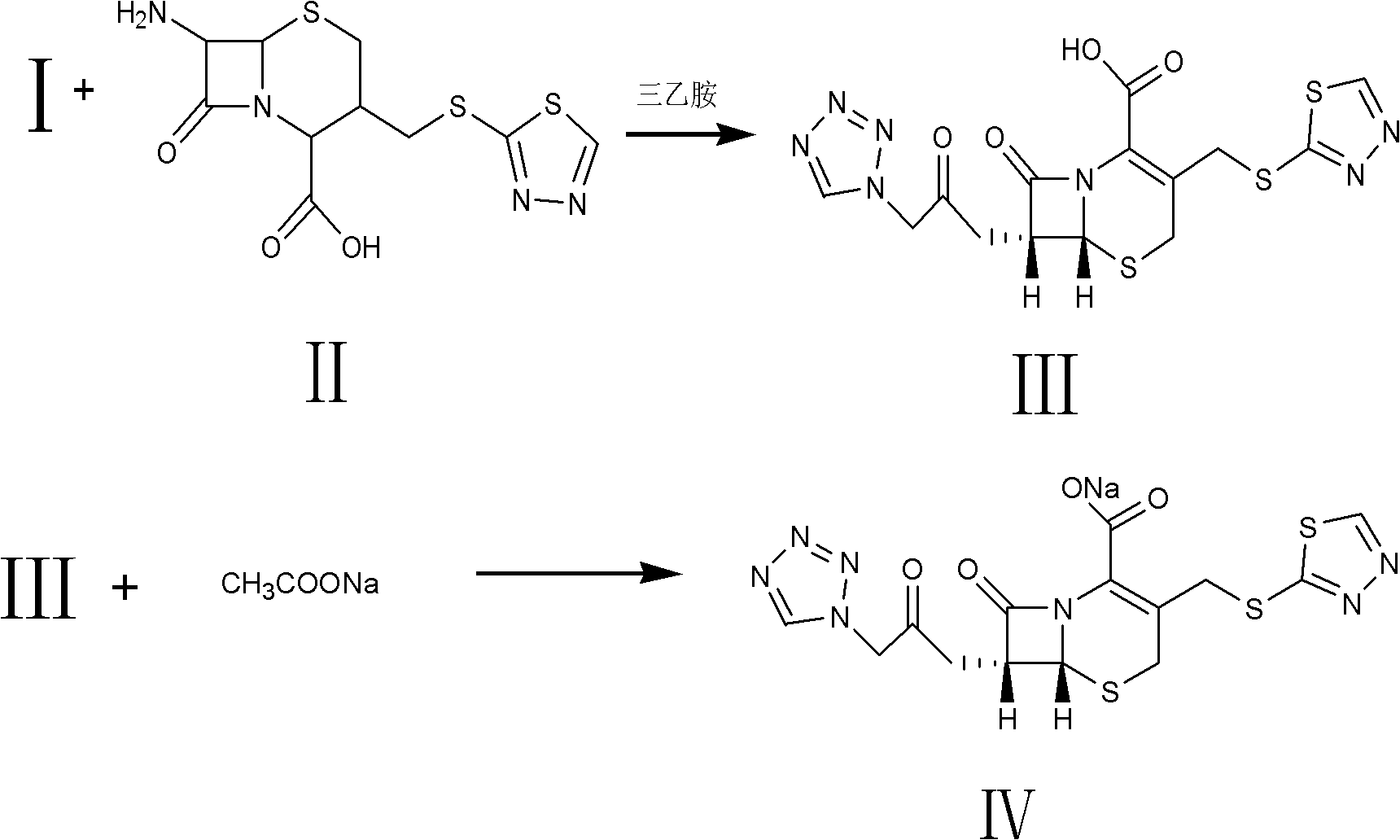
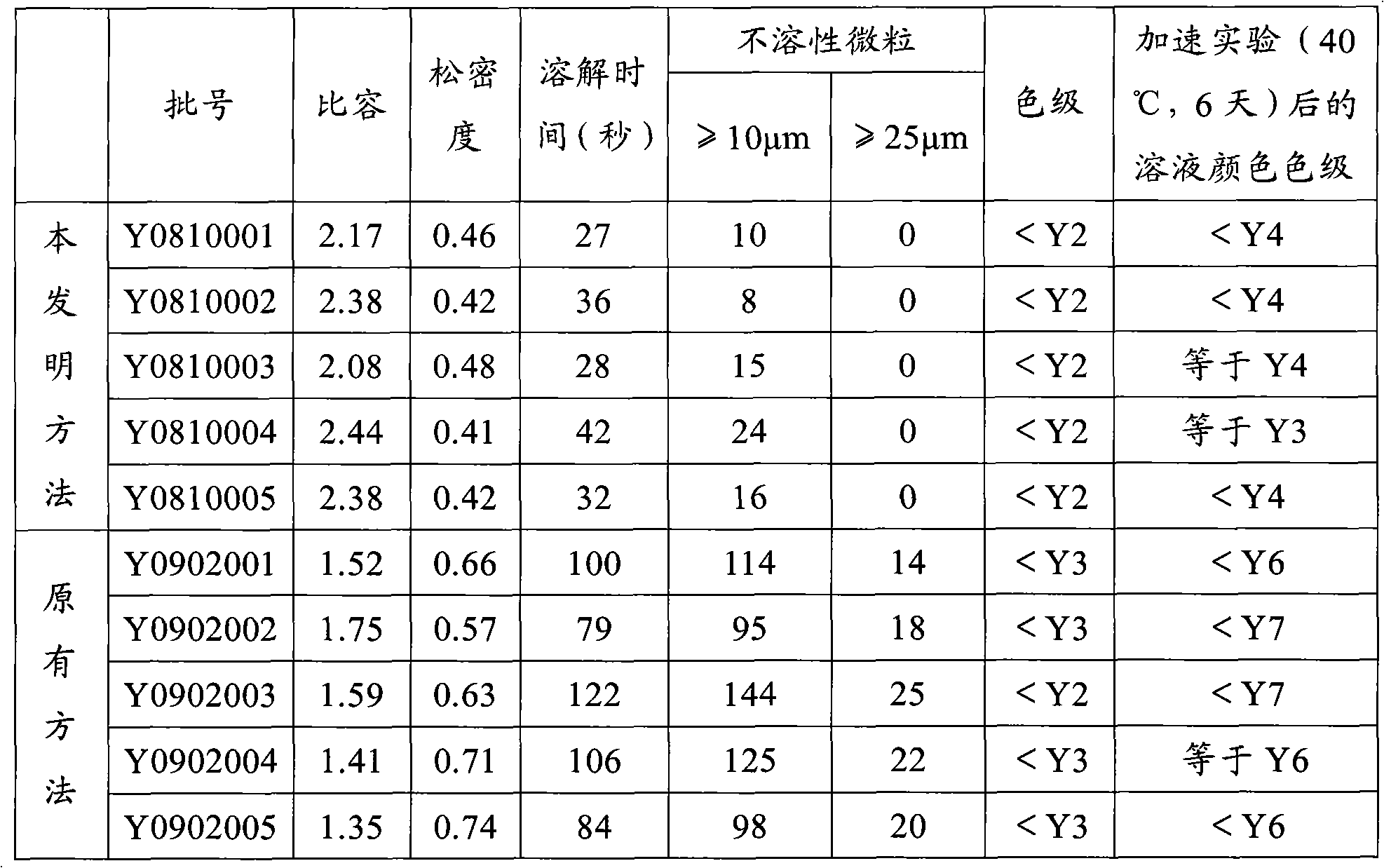
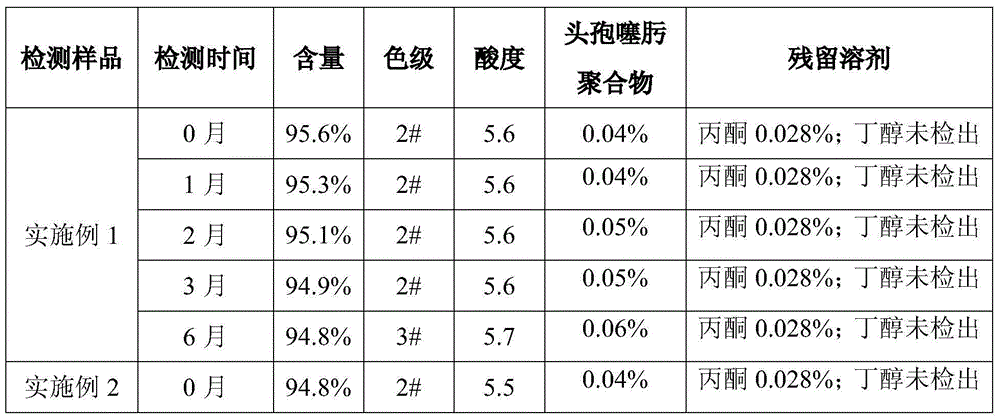
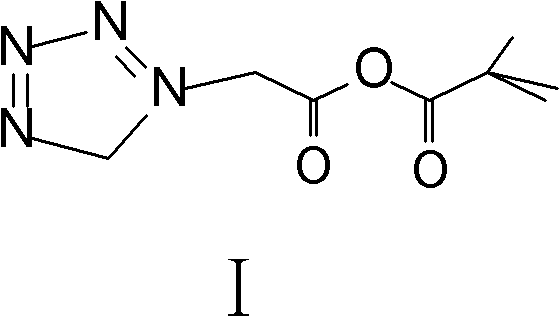
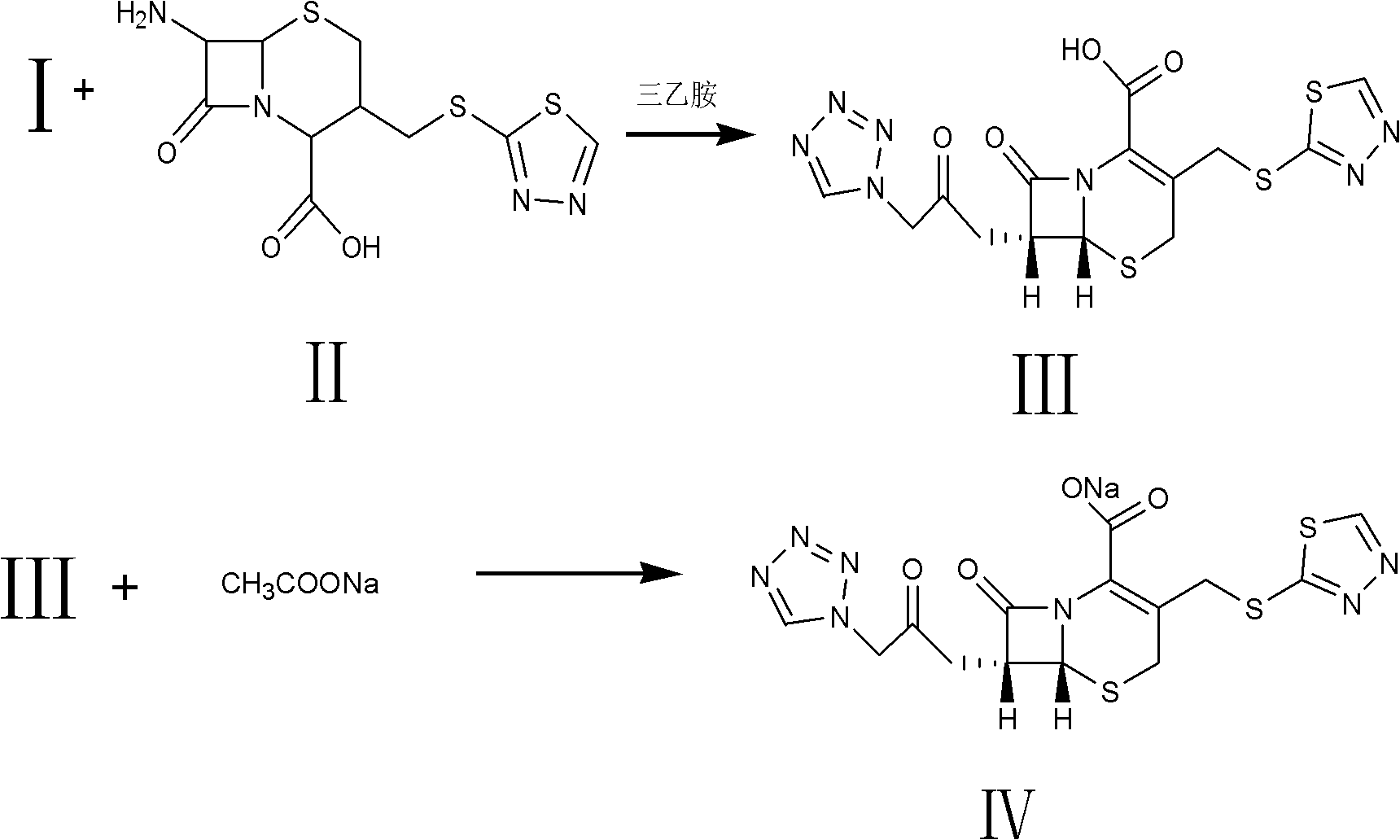
The invention relates to a method for preparing an antibacterial compound, in particular to a method for preparing a ceftezole sodium compound. The method comprises the following steps of: 1, synthesizing TZT II, and reacting 2-mercapto-1,3,4 thiadiazole and 7-aminocephalosporanic acid (ACA) to obtain TZT, wherein dimethyl carbonate is used as a reaction solvent; a boron trifluoride-dimethyl carbonate complex is used as a catalyst; after reaction, the agent used by adjusting pH of reaction liquid is sodium carbonate; the weight ratio of boron trifluoride to 7-ACA is 0.7 to 1.3; and 2, synthesizing anhydride I, namely reacting 1H-tetrazole-1-acetic acid and pivaloyl chloride to obtain anhydride; 3, synthesizing ceftezole III, namely reacting TZT II and anhydride I to obtain ceftezole; and 4, synthesizing ceftezole sodium IV, namely reacting ceftezole and sodium salt to obtain ceftezole sodium IV, wherein salt is sodium hydroxide.
Owner:哈药集团股份有限公司 +1
Method for preparing cefuroxime sodium
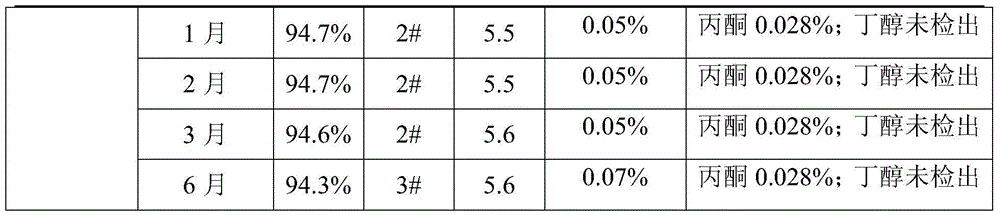
The invention provides a method for preparing cefuroxime sodium. The method comprises a step of reacting cefuroxime acid with mixed sodium salt to produce the cefuroxime sodium, wherein the mixed sodium salt comprises two or three of sodium acetate, sodium lactate and sodium iso-octoate. The product prepared by the method has the advantages of uniform crystal dispersion, greatly improved fluidity and easy packaging; the solubility of the product is greatly improved, and compared with similar products prepared by the conventional methods, the dissolution time is the shortest; the method greatly shortens the time for washing, filtering and drying the product, reduces the opportunities of powder exposure and human contact, more easily controls visible foreign matters in the product and effectively reduces the number of insoluble particles in the product; because the method improves the crystal form of the product, the crystal is easy to wash and dry, the time of the product at a high temperature is shortened and the stability of the product is improved effectively. The color grade of the product is further reduced, and the product is more stable and uniform and has better performance on indexes such as the color grade, content, impurities and the like.
Owner:LIVZON PHARM GRP INC +1
Preparation method of clavulanic acid amine salt
ActiveCN105384758AReduce solubilityHigh extraction rateOrganic compound preparationOrganic chemistry methodsOrganic solventSalting out
The invention belongs to the technical field of pharmacy, and relates to a preparation method of clavulanic acid amine salt. The method comprises the following steps: (1) extraction of a clavulanic acid aqueous solution and concentration of extract liquor: acidizing the clavulanic acid aqueous solution, then adding a salting-out agent, and extracting by an organic solvent, thus obtaining the extract liquor containing clavulanic acid; nano-filtering and concentrating by using an organic solvent-resistant film, thus obtaining a clavulanic acid extraction concentrated solution; (2) preparation of the clavulanic acid amine salt: mixing the clavulanic acid extraction concentrated solution with an organic amine donor and a cosolvent, thus obtaining clavulanic acid amine salt crystals. The salting-out agent is introduced, and the extraction rate of the clavulanic acid is increased; the organic solvent-resistant roll film is innovatively adopted for nano-filtering and concentrating, so the energy consumption is lowered; the addition of the cosolvent can effectively reduce the content of various impurities in a final clavulanic acid amine salt product. Therefore, in the quality parameter aspects of the content, the impurities, light transmittance and the like, the clavulanic acid amine salt prepared by the preparation method is remarkably superior to clavulanic acid amine salt prepared by a conventional process.
Owner:SHANXI WEIQIDA PHARMA IND
Preparation method of cefotaxime sodium
ActiveCN104086569ALow color levelImprove liquidityOrganic chemistryCeftizoximeCrystallization temperature
The invention discloses a preparation method of cefotaxime sodium. Ceftizoxime acid used as the initial raw material reacts with anhydrous sodium acetate to generate the cefotaxime sodium. In such process, purified water, butanol and acetone are selected as crystallizing solvents to control the mixing speed and crystallization temperature in the crystallization process, so that the finally obtained cefotaxime sodium has favorable flowability and can satisfy the subpackaging requirements in production. Various quality indexes of the obtained cefotaxime sodium conform to the requirements for medicinal standard, and thus, the cefotaxime sodium can satisfy the demands for clinical application.
Owner:石药集团中诺药业(石家庄)有限公司
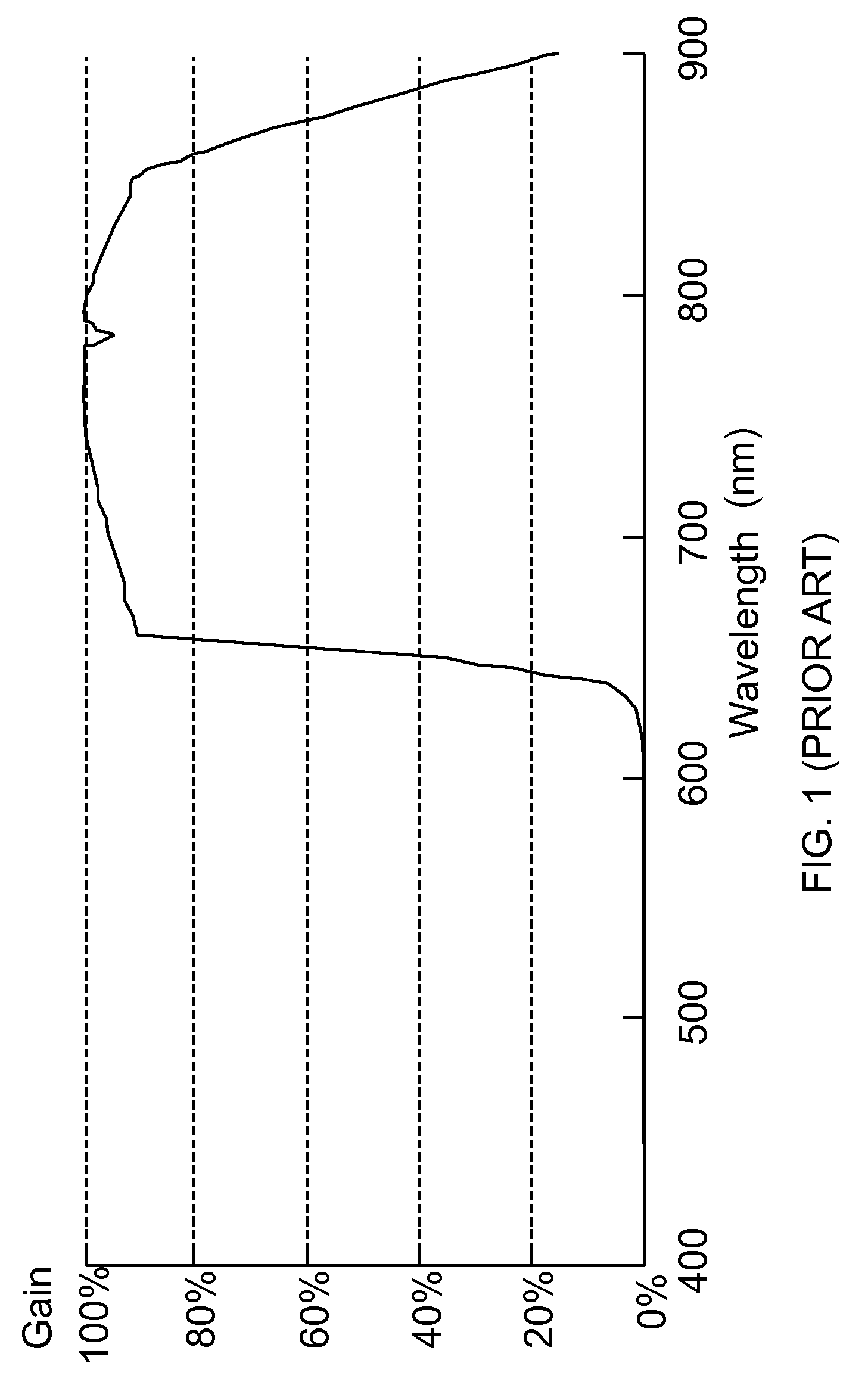
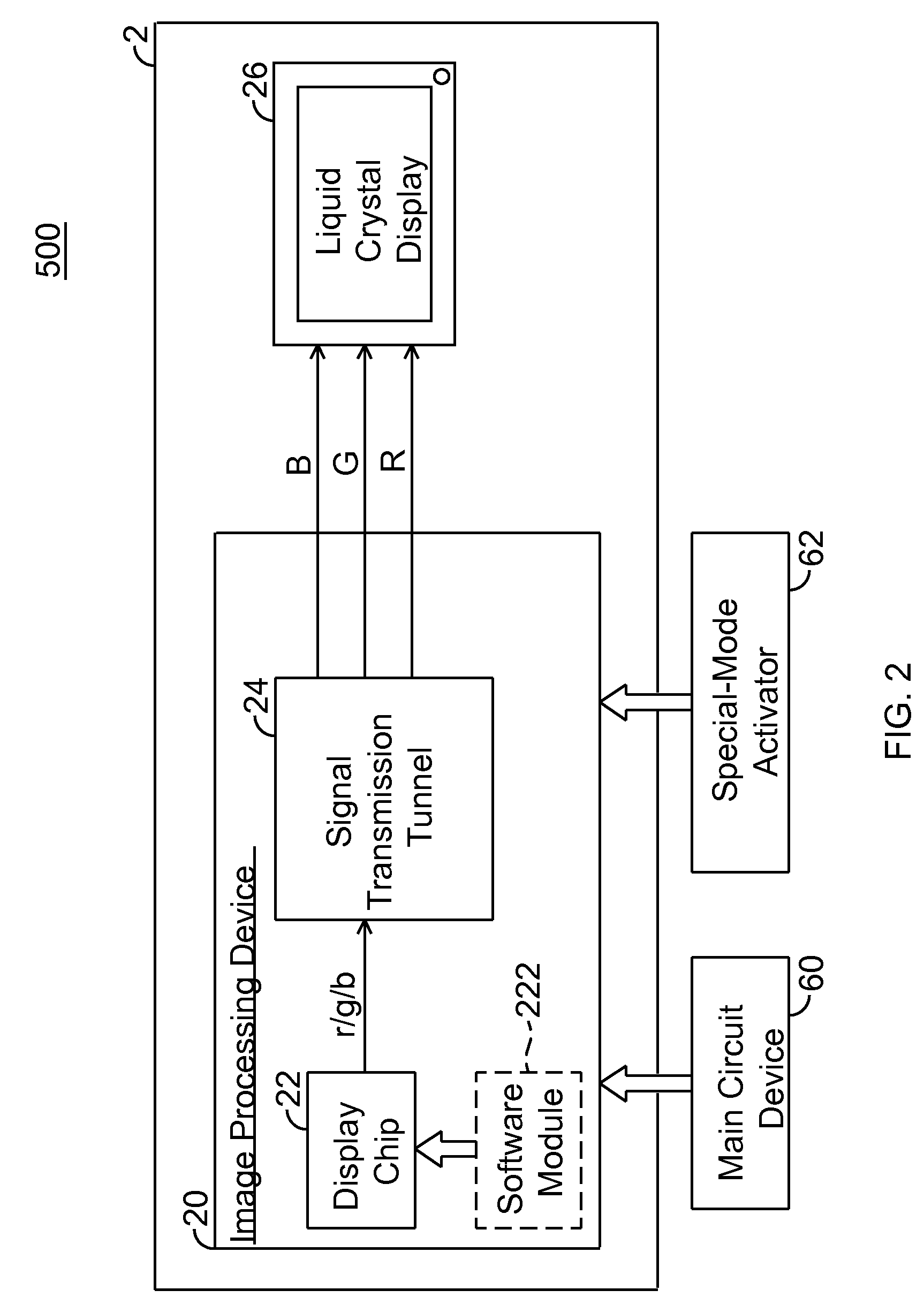
Display system and driving method thereof
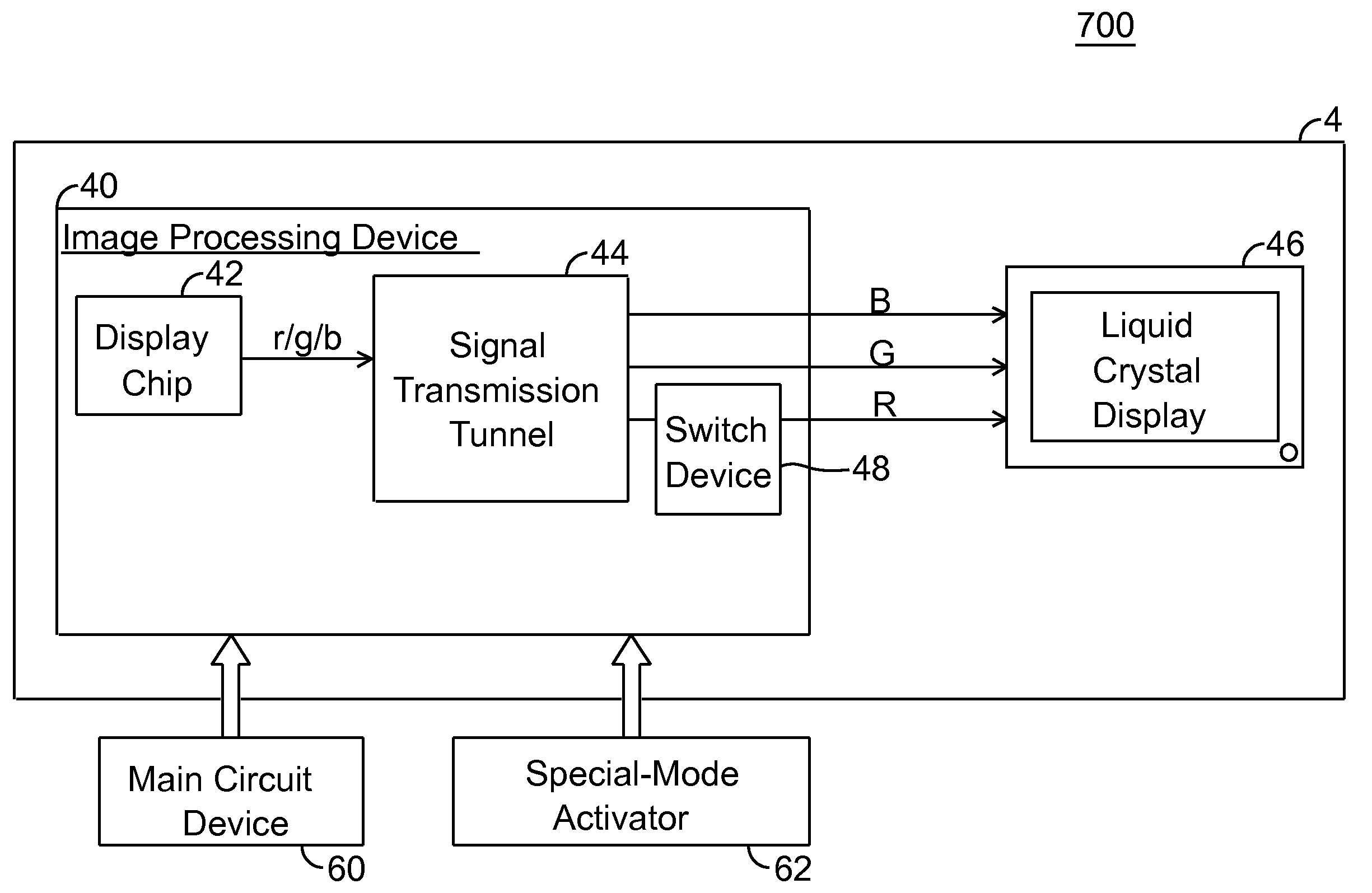
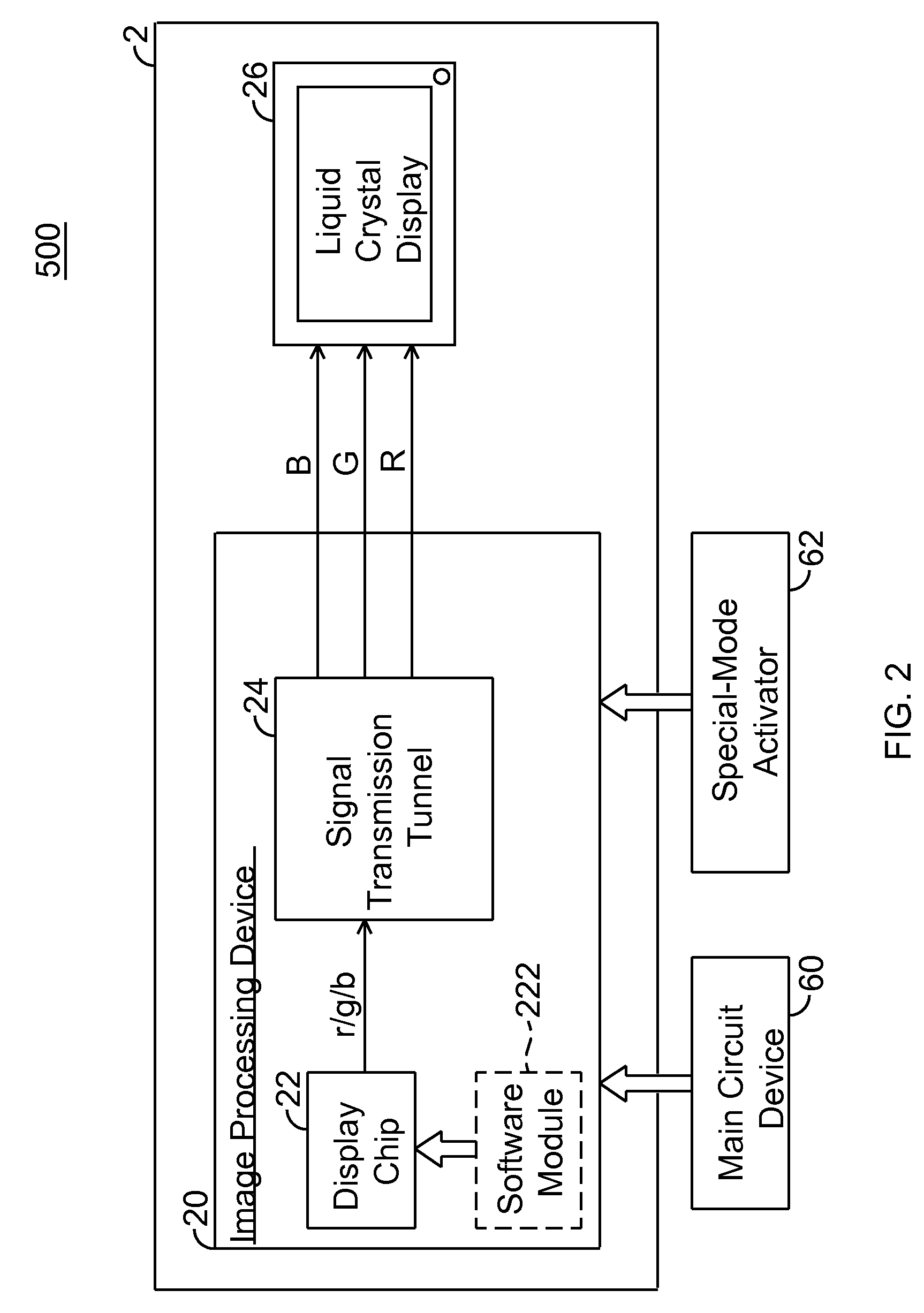
ActiveUS20100149081A1Reduce brightnessLow color levelCathode-ray tube indicatorsImaging processingDisplay device
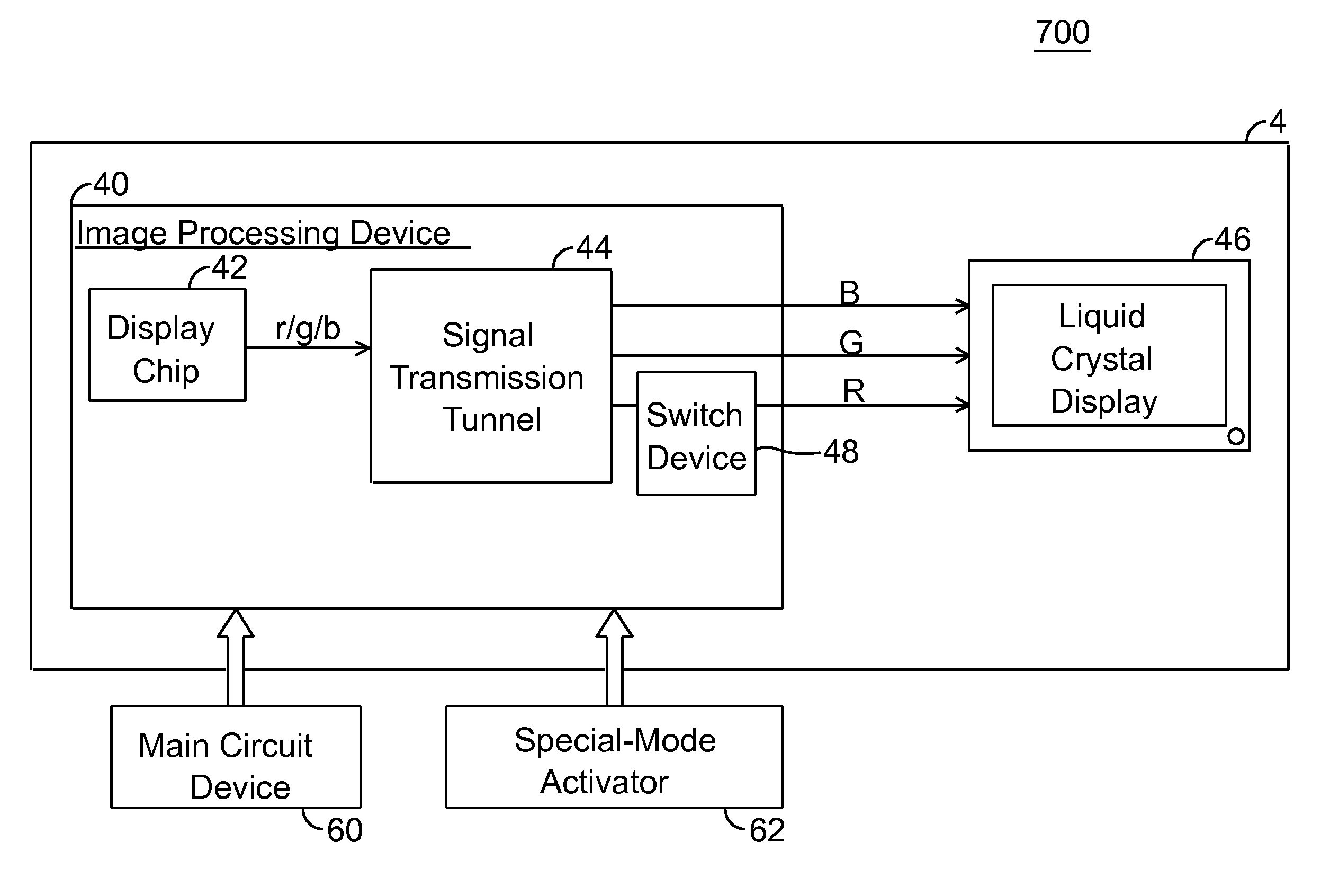
A display system and driving method thereof are capable of outputting a low luminance of red light, especially through descending a color level of red signals when displayed. The display system includes a display device and an image processing device. The image processing device outputs the red signals to the display device for displaying thereon. The color level of red signals is descended by a display chip or a switch device to allow the display device to display images with low luminance of red light, so that the display device is viewable through a night-vision device.
Owner:GETAC TECH CORP
Method utilizing coupling reaction crystallization to prepare cefuroxime sodium
InactiveCN102617604AReduce adsorption filtration processReduce degradationOrganic chemistryActivated carbonFiltration
The invention relates to a method utilizing coupling reaction crystallization to prepare cefuroxime sodium, which comprises dissolving cefuroxime acid in a mixed solvent at 20-30 DEG C to prepare a solution with the concentration to be 0.025g / ML-0.1g / Ml; adding an alkaline sodium salt water solution into the solution; mixing for 10-20min at constant temperature to enable a reaction to be complete, and adding cefuroxime sodium seed crystal; adding an elution agent after 10-20min; cooling the temperature of the solution to 0-5 DEG C, and keeping at the constant temperature for 0.5-2h; filtering, washing and drying the obtained suspension, and obtaining a cefuroxime sodium product. The method reduces the adsorption filtration process of activated carbon and avoids loss of yield. The method achieves coupling of reactions and crystallization, and the methods of elution crystallization and cooling crystallization are combined with each other in the crystallization process, the crystallization process is controlled easily, the particle size of the product is uniform, the liquidity is greatly improved, the purity is higher than 99.5%, and the yield is over 92%.
Owner:TIANJIN UNIV +1
Method for preparing cefmenoxime hydrochloride
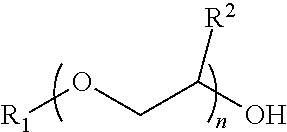
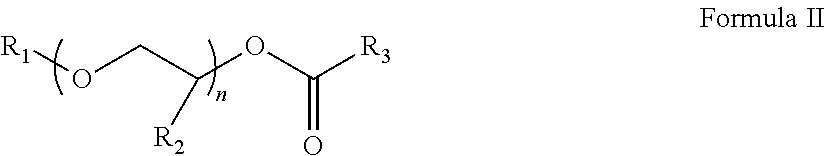
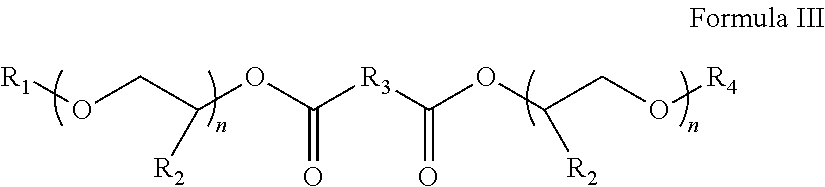
InactiveCN102675344AHigh yieldHigh purityOrganic chemistryMethylene DichlorideCefmenoxime Hydrochloride
The invention discloses a method for preparing cefmenoxime hydrochloride, which comprises the following steps: uniformly mixing 7-ACT hydrochloride shown in a formula II and AE active ester shown in a formula III under the condition that methylene dichloride and a solvent exist for condensation reaction and obtaining the cefmenoxime hydrochloride shown in a formula I after reaction. The method has the benefits that the cefmenoxime hydrochloride can be synthesized in one step, the cefmenoxime hydrochloride is not required to be separated, a reversely-dropping crystallization method is adopted, the synthetic line is short, the cost is low, the reaction conditions are wild, and the production is convenient; and the cefmenoxime hydrochloride product is high in yield and purity and low in color grade and has an important application value.
Owner:SHANDONG LUKANG PHARMA
Refining method of cefathiamidine
The invention discloses a refining method of cefathiamidine, belonging to the chemical pharmacy field. The refining method comprises the steps of dissolving a cefathiamidine crude product by virtue ofa dissolving agent, adding a stable adjusting agent, and carrying out secondary decolorization by virtue of an aluminum oxide adsorption column. According to the refining method, the color grade of cefathiamidine can be effectively decreased, obtained cefathiamidine crystal particles are uniform, high in purity and good in stability, the product yield is high, and the preparation method is easy,convenient and controllable and suitable for large-scale industrial production.
Owner:NORTH CHINA PHARMA HEBEI HUAMIN PHARMA
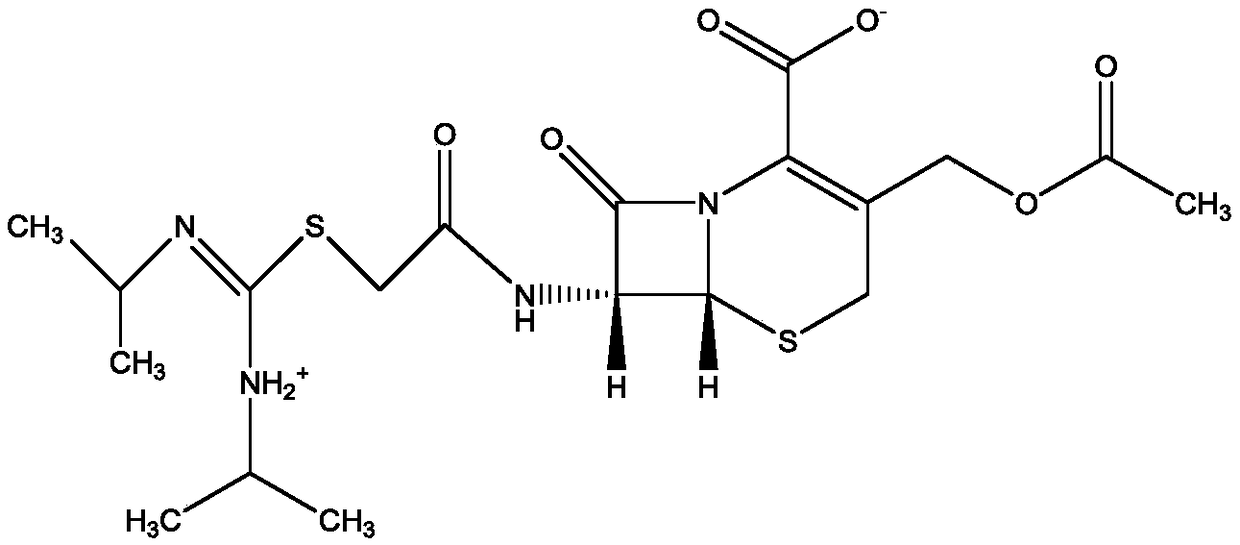
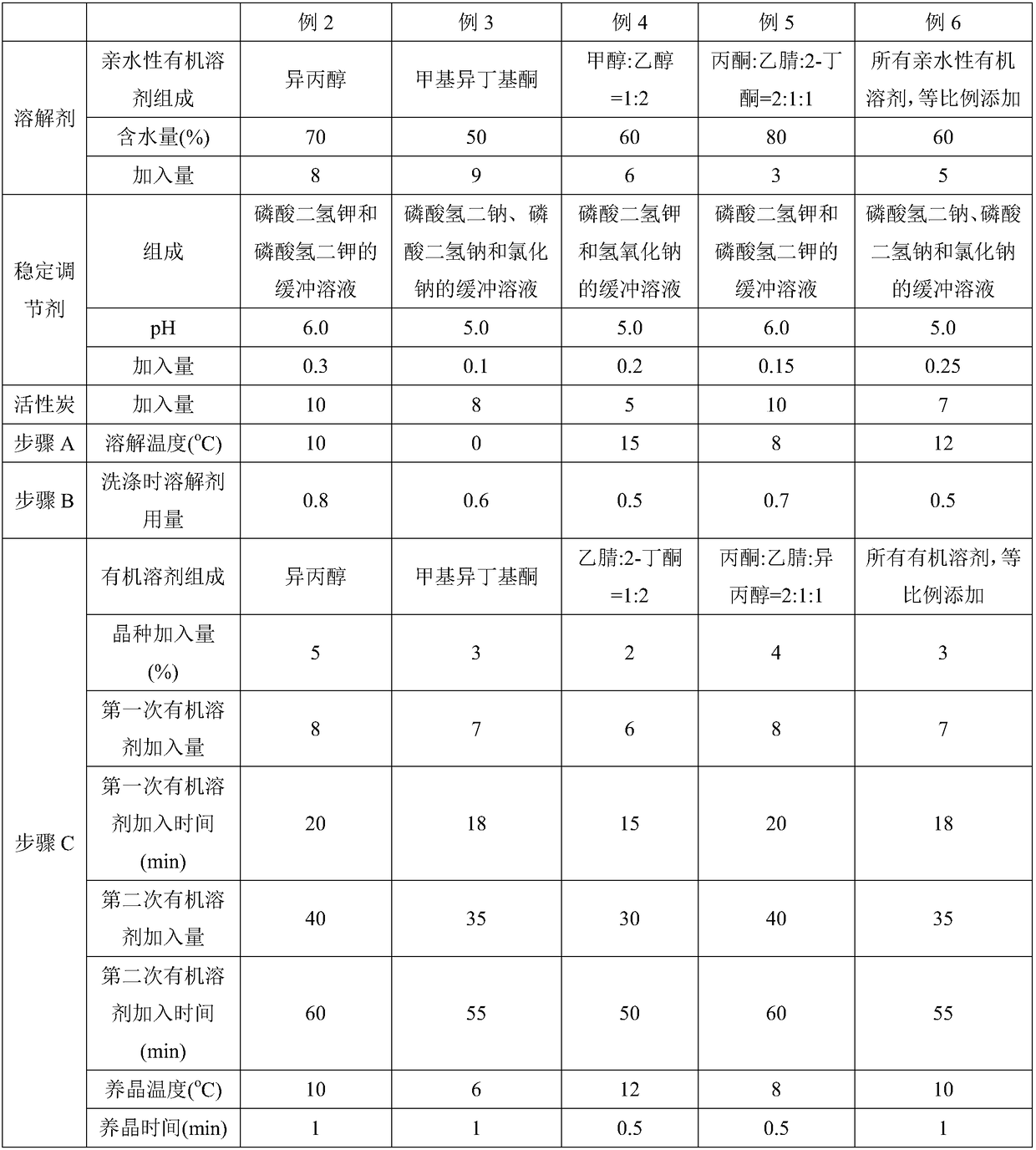
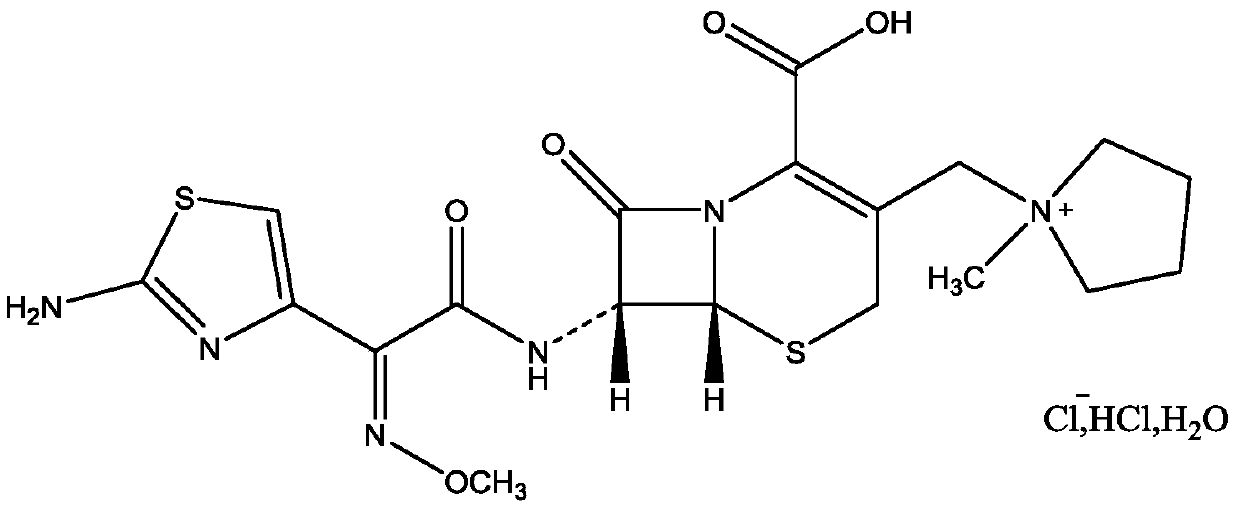
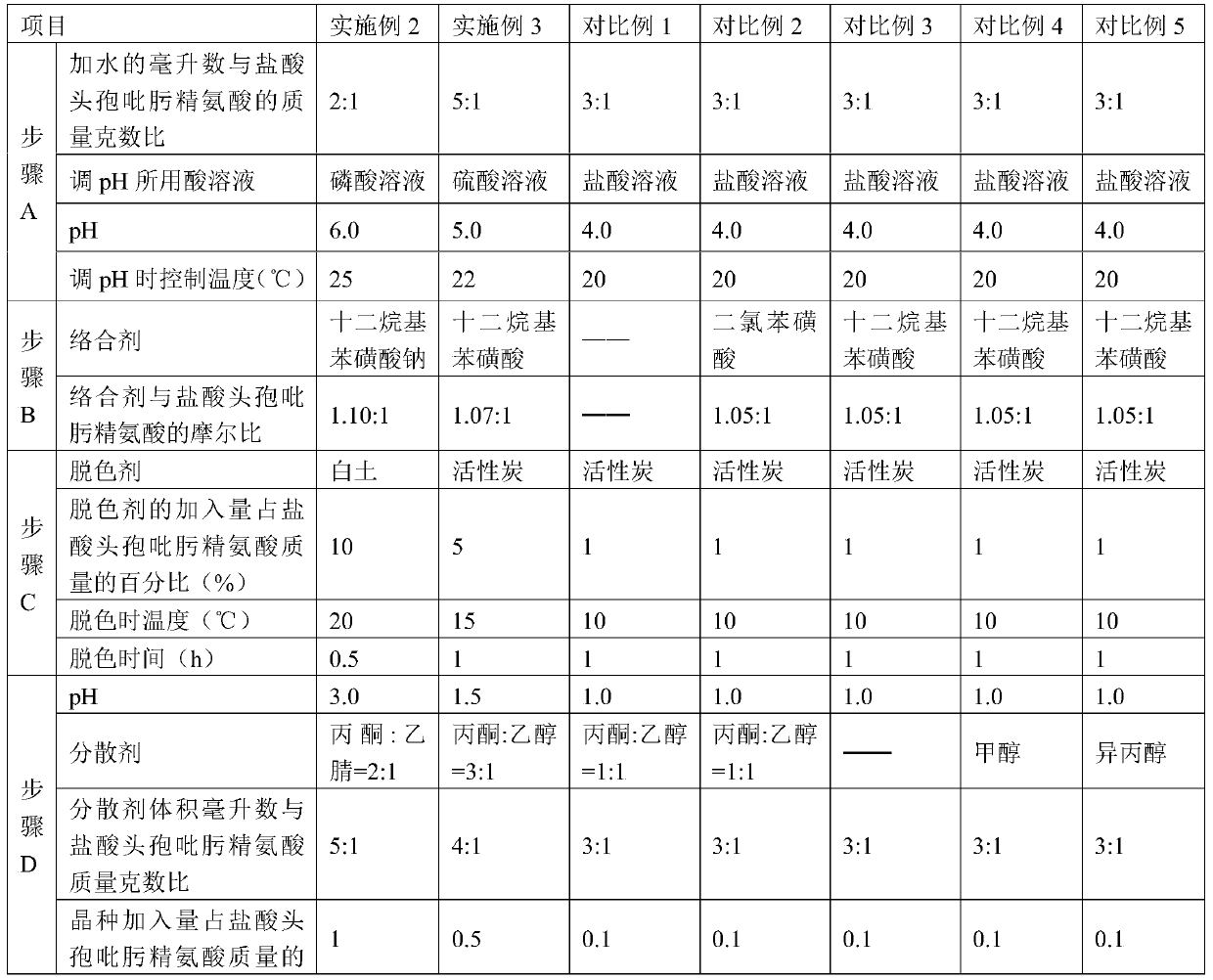
Purification method of cefepime dihydrochloride
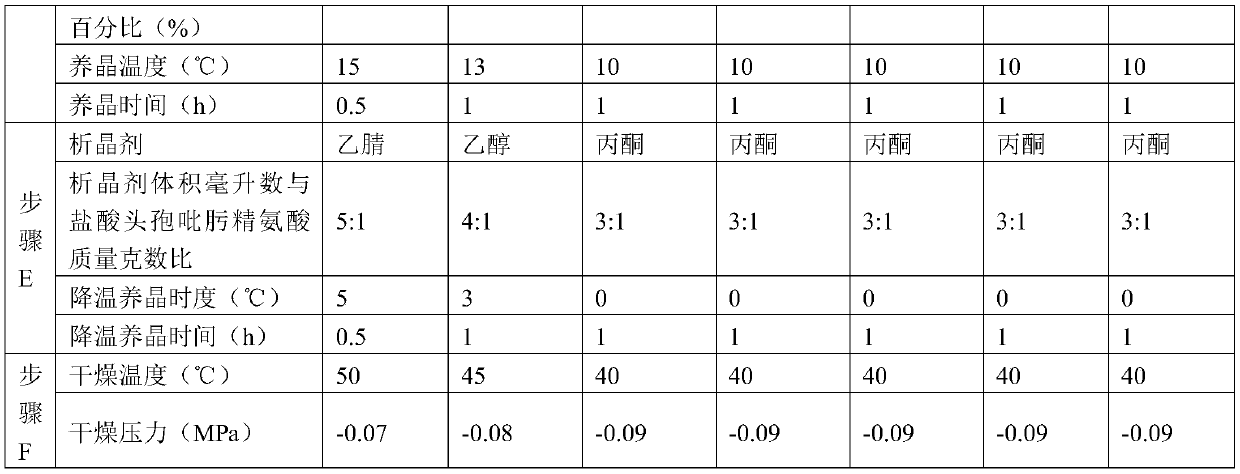
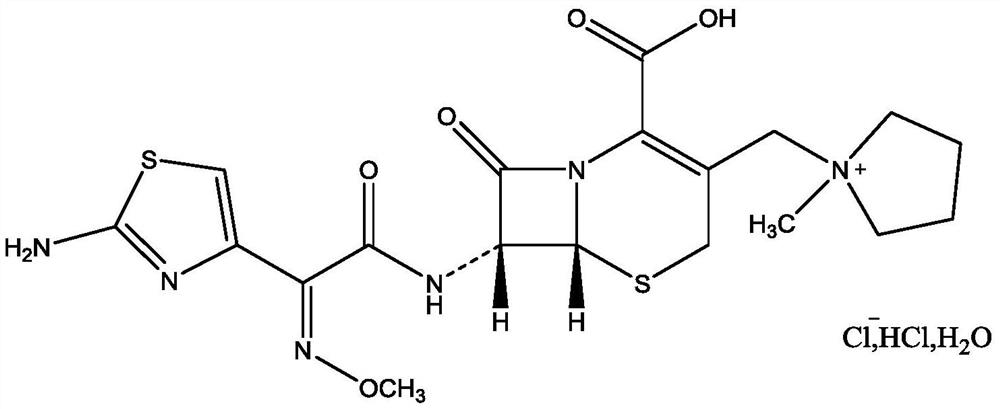
ActiveCN109776572AIncrease contentLess impuritiesOrganic chemistryCefepime hydrochloridePurification methods
The invention discloses a purification method of cefepime dihydrochloride, and belongs to the field of chemical pharmacy. The method comprises the following steps: cefepime dihydrochloride arginine istaken as a raw material, a complexing agent is added in the dissolving solution of the cefepime dihydrochloride arginine for decoloration; and a dispersant and a crystallization agent are added intothe decolorated solution for crystallization, so as to finally obtain the cefepime dihydrochloride. The purification method can enable the cefepime dihydrochloride arginine which does not meet the quality standard to be fully recycled, therefore the prepared cefepime dihydrochloride has the advantages of high content, less impurities and high stability; and the preparation method is simple, energy-saving and environmental-friendly, thereby being suitable for large-scale industrial production.
Owner:NORTH CHINA PHARMA HEBEI HUAMIN PHARMA
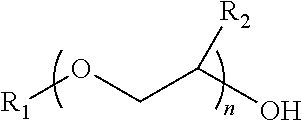
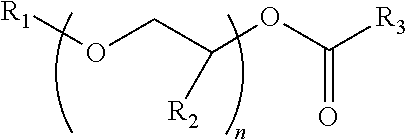
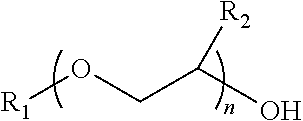
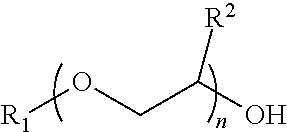
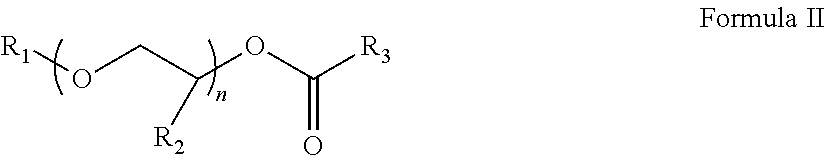
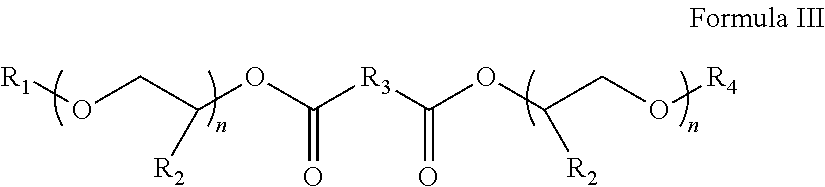
Process for producing low VOC coalescing aids
ActiveUS9908839B2Low color levelOrganic compound preparationCarboxylic acid esters preparationGlycol ethersPhosphoric acid
A process comprising reacting a mono- or di-carboxylic acid and / or acid anhydride with a glycol ether in the presence of phosphoric acid to produce a glycol ether ester product having low color and low VOC content.
Owner:DOW GLOBAL TECH LLC
Process for producing low VOC coalescing aids
ActiveUS9809529B2Low color levelLow levelOrganic compound preparationCarboxylic acid esters preparationBenzoic acidGlycol ethers
A process comprising reacting a benzoic acid with a glycol ether in the presence of phosphoric acid to produce a glycol ether ester product having low color, low odor, and low VOC content.
Owner:DOW GLOBAL TECH LLC
Method for refining cefpiramide acid
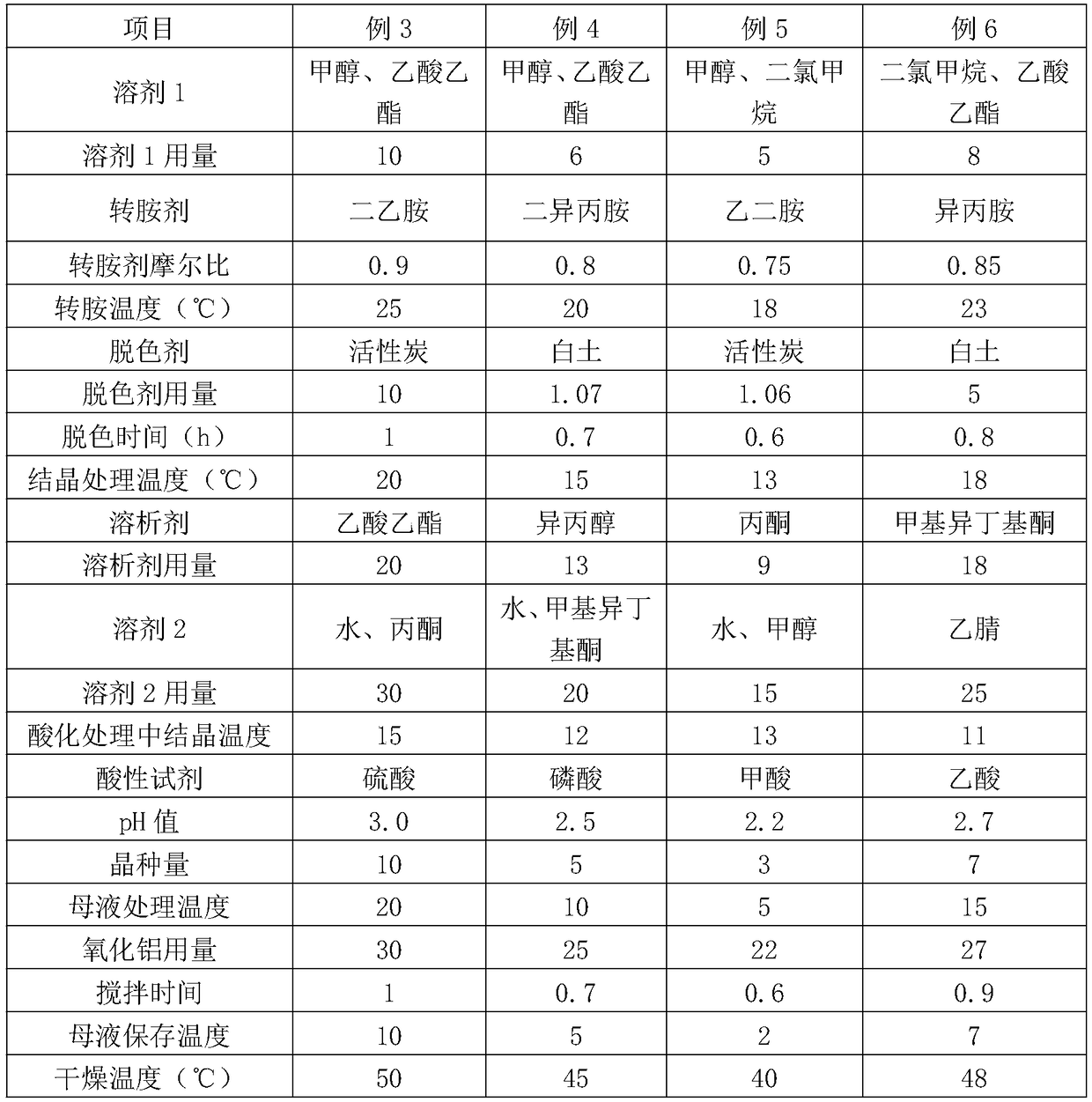
The invention discloses a method for refining cefpiramide acid, and belongs to the technical field of medicines. The method comprises the following steps: A, putting crude cefpiramide acid into a solvent 1, adding a transaminating agent, stirring and dissolving the mixture to clarification, so as to obtain a cefpiramide salt solution; B, adding a decolorizing agent into the cefpiramide salt solution, filtering and collecting filtrate; C, controlling the filtrate temperature to be 10-20 DEG C, adding a dissolution agent, filtering and collecting a solid crystal; D, putting the solid crystal into a solvent 2, controlling the temperature to be 10-15 DEG C, adding an acidic reagent to adjust the pH value to 2.0-3.0, adding a seed crystal, crystallizing, filtering and collecting the solid crystal of cefpiramide acid and mother liquor; and E, washing the solid crystal of cefpiramide acid and drying to obtain refined cefpiramide acid. The cefpiramide acid prepared with the method is high in purity, few in impurities and low in color grade; and the refining method provided by the invention has the advantages of being simple in process, environmentally friendly, and suitable for large-scaleindustrial production, saving energy, and the like.
Owner:NORTH CHINA PHARMA HEBEI HUAMIN PHARMA
Method for preparing cefuroxime sodium
The invention provides a method for preparing cefuroxime sodium. The method comprises a step of reacting cefuroxime acid with mixed sodium salt to produce the cefuroxime sodium, wherein the mixed sodium salt comprises two or three of sodium acetate, sodium lactate and sodium iso-octoate. The product prepared by the method has the advantages of uniform crystal dispersion, greatly improved fluidityand easy packaging; the solubility of the product is greatly improved, and compared with similar products prepared by the conventional methods, the dissolution time is the shortest; the method greatly shortens the time for washing, filtering and drying the product, reduces the opportunities of powder exposure and human contact, more easily controls visible foreign matters in the product and effectively reduces the number of insoluble particles in the product; because the method improves the crystal form of the product, the crystal is easy to wash and dry, the time of the product at a high temperature is shortened and the stability of the product is improved effectively. The color grade of the product is further reduced, and the product is more stable and uniform and has better performanceon indexes such as the color grade, content, impurities and the like.
Owner:LIVZON PHARM GRP INC +1
Display system and driving method thereof
ActiveUS8102341B2Reduce brightnessLow color levelCathode-ray tube indicatorsDisplay deviceComputer science
A display system and driving method thereof are capable of outputting a low luminance of red light, especially through descending a color level of red signals when displayed. The display system includes a display device and an image processing device. The image processing device outputs the red signals to the display device for displaying thereon. The color level of red signals is descended by a display chip or a switch device to allow the display device to display images with low luminance of red light, so that the display device is viewable through a night-vision device.
Owner:GETAC TECH CORP
A kind of refining method of cefathiamidine
ActiveCN108948048BLow color levelUniform particlesOrganic chemistryPhysical chemistryChemical engineering
The invention discloses a method for refining cefathiamidine, belonging to the field of chemical pharmacy, comprising dissolving crude cefathiamidine with a dissolving agent, adding a stabilizing regulator, and using an alumina adsorption column for secondary decolorization; the invention can effectively reduce the The color grade of cefathiamidine, the obtained cefathiamidine crystal particles are uniform, the purity is high, the stability is good, and the product yield is high, the preparation method is simple and easy to control, and it is suitable for large-scale industrial production.
Owner:NORTH CHINA PHARMA HEBEI HUAMIN PHARMA
A kind of purification method of cefepime hydrochloride
Owner:NORTH CHINA PHARMA HEBEI HUAMIN PHARMA
Method for preparing ceftezole sodium compound
ActiveCN102617606BReduce pollutionHigh yieldAntibacterial agentsOrganic chemistryCeftezole SodiumSolvent
The invention relates to a method for preparing an antibacterial compound, in particular to a method for preparing a ceftezole sodium compound. The method comprises the following steps of: 1, synthesizing TZT II, and reacting 2-mercapto-1,3,4 thiadiazole and 7-aminocephalosporanic acid (ACA) to obtain TZT, wherein dimethyl carbonate is used as a reaction solvent; a boron trifluoride-dimethyl carbonate complex is used as a catalyst; after reaction, the agent used by adjusting pH of reaction liquid is sodium carbonate; the weight ratio of boron trifluoride to 7-ACA is 0.7 to 1.3; and 2, synthesizing anhydride I, namely reacting 1H-tetrazole-1-acetic acid and pivaloyl chloride to obtain anhydride; 3, synthesizing ceftezole III, namely reacting TZT II and anhydride I to obtain ceftezole; and 4, synthesizing ceftezole sodium IV, namely reacting ceftezole and sodium salt to obtain ceftezole sodium IV, wherein salt is sodium hydroxide.
Owner:哈药集团股份有限公司 +1
Preparation method of sulbactam acid
The invention discloses a preparation method of sulbactam acid, and belongs to the field of preparation of pharmaceutical intermediates. The preparation method comprises the steps of: adopting sodiumsulbacetanate as a raw material, adding a dissolution-assistant agent to a concentrated liquid, introducing carbon dioxide at the same time, and performing crystallization so as to obtain sulbatam. Through the method, sodium sulbacetanate which does not meet the quality standard can be recycled and utilized fully, and the prepared sulbactam acid has the advantages of high content, less impurities,low color grade, good fluidity and good stability; and the preparation method is simple, energy-saving and environmentally friendly, and is suitable for large-scale industrial production.
Owner:NORTH CHINA PHARMA HEBEI HUAMIN PHARMA
The preparation method of clavulanic acid amine salt
ActiveCN105384758BReduce solubilityHigh extraction rateOrganic compound preparationOrganic chemistry methodsOrganosolvAqueous solution
The invention belongs to the technical field of pharmacy, and relates to a preparation method of clavulanic acid amine salt. The method comprises the following steps: (1) extraction of a clavulanic acid aqueous solution and concentration of extract liquor: acidizing the clavulanic acid aqueous solution, then adding a salting-out agent, and extracting by an organic solvent, thus obtaining the extract liquor containing clavulanic acid; nano-filtering and concentrating by using an organic solvent-resistant film, thus obtaining a clavulanic acid extraction concentrated solution; (2) preparation of the clavulanic acid amine salt: mixing the clavulanic acid extraction concentrated solution with an organic amine donor and a cosolvent, thus obtaining clavulanic acid amine salt crystals. The salting-out agent is introduced, and the extraction rate of the clavulanic acid is increased; the organic solvent-resistant roll film is innovatively adopted for nano-filtering and concentrating, so the energy consumption is lowered; the addition of the cosolvent can effectively reduce the content of various impurities in a final clavulanic acid amine salt product. Therefore, in the quality parameter aspects of the content, the impurities, light transmittance and the like, the clavulanic acid amine salt prepared by the preparation method is remarkably superior to clavulanic acid amine salt prepared by a conventional process.
Owner:SHANXI WEIQIDA PHARMA IND
Preparation method of cefotaxime acid
The invention discloses a preparation method of cefotaxime acid, which belongs to the technical field of medicine, and comprises the following steps: by taking unqualified cefotaxime sodium as a raw material, dissolving the cefotaxime sodium, adding a protective agent and a phosphate solution, decoloring, dropwise adding an acid reagent to adjust the pH value of the feed liquid, adding a cefotaxime acid seed crystal, growing the crystal, filtering, and drying to obtain the cefotaxime acid. And after crystal growing is finished, continuously dropwise adding an acid reagent and water to adjust the pH value, cooling, growing the crystal, filtering and drying to obtain the cefotaxime acid. The cefotaxime acid prepared by the method disclosed by the invention has the advantages of energy conservation, environmental protection, recyclable solvent and simple operation, and the prepared cefotaxime acid has the advantages of good quality, high product content and low impurity content, and solves the problem that reworking is required for producing dirty powder due to unqualified products such as content, impurities and the like of cefotaxime sodium sometimes.
Owner:NORTH CHINA PHARMA HEBEI HUAMIN PHARMA
A kind of refining method of cefanixime dibenzylethylenediamine salt
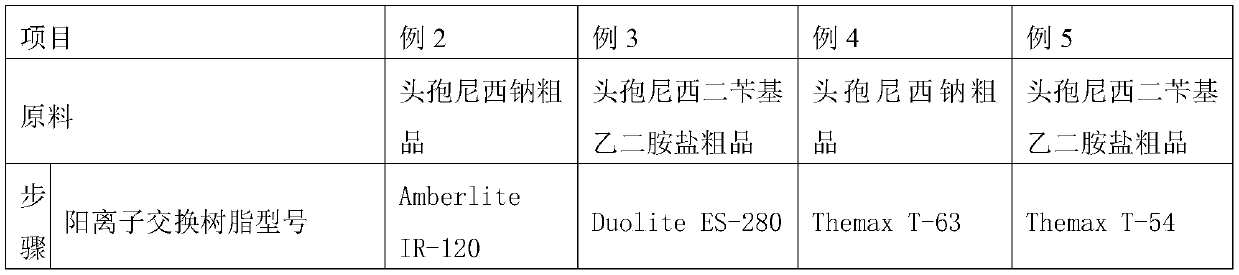
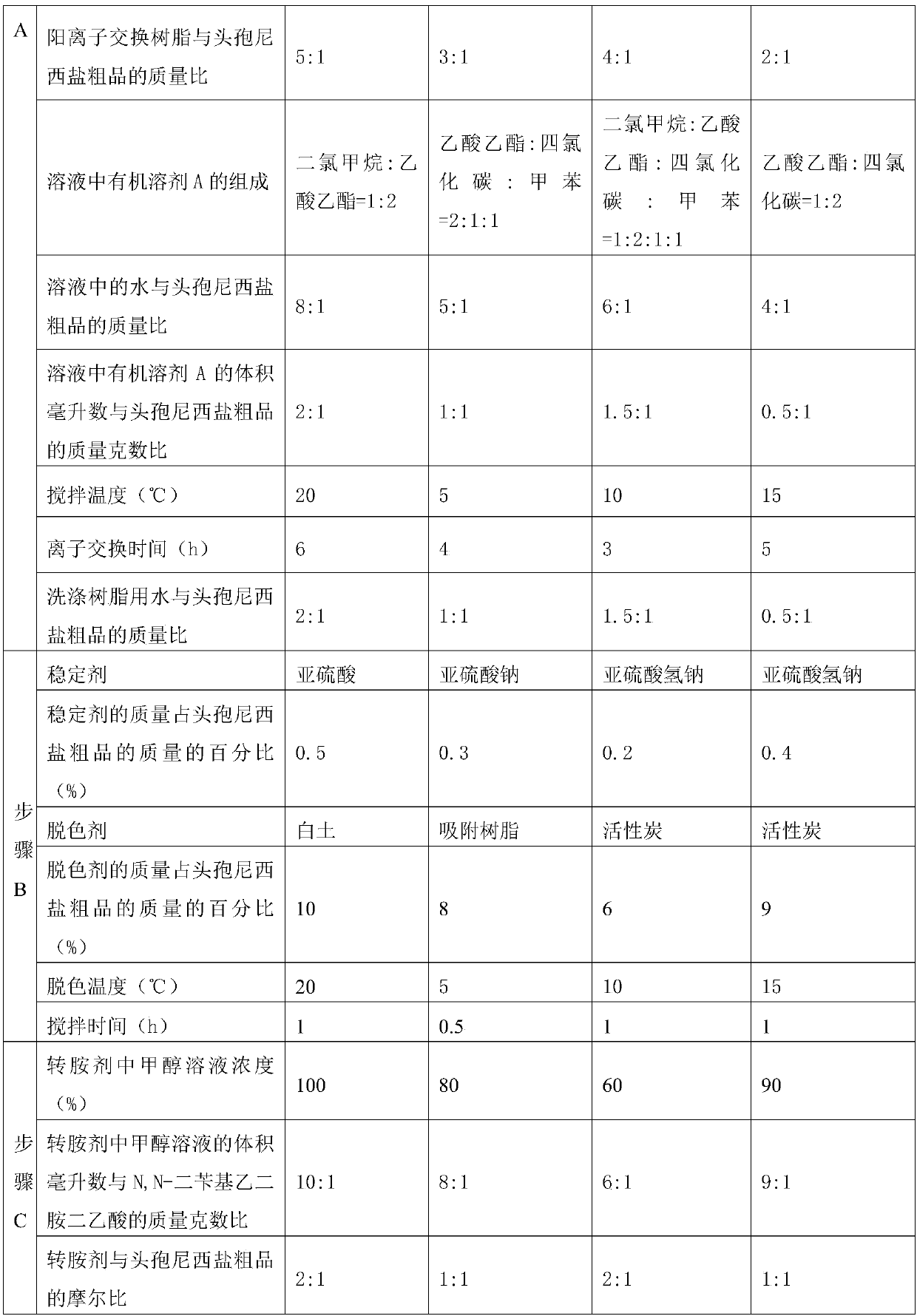
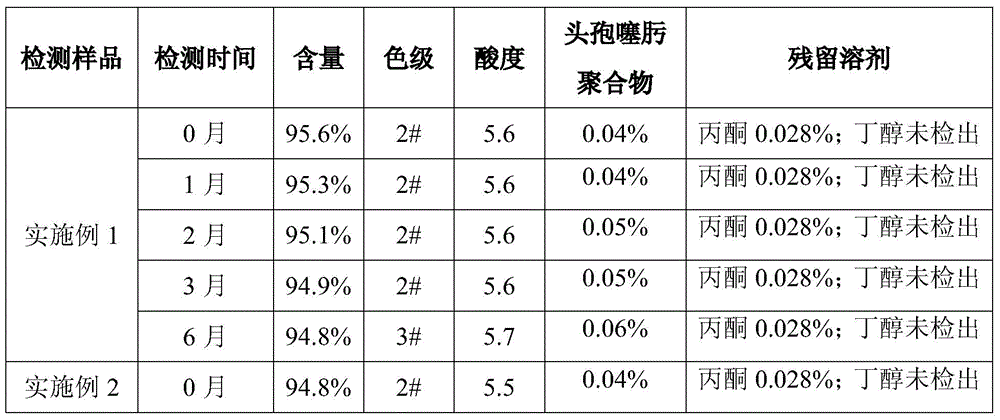
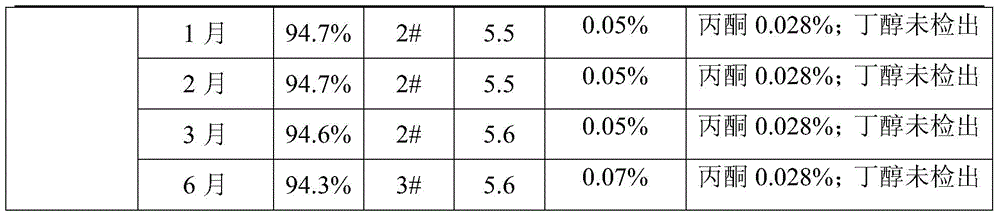
ActiveCN109232610BHigh purityLess impuritiesOrganic compound preparationAmino compound preparationIon exchangeProcess engineering
The invention discloses a method for refining cefanizidime dibenzylethylenediamine salt, which belongs to the technical field of medicine. The crude product of cefanizidime salt is ion-exchanged with a resin, decolorized with a decolorizing agent, and then synthesized with a transamination agent. Salt reaction, and then crystallization with a precipitating agent to obtain the refined cefanixime dibenzylethylenediamine salt. The cefanisidine dibenzylethylenediamine salt prepared by the present invention has high purity, few impurities and low color grade, and the refining method of the present invention has the advantages of simple process, energy saving and environmental protection, suitable for large-scale industrial production, and the like.
Owner:NORTH CHINA PHARMA HEBEI HUAMIN PHARMA
A kind of preparation method of cefotaxime sodium
The invention discloses a preparation method of cefotaxime sodium. Ceftizoxime acid used as the initial raw material reacts with anhydrous sodium acetate to generate the cefotaxime sodium. In such process, purified water, butanol and acetone are selected as crystallizing solvents to control the mixing speed and crystallization temperature in the crystallization process, so that the finally obtained cefotaxime sodium has favorable flowability and can satisfy the subpackaging requirements in production. Various quality indexes of the obtained cefotaxime sodium conform to the requirements for medicinal standard, and thus, the cefotaxime sodium can satisfy the demands for clinical application.
Owner:石药集团中诺药业(石家庄)有限公司
Method for recrystallizing cefuroxime sodium
InactiveCN101967156BImprove solubilityHigh recrystallization efficiencyOrganic chemistryDark colorCefuroxime Sodium
The invention relates to a method for recrystallizing cefuroxime sodium, which is used for solving the problem of the refining of cefuroxime sodium raw material medicaments with darker colors. In the technical scheme, white crystalline powder recrystallized by the cefuroxime sodium is prepared by the following four steps of: a, preparing crude product solution; b, preparing a crystallization solvent; c, crystallizing; and d, discharging. By the method, the yield is over 83 percent, and the color level and the pH value of final products meet the pharmacopoeial standard; and the method is suitable for recrystallizing the raw material medicaments with unqualified color levels.
Owner:石药集团中诺药业(石家庄)有限公司
Process for producing low VOC coalescing aids
ActiveUS20170113997A1Low color levelOrganic compound preparationCarboxylic acid esters preparationGlycol ethersPhosphoric acid
A process comprising reacting a mono- or di-carboxylic acid and / or acid anhydride with a glycol ether in the presence of phosphoric acid to produce a glycol ether ester product having low color and low VOC content.
Owner:DOW GLOBAL TECH LLC
Purification method of cefpiramide acid
ActiveCN111072688APromote crystallizationHigh purityOrganic chemistryMedicinal chemistryPharmacology
The invention relates to the technical field of medicine purification, and particularly discloses a purification method of cefpiramide acid. The method comprises the following steps: mixing an alcoholsolvent with a cefpiramic acid crude product, and then adding triethylamine to obtain a cefpiramic acid triethylamine salt dissolving solution; adding a precipitant into the cefpiramide acid amine salt dissolving solution, mixing, stirring, centrifugally filtering and washing to obtain cefpiramide acid triethylamine salt; and uniformly mixing the cefpiramic acid triethylamine salt with purified water, decolorizing, performing filtration, adjusting the pH value of the collected filtrate to 2-2.5, crystallizing, washing, and carrying out vacuum drying to obtain the refined cefpiramic acid. Therefined cefpiramide acid has the advantages of high purity, high yield, low color grade, simple operation and low production cost, and is suitable for large-scale industrial production.
Owner:湖北凌晟药业股份有限公司
Method for synthesizing ceftizoxime acid by one-pot method
The invention discloses a method for synthesizing ceftizoxime acid by a one-pot method. The method comprises the following steps: reacting 7-ANCA and AE-active ester in a solvent system of water and tetrahydrofuran in the presence of organic alkali, regulating the pH value of the reaction system to 2-3 after the reaction is finished, and carrying out crystal growing, filtering, washing and dryingto obtain the ceftizoxime acid. According to the method, the ceftizoxime acid is synthesized by adopting the one-pot method, the process conditions are mild, an extracting agent does not need to be added for extraction separation, an adsorbent does not need to be added, acid is directly used for neutralization and crystallization after reaction, operation is convenient, and the solvent can be recycled.
Owner:苏州盛达药业有限公司
Method for purifying cefepime hydrochloride
The invention discloses a method for purifying cefeprime hydrochloride and belongs to the field of chemical pharmaceutical. The method includes: taking a coarse product of the cefeprime hydrochlorideas a raw material; adjusting the pH value of solving liquid of the coarse product of the cefeprime hydrochloride and adding a dispersing agent to obtain the cefeprime hydrochloride after crystallization of isoelectric points. The purity of the cefeprime hydrochloride can be improved, and the prepared cefeprime hydrochloride has the advantages of low color level, uniform particle size and good fluidity.
Owner:NORTH CHINA PHARMA HEBEI HUAMIN PHARMA
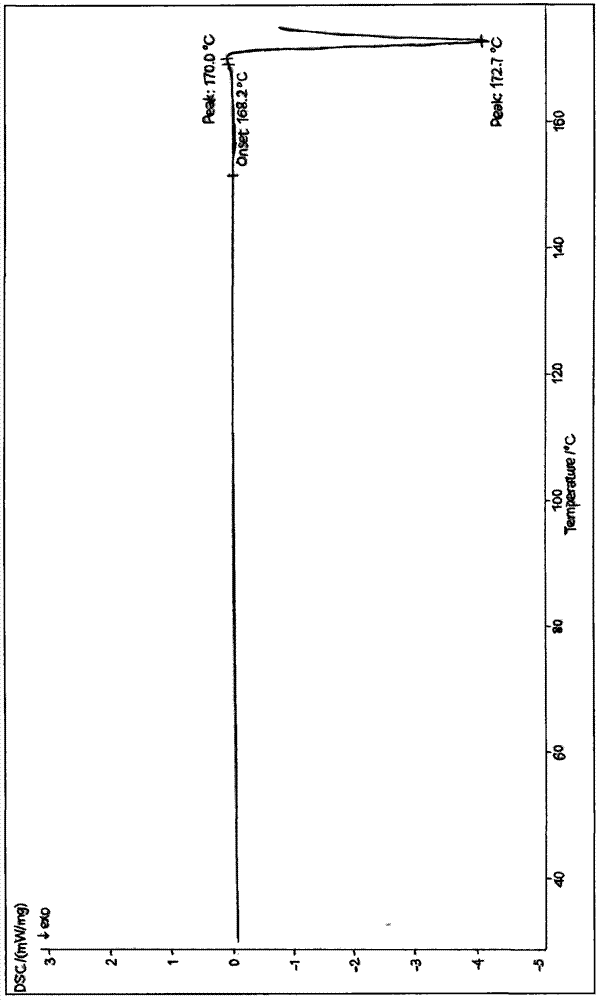
Cefoxitin anhydrous crystal, preparation method thereof and method for preparing cefoxitin sodium by using same
The invention provides a cefoxitin anhydrous crystal. Peaks exist at 2theta angles of 12.0 degrees, 15.7 degrees, 18.8 degrees and 22.6 degrees of the X-ray diffraction pattern of the crystal. In a differential thermal analysis diagram, an endothermic peak exists at 170.0 degrees and exothermic peak exists at 172.7 degrees. The invention additionally provides a method for preparing the cefoxitin anhydrous crystal and a method for preparing cefoxitin sodium by using the cefoxitin anhydrous crystal. The impurity content of the cefoxitin anhydrous crystal is low, the color level of the cefoxitinsodium is reduced and the application prospect of cefoxitin acid drugs is improved.
Owner:SHENZHEN SALUBRIS PHARMA CO LTD
Process for producing low VOC coalescing aids
ActiveUS20170129843A1Low color levelLow levelOrganic compound preparationCarboxylic acid esters preparationBenzoic acidPhosphoric acid
A process comprising reacting a benzoic acid with a glycol ether in the presence of phosphoric acid to produce a glycol ether ester product having low color, low odor, and low VOC content.
Owner:DOW GLOBAL TECH LLC
A kind of purification method of cefepime hydrochloride
Owner:NORTH CHINA PHARMA HEBEI HUAMIN PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com