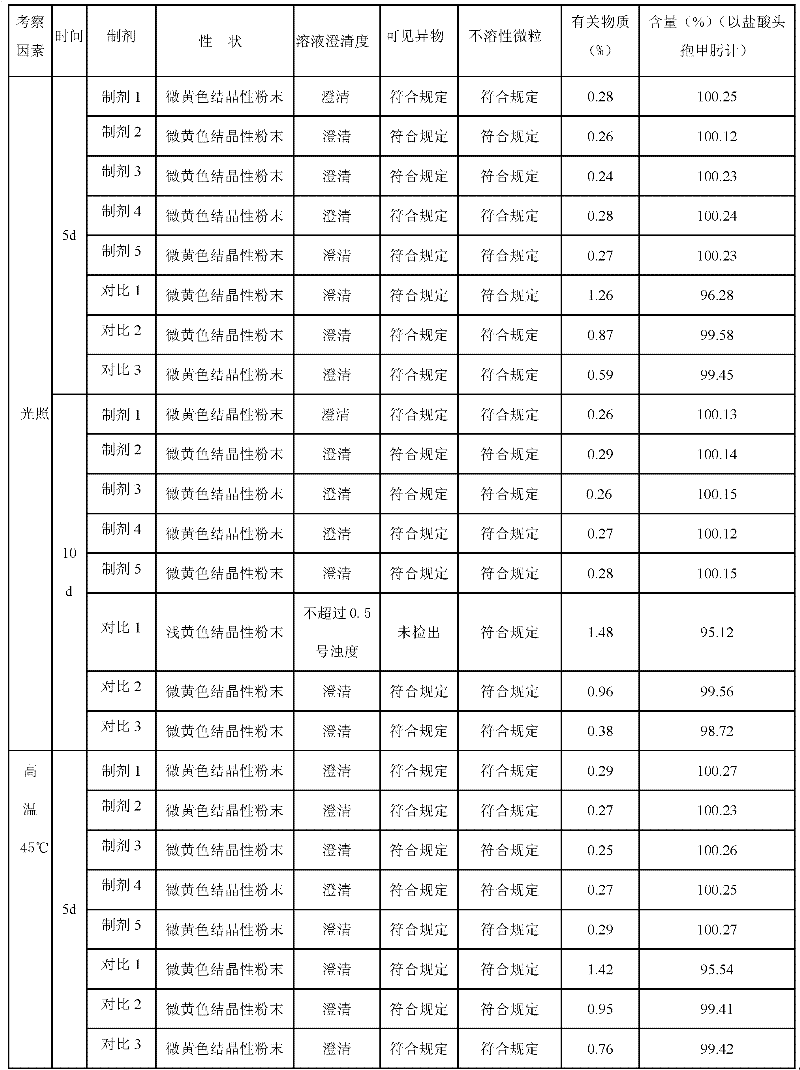
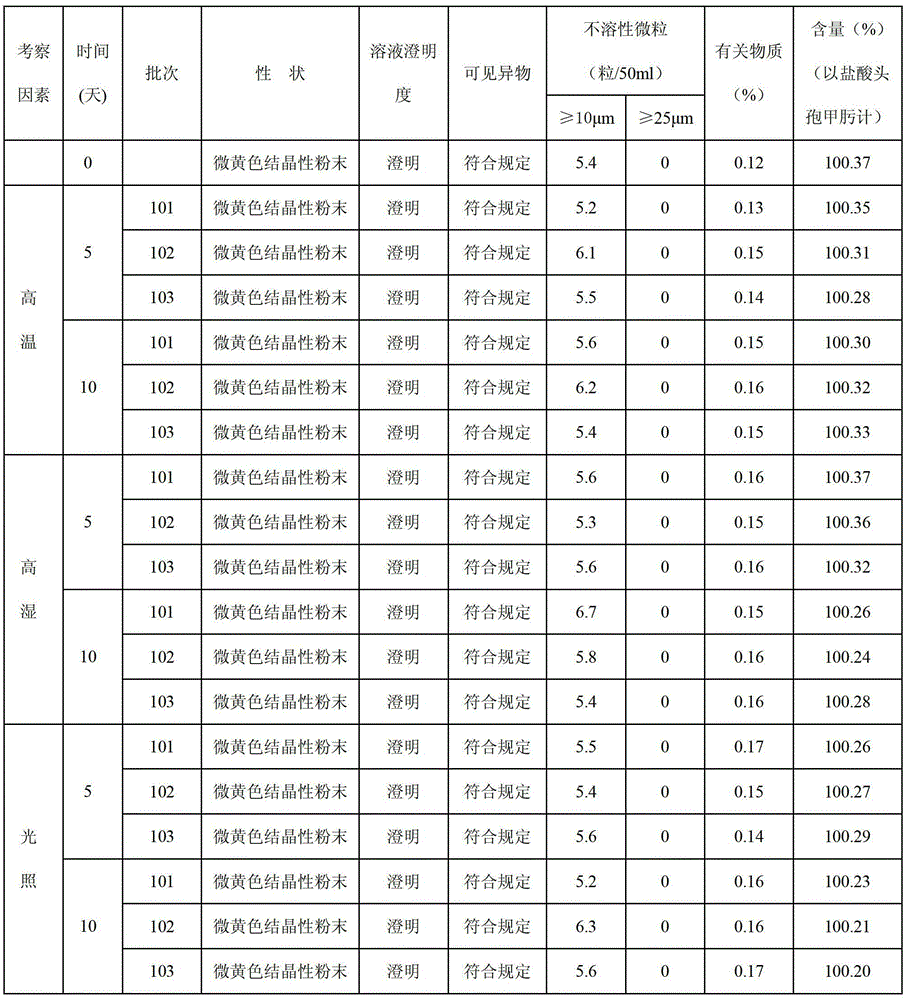
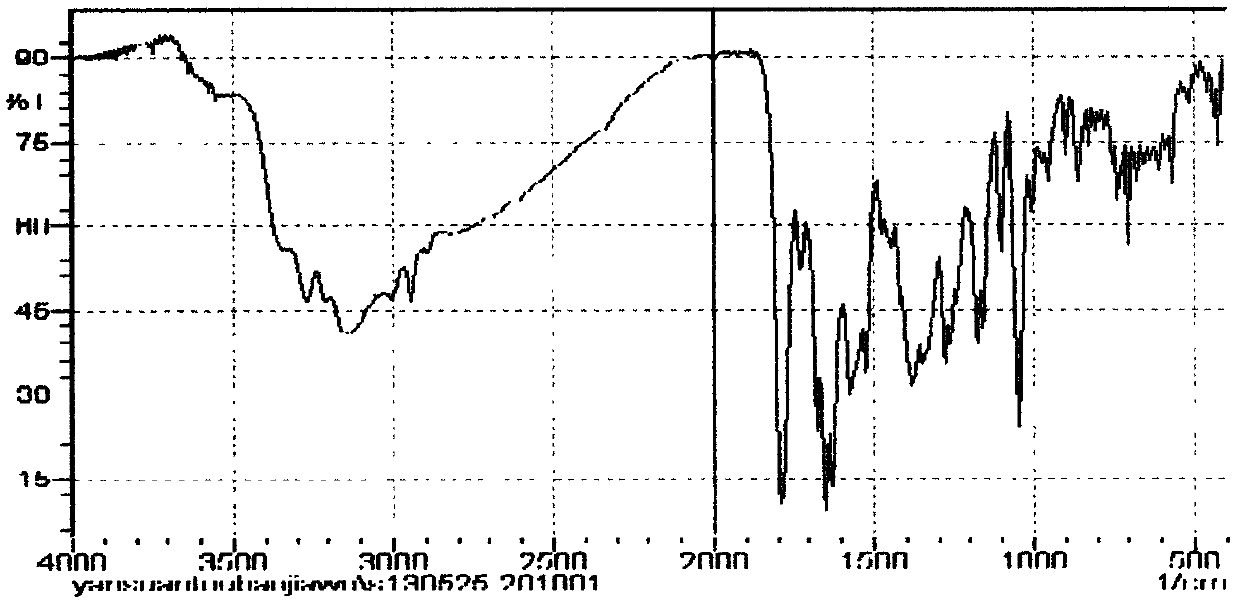
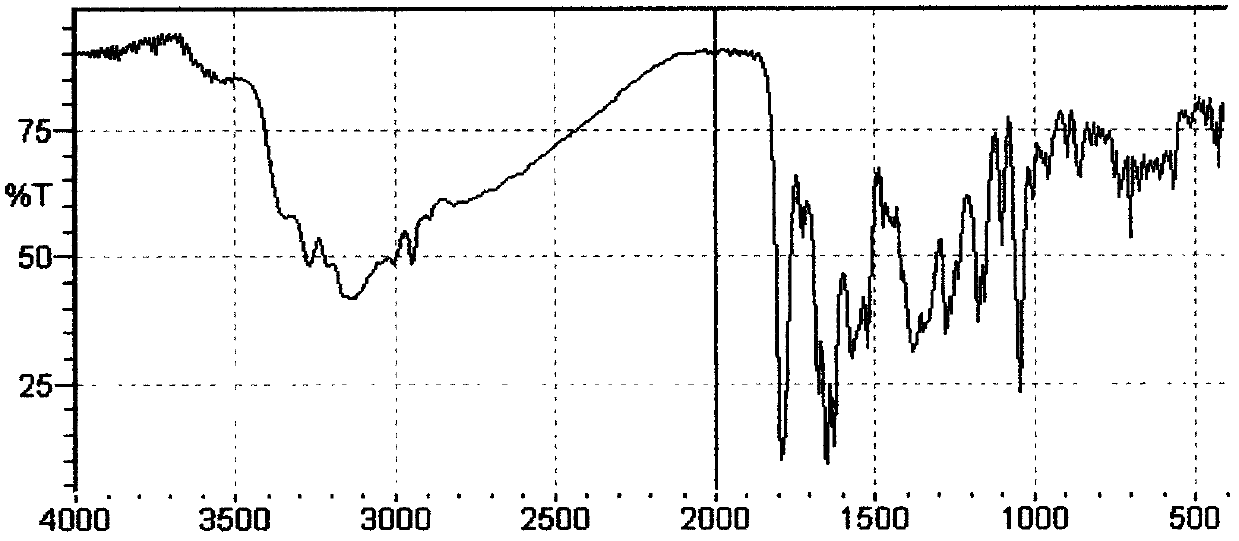
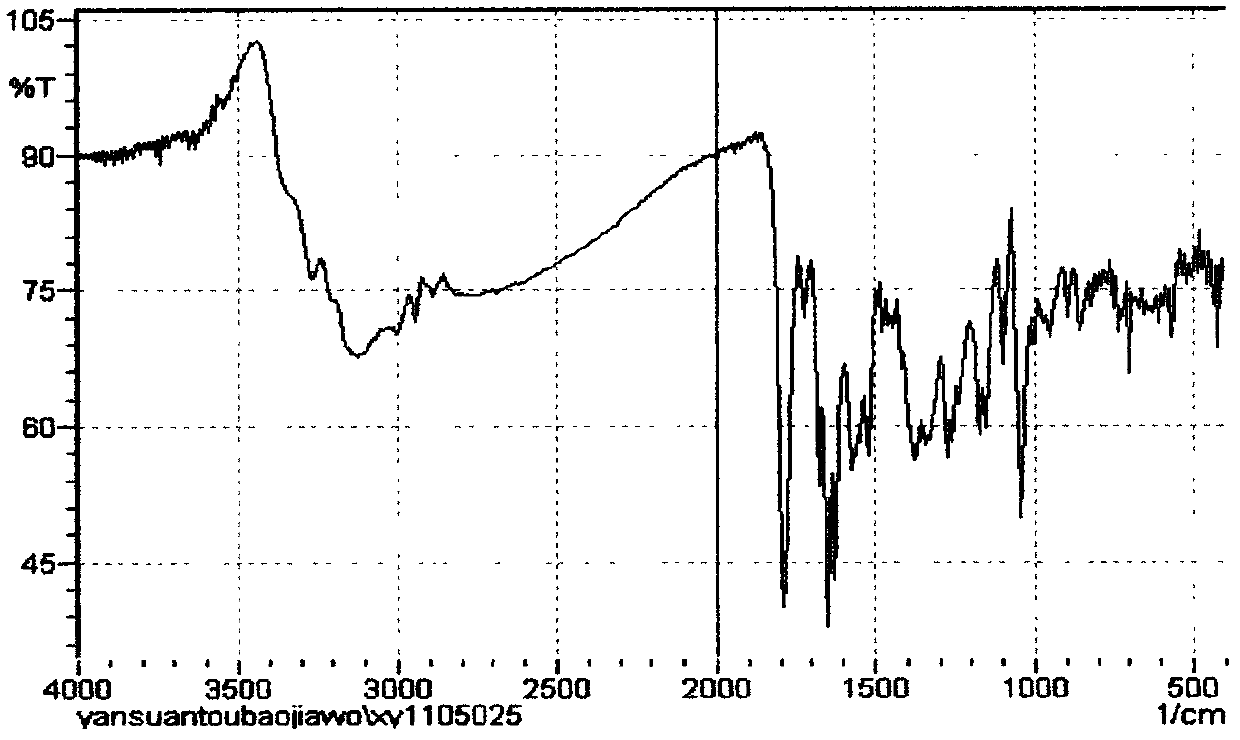
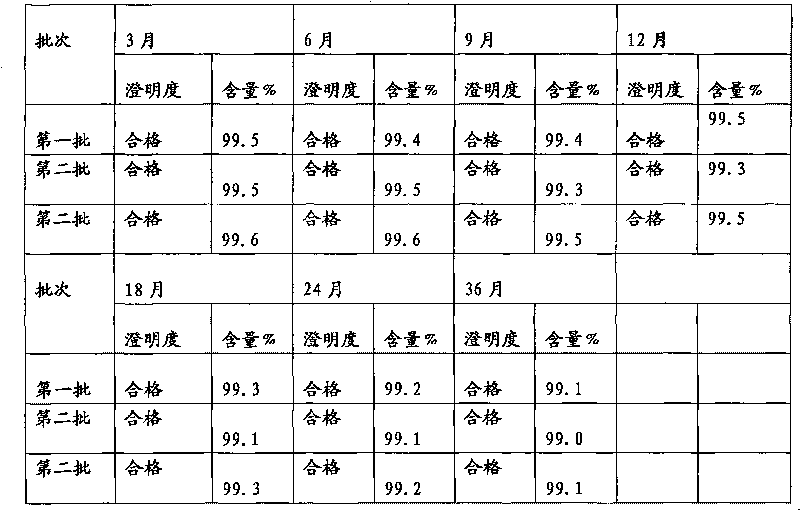
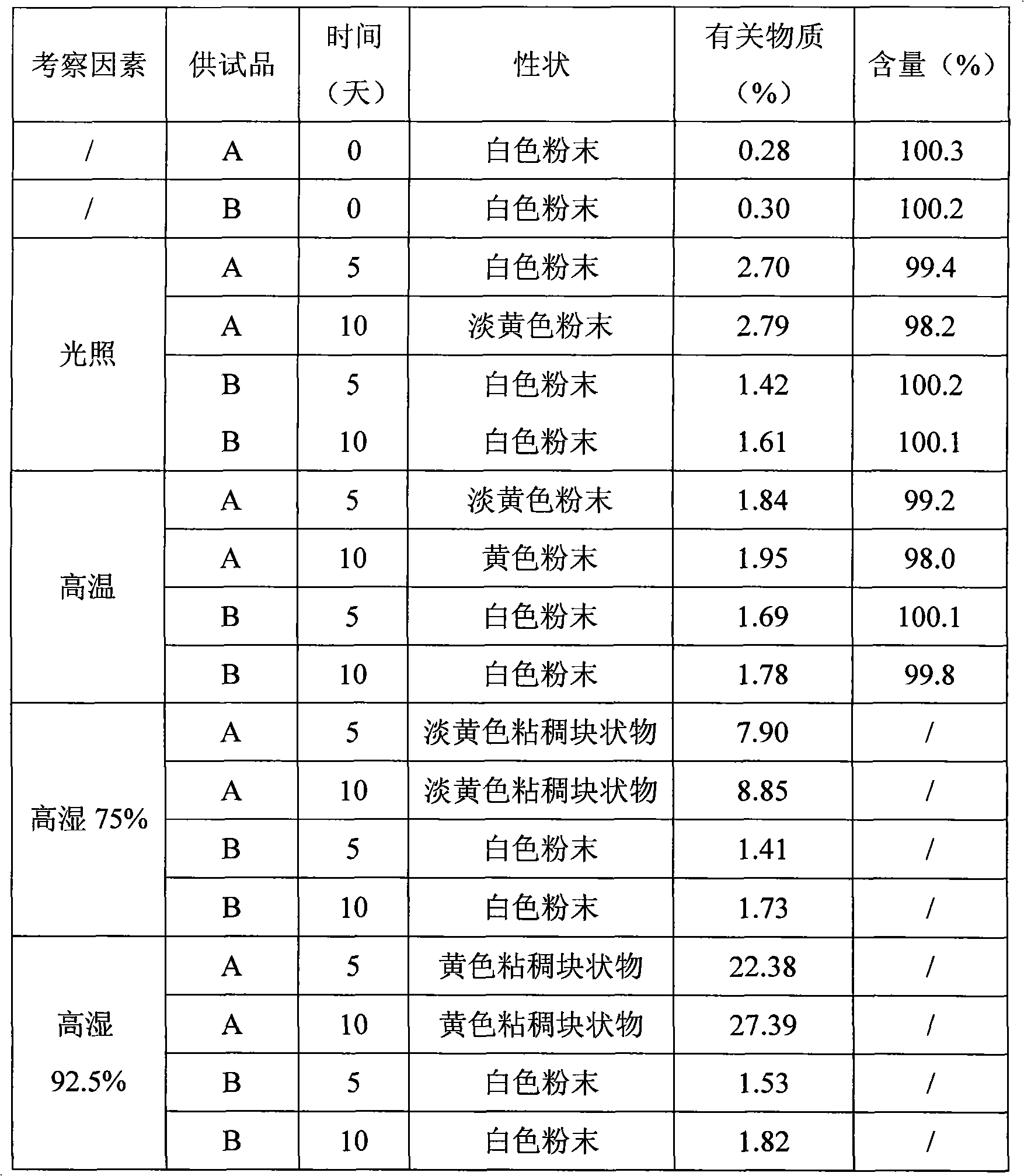
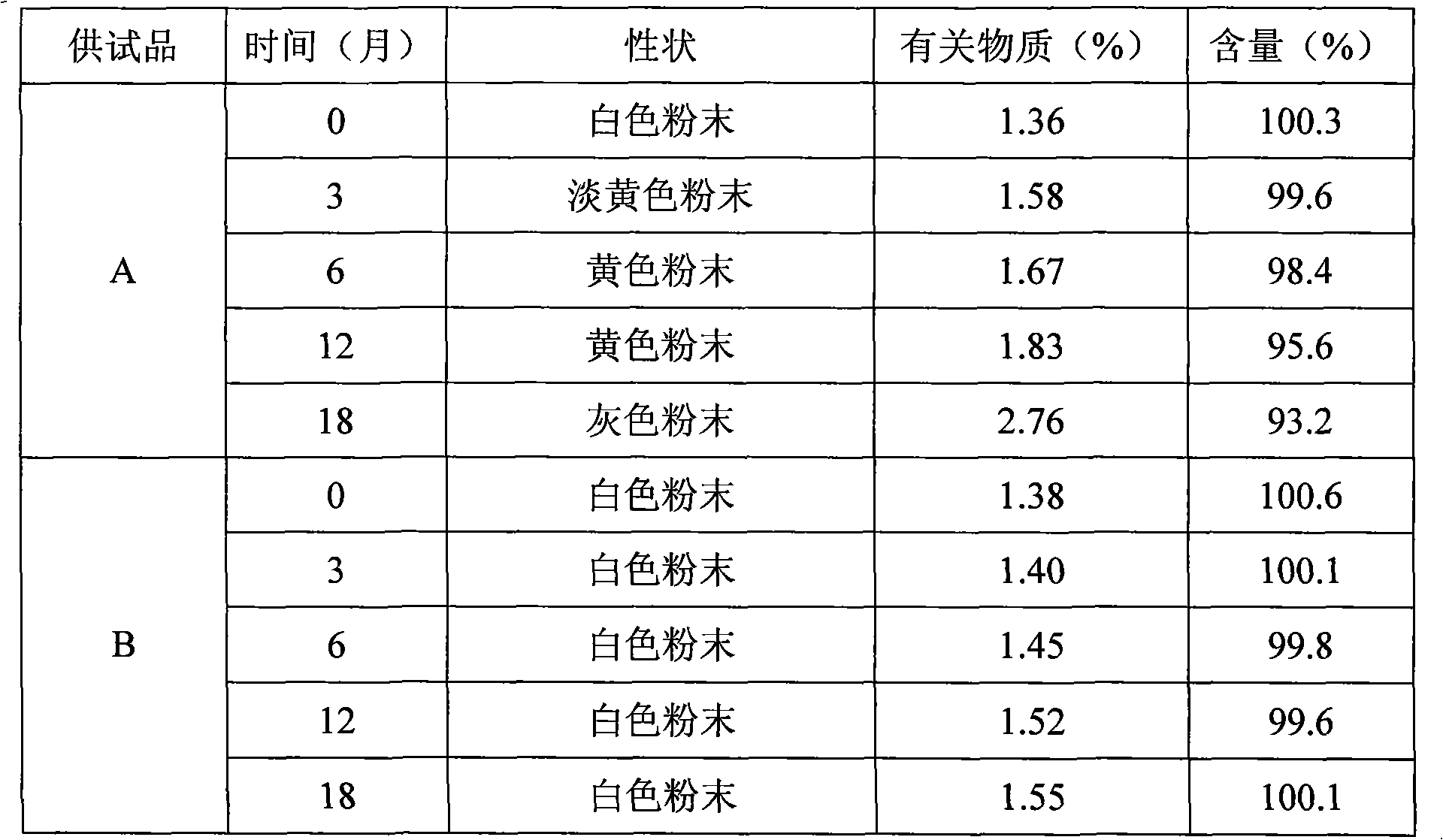
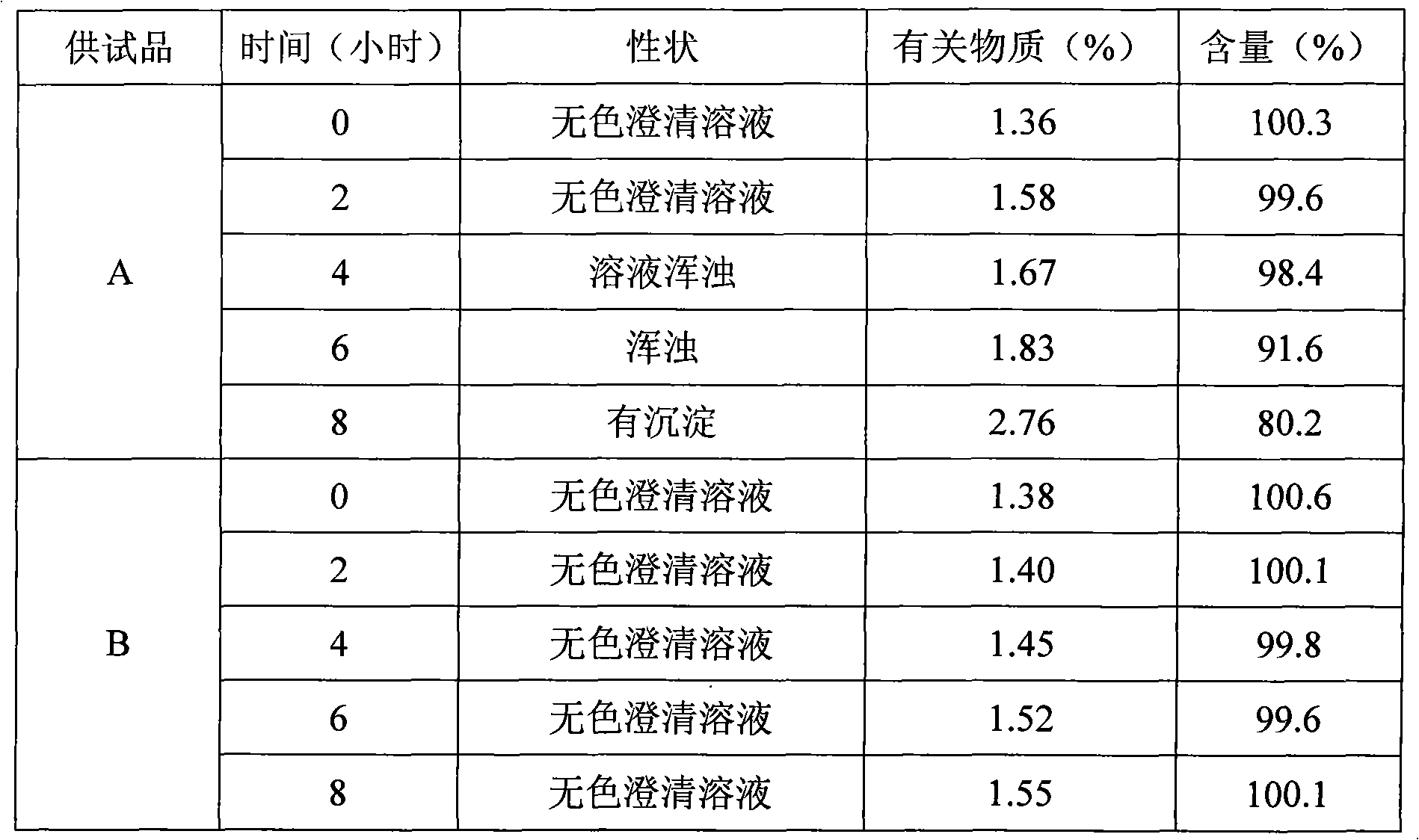
Patents
Literature
61 results about "Cefmenoxime Hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
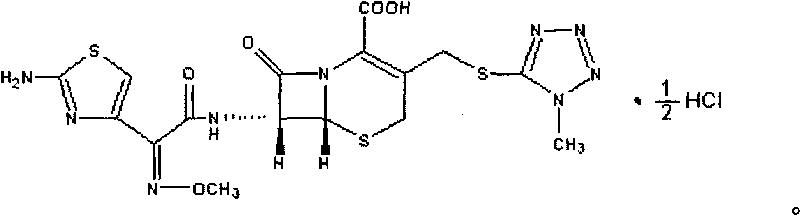
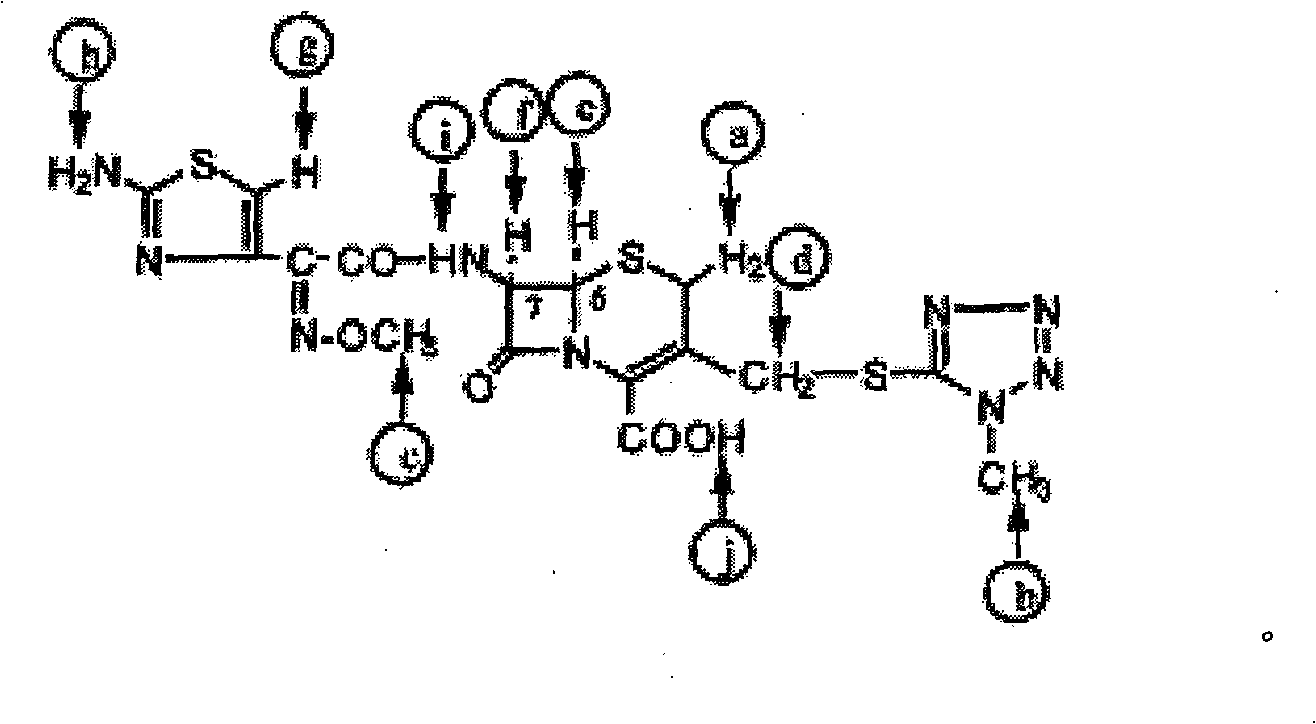
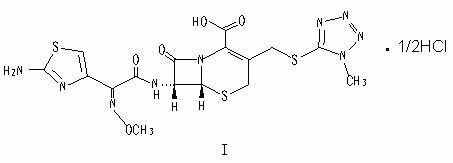
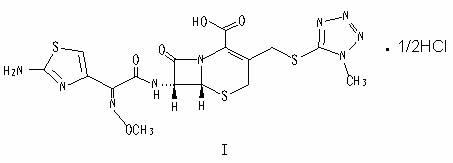
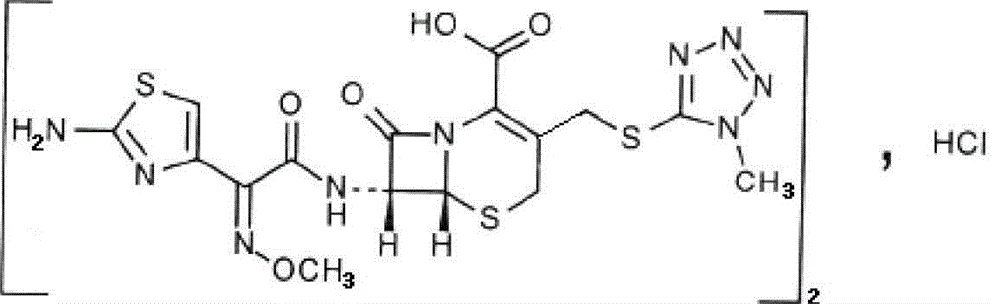
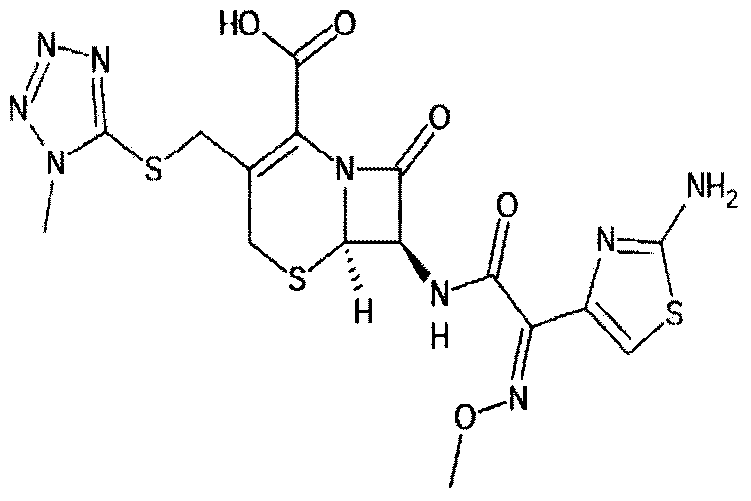
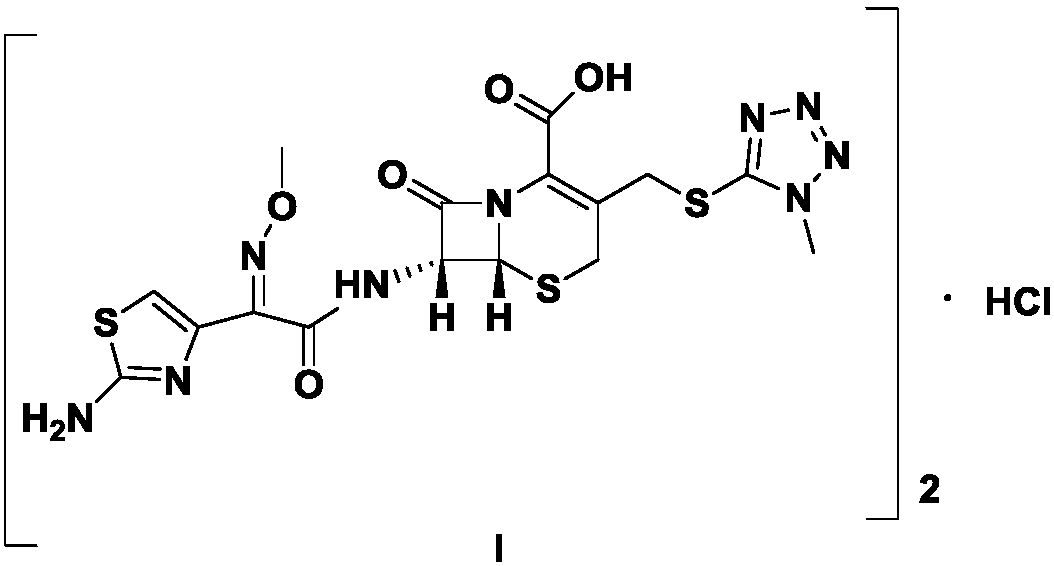
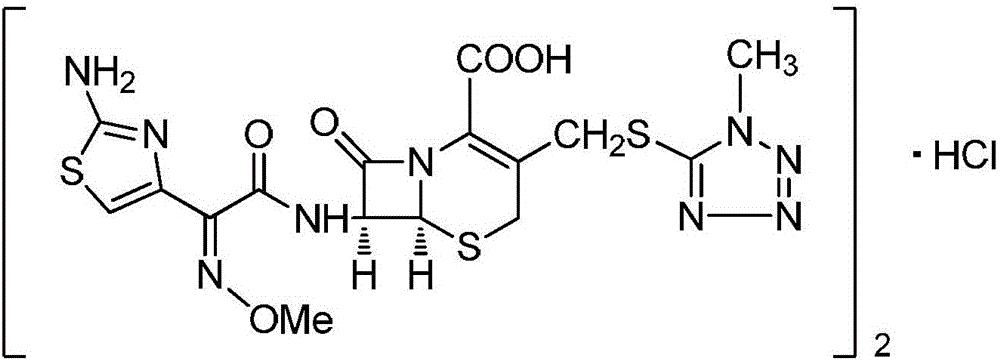
The hydrochloride salt form of cefmenoxime, a third-generation, semi-synthetic, beta-lactam cephalosporin antibiotic with antibacterial activity. Cefmenoxime binds to penicillin-binding proteins (PBPs), transpeptidases that are responsible for crosslinking of peptidoglycan. By preventing crosslinking of peptidoglycan, cell wall integrity is lost and cell wall synthesis is halted.
Cefmenoxime hydrochloride composition powder injection and manufacturing method thereof
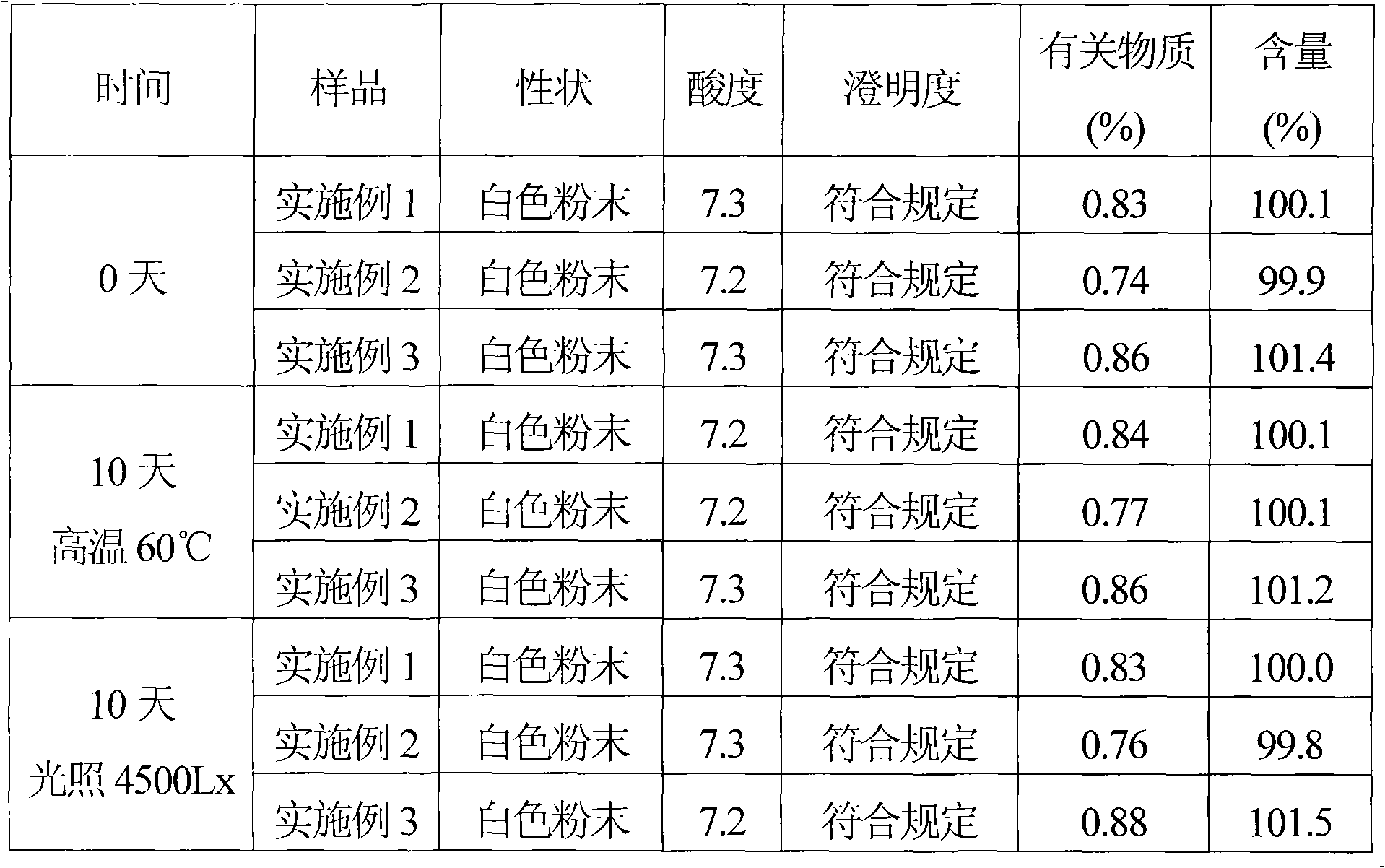
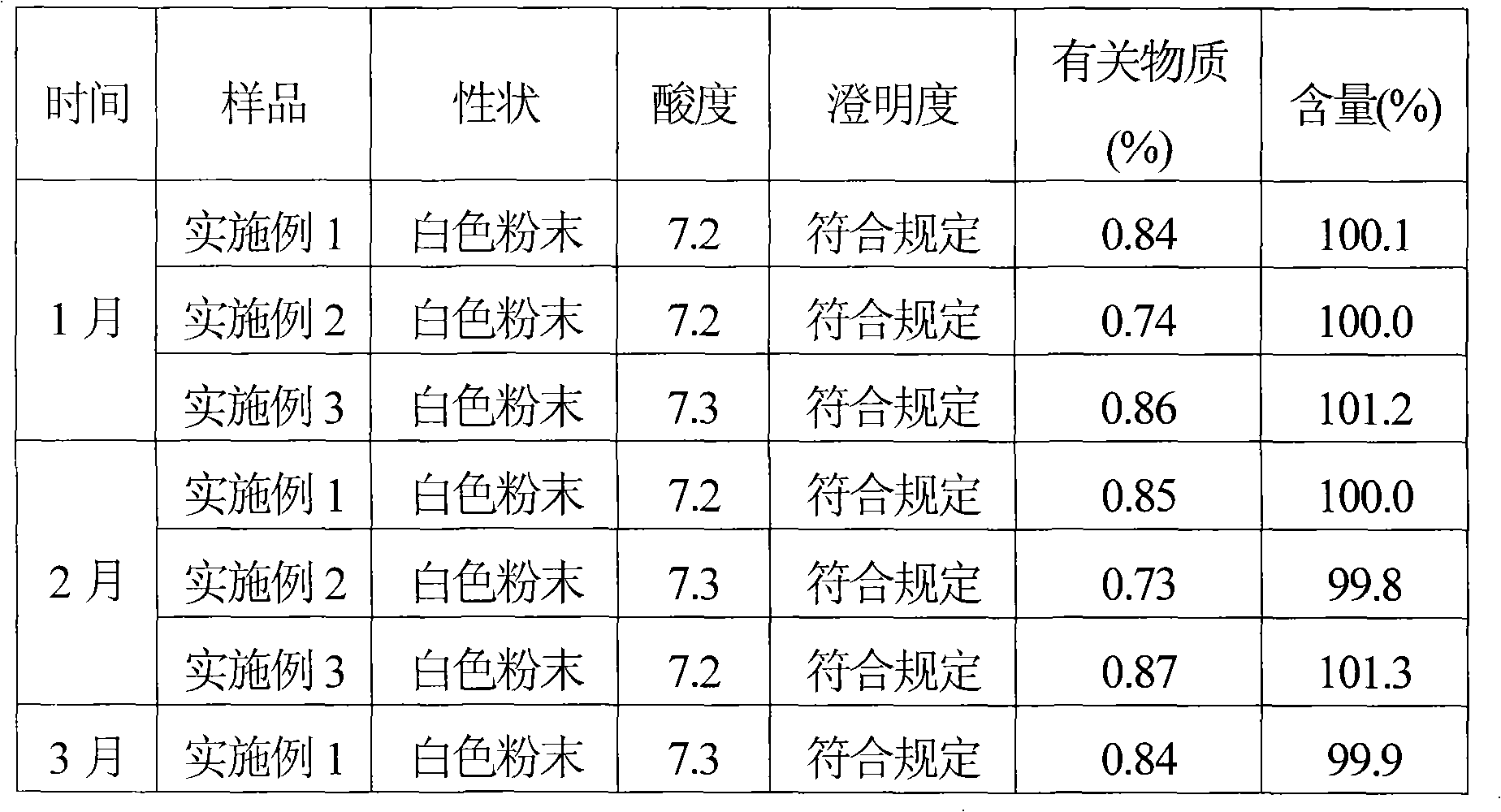
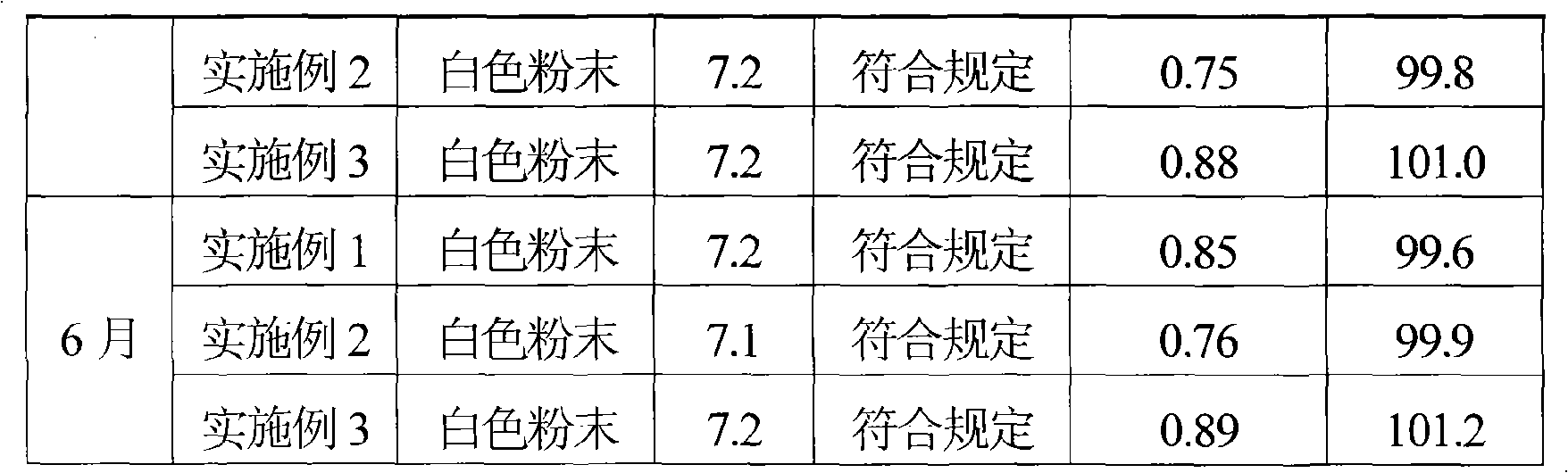
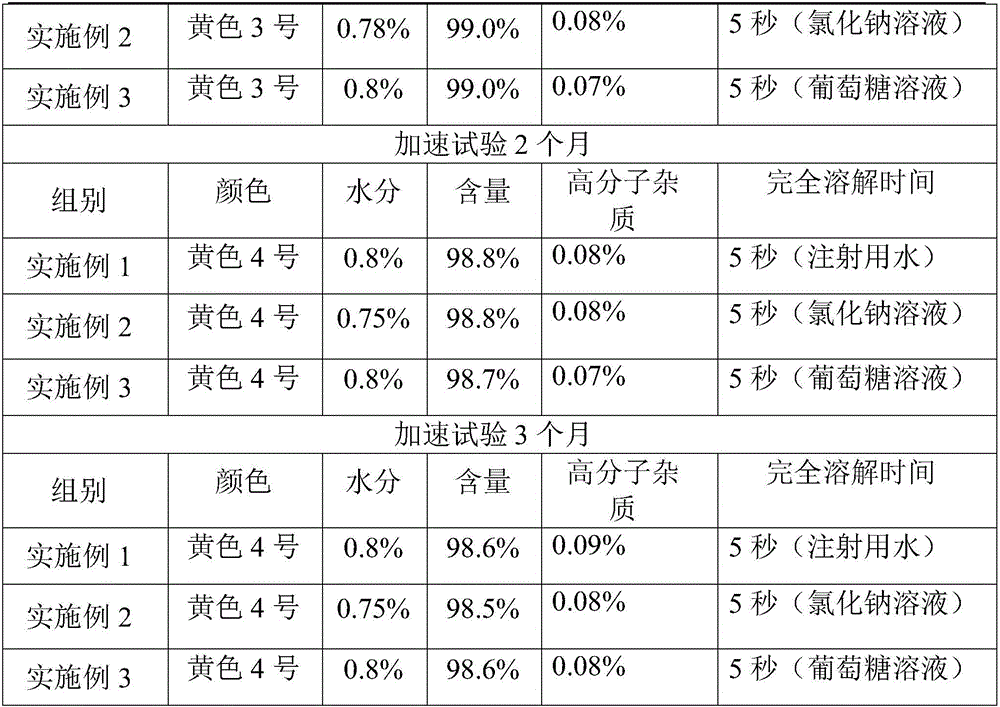
ActiveCN101569628AQuality improvementImprove solubilityAntibacterial agentsOrganic active ingredientsDissolutionCefmenoxime Hydrochloride
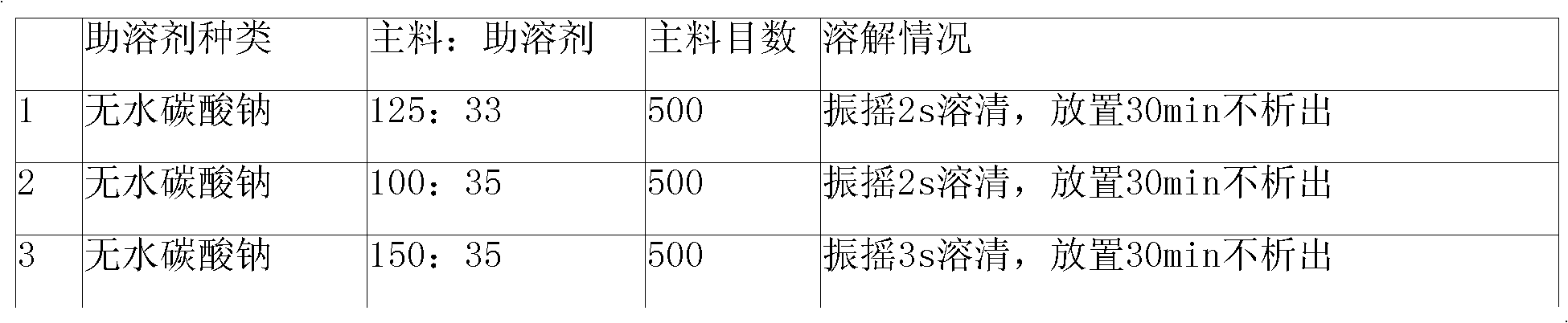
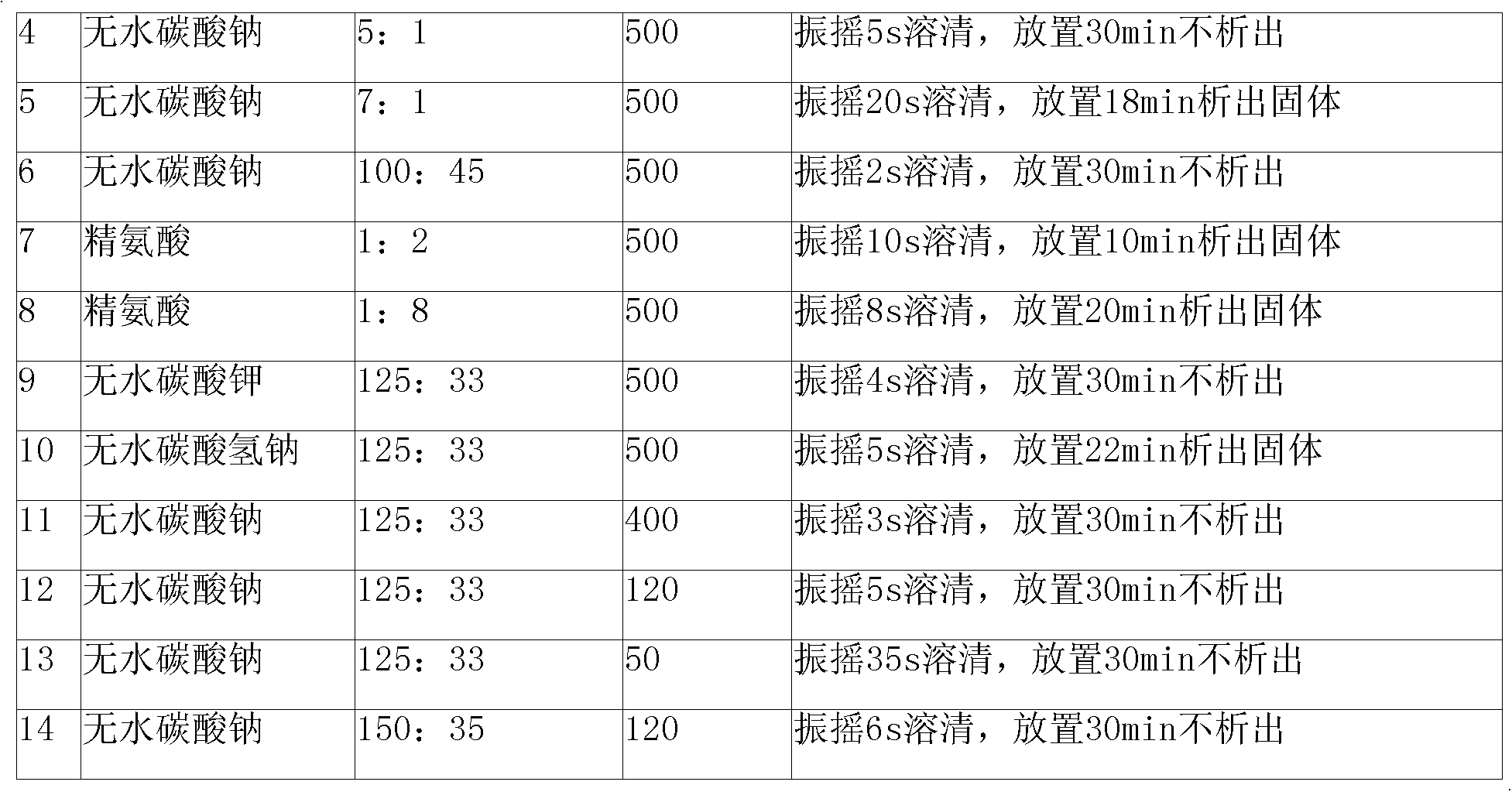
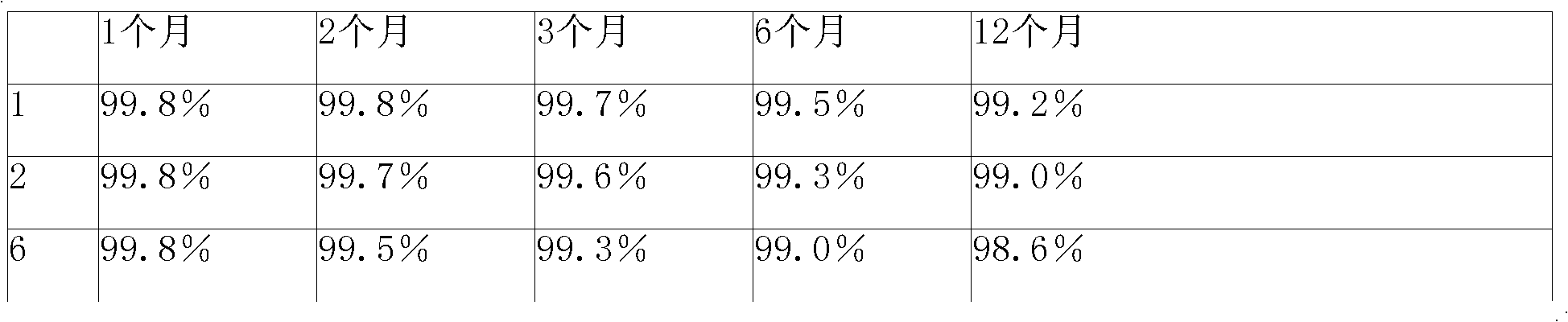
The invention relates to a cefmenoxime hydrochloride composition powder injection, which comprises the following components: 100-150 parts of cefmenoxime hydrochloride, 30-35 parts of natrium carbonicum calcinatum, preferably 125 parts of cefmenoxime hydrochloride and 33 parts of natrium carbonicum calcinatum. The grain size of the cefmenoxime hydrochloride is 400-600 meshes, which is preferably 500 meshes. The manufacturing method of the cefmenoxime hydrochloride composition powder injection comprises the steps as follows: 100-150 parts of cefmenoxime hydrochloride and 30-35 parts of natrium carbonicum calcinatum are respectively sieved, evenly mixed and pulverized until the grain size of the cefmenoxime hydrochloride is 400-600 meshes and is preferably 500 meshes; the obtained powder is sub-packaged into sterilized vials and is plugged. The cefmenoxime hydrochloride composition powder injection prepared by the invention has the advantages of fine quality, rapid dissolution and good stability after being prepared into solution.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
Process for preparing instantly-dissolving cefmenoxime hydrochloride
The invention discloses a process for preparing instantly-dissolving cefmenoxime hydrochloride, which comprises the following steps: 1) adding water into a coarse product of cefmenoxime hydrochloride to prepare a suspension, adding sodium carbonate into the suspension to dissolve the cefmenoxime hydrochloride, and thus obtaining clear liquor; 2), decolorizing the clear liquor by using active carbon, washing the clear liquor and mixing the washing liquor to obtain solution A; 3) adjusting the pH value of the solution A to 0.8 to 1.2 by using hydrochloric acid, filtering the solution A and collecting the filtrate to obtain solution B; and 4), adjusting the pH value of the solution B to 1.3 to 1.75 by using an alkalescent solution, stirring the solution B for crystallization, filtering the solution after the crystallization is finished, washing crystals and drying the crystals under vacuum to obtain the instantly-dissolving cefmenoxime hydrochloride. Compared with the prior art, the method has the advantages that: the pH value is adjusted at two stages and the crystallization is performed under a condition of a pH value of 1.3 to 1.75, so the precipitated crystals have a proper particle size and good dispersibility, can be charged conveniently and separately and can dissolve in a short time; the operation process is simplified; the risks of producing visible foreign matters are reduced greatly; the production efficiency is improved; the cost is reduced; and the clinic use and operation are convenient.
Owner:桂林澳林制药有限责任公司
High-purity cefmenoxime hydrochloride compound
InactiveCN101798314AHigh purityImprove product qualityOrganic chemistryActivated carbonChromatographic separation
The invention relates to a cefmenoxime hydrochloride compound, which is prepared through acid-base reaction, activated carbon adsorption, separation and purification of prepared chromatography so as to achieve the aim of purification and obtain the high-purity cefmenoxime hydrochloride compound finally. The invention optimizes the product quality, and guarantees safety of clinical medication.
Owner:HAINAN LINGKANG PHARMA CO LTD
Novel method for preparing cefmenoxime hydrochloride compound
The invention relates to a novel method for preparing a cefmenoxime hydrochloride compound. The method comprises the following steps of: 1) adding a cefmenoxime hydrochloride-insoluble solvent into a raw material, namely cefmenoxime hydrochloride, controlling the temperature to be not more than 30 DEG C, violently stirring, filtering, washing a filter cake by using the cefmenoxime hydrochloride-insoluble solvent at the temperature of not more than 20 DEG C, and performing vacuum drying or air-drying; 2) putting the filter cake into ammonia water, mildly stirring, controlling the pH value to be not more than 9 so as to obtain an ammonia water solution of cefmenoxime acid, and filtering a precipitate; 3) slowly adding hydrochloric acid at the concentration of between 0.5 and 4mol / L into the ammonia water solution of the cefmenoxime acid, controlling the temperature to be 30 to 60 DEG C, finally controlling the pH value to be 0.5 to 3.0, keeping for 30 minutes to 5 hours so as to slowly precipitate crystals, gradually reducing the temperature to the lowest 10 DEG C, standing, crystallizing, performing suction filtration, and performing vacuum drying to obtain a refined product of the cefmenoxime hydrochloride; and 4) optionally, returning crystallization mother liquor obtained after the crystals are precipitated to step 3). By the method, the purity of the cefmenoxime hydrochloride is greatly improved, the content of related substances is remarkably reduced, the quality of preparation products is improved, and the toxic or side effect is reduced.
Owner:LIONCO PHARM GRP CO LTD
Cefmenoxime hydrochloride preparation for injection and preparation method thereof
InactiveCN101444514AImprove securityReduce adverse reactionsAntibacterial agentsOrganic active ingredientsWater bathsTreatment effect
The invention provides a cefmenoxime hydrochloride preparation for injection and a preparation method thereof. The main components of the cefmenoxime hydrochloride preparation are soyabean lecithin for injection, cholesterin and cefmenoxime, and the weight ratio of the soyabean lecithin, the cholesterin and the cefmenoxime is 10-2:4-1:1. The preparation method comprises the following steps: dissolving the soyabean lecithin and the cholesterin with absolute ethyl alcohol, adding buffer solution for complete hydration, and filtering with a microporous filter membrane of 0.8mum twice to obtain a blank liposome, then adding the cefmenoxime and NaHCO3 solution, adding water for injection after being evenly mixed, preserving heat in a water bath, and promptly cooling with cold water to obtain a cefmenoxime liposome preparation. The cefmenoxime hydrochloride preparation can achieve the same curative effect as the conventional cefmenoxime for injection at a low dose of the cefmenoxime and reduces adverse reaction caused by the cefmenoxime, thus causing the products to be safer and more effective.
Owner:HAINAN LINGKANG PHARMA CO LTD
High-purity cefmenoxime hydrochloride and preparation thereof
InactiveCN101348494AHigh purityNo pollution in the processAntibacterial agentsOrganic active ingredientsCefmenoxime HydrochloridePowder injection
Owner:HAINAN SHU ER PHARMA RES
Cefmenoxime hydrochloride micro-powder and preparation method and device thereof
ActiveCN102180891ASmall granularityFast granularityOrganic chemistryGrain treatmentsPharmacyCefmenoxime Hydrochloride
The invention relates to the field of pharmacy, in particular to cefmenoxime hydrochloride micro-powder and a preparation method and a device thereof. The particle size of the cefmenoxime hydrochloride micro-powder is greater than 400 meshes. The method for micronization of cefmenoxime hydrochloride raw materials includes the following steps: the dried cefmenoxime hydrochloride crystalline powderraw materials mutually collide with each other to be smashed after acceleration by using supersonic airflow in a jet mill; the supersonic airflow is pushed by pressure of 0.4-0.8 MPa compressed air, and the temperature of the compressed air is less than or equal to 40 DEG C; the smashed materials enter a classification area along with airflow, the materials meeting the requirement on the particlesize pass through a classification wheel with set rotating speed, and the materials not meeting the requirement on the particle size return to a smashing area to be continuously smashed; and the particle size of the materials meeting the requirement on the particle size is greater than 500 meshes. The micronized cefmenoxime hydrochloride raw material has the advantages of fine granularity, high dissolving speed, convenience in use, reduced relevant materials and the like.
Owner:ZHEJIANG JIANFENG PHARM CO LTD
Cefmenoxime hydrochloride compound used for injection
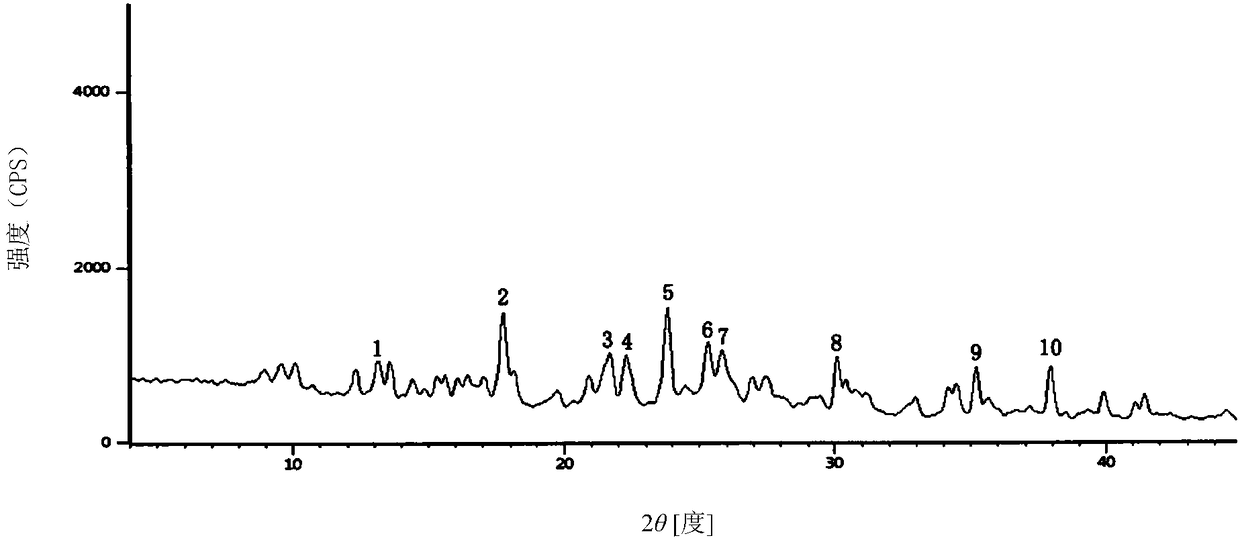
ActiveCN102408439AEasy to prepareFast dissolutionOrganic chemistryCrystallographyCefmenoxime Hydrochloride
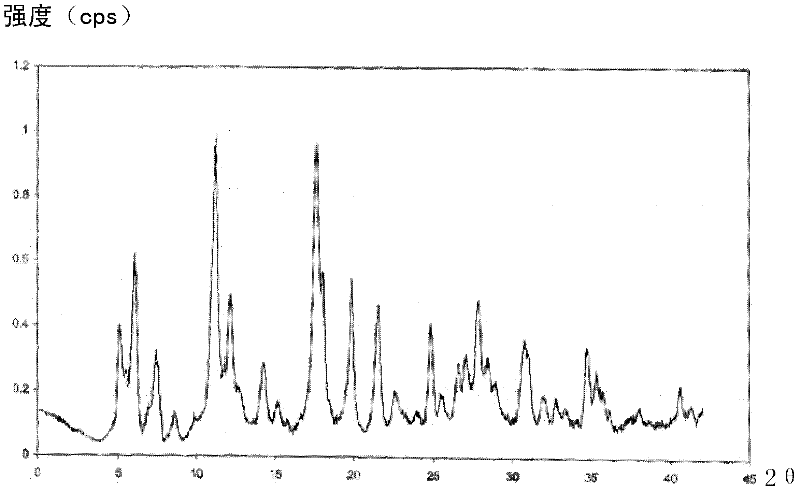
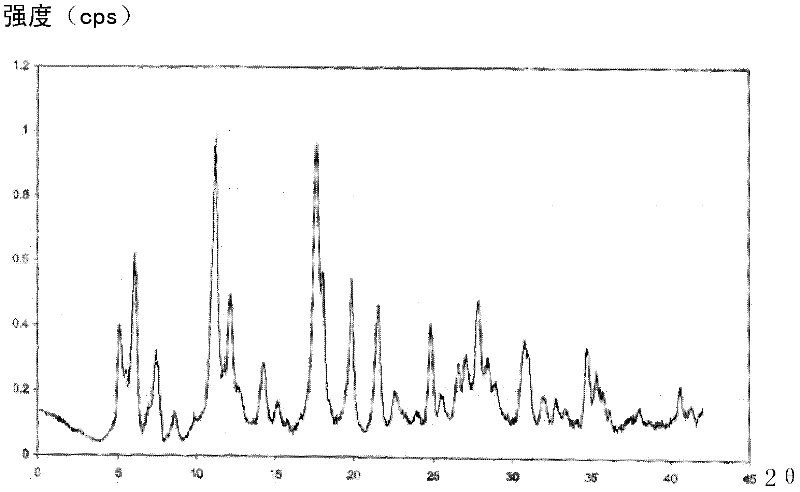
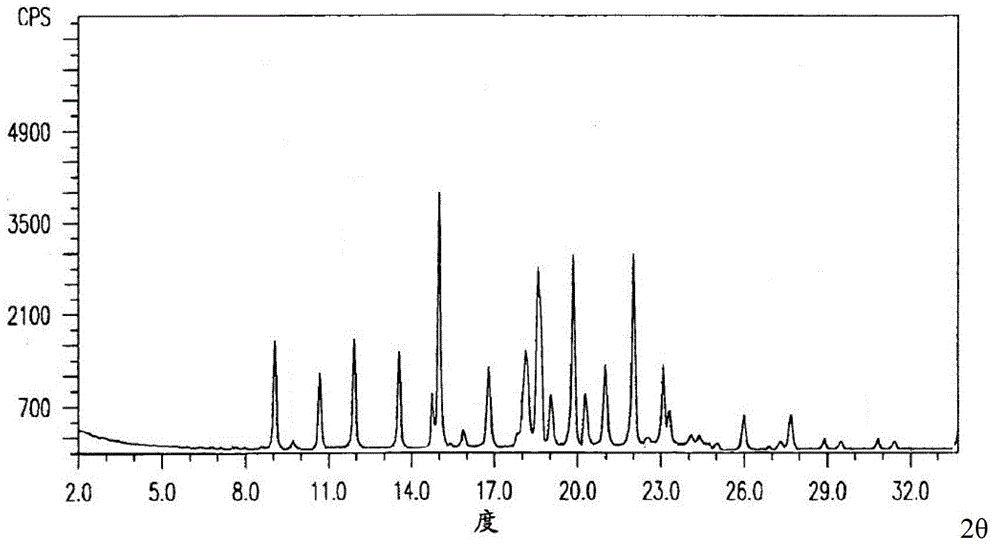
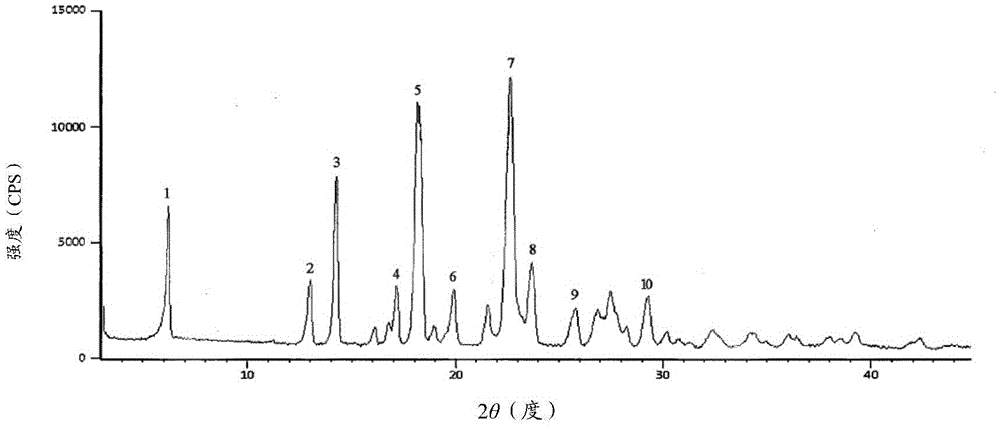
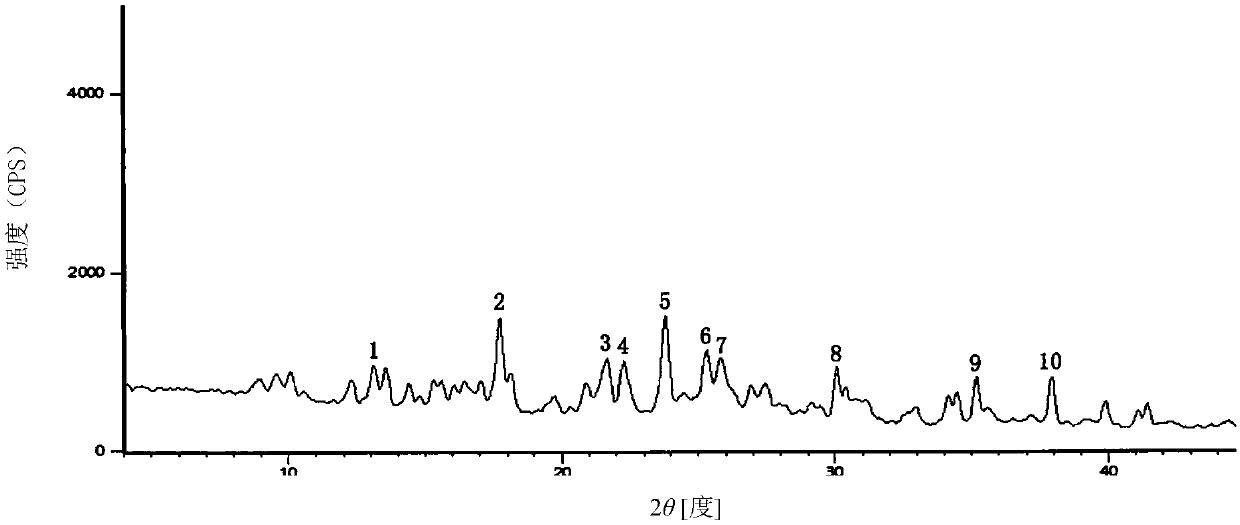
The invention relates to a cefmenoxime hydrochloride compound used for injection which is a crystal. X-ray powder obtained through Cu-K alpha ray measurement is diffracted at a 2 theta of 6.0 degree, 7.4 degree, 11.0 degree, 12.2 degree, 17.5 degree, 19.8 degree, 21.6 degree, 24.8 degree and 27.7 degree with characteristic peaks shown. The main granularity of the cefmenoxime hydrochloride crystal is 30-45 Mum and the distribution width of the cefmenoxime hydrochloride crystal is 25-75 Mum. Injections prepared through the cefmenoxime hydrochloride compound not only are rapid to dissolve and applicable to clinical application, but also have excellent stability, and are safe and reliable.
Owner:桂林澳林制药有限责任公司
Preparation method of cefmenoxime hydrochloride
ActiveCN102167705AResidue reductionShorten production timeOrganic chemistryCarboxylic acidCefmenoxime Hydrochloride
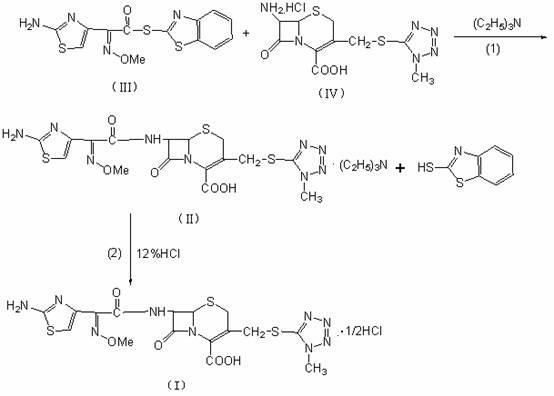
The invention relates to a novel preparation method of cefmenoxime hydrochloride, which comprises the steps of taking 7-ATCA.HCl as a starting raw material, performing acylation reaction with AE-active ester to produce 7-(alpha-(2- aminothiazole-4-group)-Z-2-methoxy imino group acetamido)-3-cephem-4-carboxylic acid, and finally reacting with hydrochloric acid to produce the cefmenoxime hydrochloride. The method is simple to operate, has high yield, greatly shortens technology time, and is extremely good for indusial production. The cefmenoxime hydrochloride prepared by the method is stable inquality, and the color grade and the impurity level of the cefmenoxime hydrochloride prepared by the method are better than those of cefmenoxime hydrochloride prepared by other methods.
Owner:苏州盛达药业有限公司
Cefmenoxime hydrochloride composition for injection and preparation thereof
ActiveCN102499922AParticle stabilityPromote generationAntibacterial agentsOrganic active ingredientsCrystal size distributionK-alpha
The invention relates to a cefmenoxime hydrochloride composition for injection which consists of 10 parts by weight of cefmenoxime hydrochloride and 1.5 to 2.5 parts by weight of anhydrous sodium carbonate, preferably 10 parts by weight of cefmenoxime hydrochloride and 1.75 to 1.8 parts by weight of anhydrous sodium carbonate. The cefmenoxime hydrochloride is crystal, having characteristic peaks at the diffraction angles 2 theta of 6.0 degrees, 7.4 degrees, 11.0 degrees, 12.2 degrees, 17.5 degrees, 19.8 degrees, 21.6 degrees, 24.8 degrees and 27.7 degrees in the X-ray powder diffraction measured using a Cu K-alpha ray. The main crystal size of cefmenoxime hydrochloride is 30 to 45 microns and the crystal size distribution width is 25 to 75 microns. The cefmenoxime hydrochloride composition of the invention is in the dosage form of injection which is dissolved quickly, suitable for clinic application, good in stability and reliable in safety.
Owner:桂林澳林制药有限责任公司
Cefmenoxime hydrochloride compound for injection and pharmaceutical composition thereof
InactiveCN103145735AImprove stabilityAntibacterial agentsOrganic active ingredientsChemical compoundCefmenoxime Hydrochloride
The invention relates to a cefmenoxime hydrochloride compound for injection. The cefmenoxime hydrochloride compound is a crystal; an X-ray powder diffraction pattern measured by Cu-Kalpha rays is shown in a figure 1; and the crystal main granularity of the hydrochloric cefmenoxime is 195-230 microns, and the distribution width is between 150 and 280 microns. The invention also relates to a pharmaceutical composition of the cefmenoxime hydrochloride compound, which comprises 10 parts by weight of hydrochloric cefmenoxime crystal, and 1.5-2.0 parts by weight of anhydrous sodium carbonate, preferably 10 parts by weight of the hydrochloric cefmenoxime crystal and 1.75 parts by weight of anhydrous sodium carbonate. The injection prepared by using the cefmenoxime hydrochloride compound is quickly dissolved, and is good in flowability, good in stability and safe and reliable in clinical application.
Owner:四川省惠达药业有限公司
Method for preparing cefmenoxime hydrochloride
InactiveCN102675344AHigh yieldHigh purityOrganic chemistryMethylene DichlorideCefmenoxime Hydrochloride
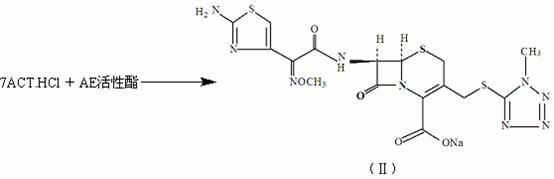
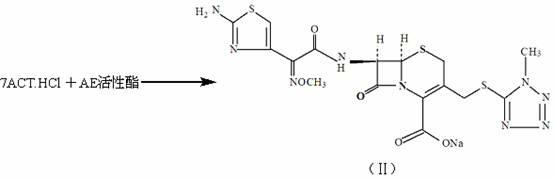
The invention discloses a method for preparing cefmenoxime hydrochloride, which comprises the following steps: uniformly mixing 7-ACT hydrochloride shown in a formula II and AE active ester shown in a formula III under the condition that methylene dichloride and a solvent exist for condensation reaction and obtaining the cefmenoxime hydrochloride shown in a formula I after reaction. The method has the benefits that the cefmenoxime hydrochloride can be synthesized in one step, the cefmenoxime hydrochloride is not required to be separated, a reversely-dropping crystallization method is adopted, the synthetic line is short, the cost is low, the reaction conditions are wild, and the production is convenient; and the cefmenoxime hydrochloride product is high in yield and purity and low in color grade and has an important application value.
Owner:SHANDONG LUKANG PHARMA
Method for preparing sterile cefmenoxime hydrochloride compound
The invention discloses a method for preparing sterile cefmenoxime hydrochloride compound. The method includes: using 7-ACT.HCL which can be easily purchased at market as initial raw materials to be in condensation reaction with AE (activity ester) to generate 7-[alpha-(2-aminothianole-4-thiazolyl)-Z-2-methoxy-iminoacetamido]-3-(1-methyl-1H-5-tetrazolyl-sulfur methyl)-3-cephem-4-carboxylic acid (namely cefmenoxime sodium salt) which is further in action with 10% hydrochloric acid to generate cefmenoxime hydrochloride. The method is simple in operation, high in yield, cost-saving and stable in quality.
Owner:HARBIN PHARMA GRP CO LTD GENERAL PHARMA FACTORY
Cefmenoxime hydrochloride/anhydrous sodium carbonate pharmaceutical composition liposome injection
InactiveCN101785758ATargetedGood clinical effectAntibacterial agentsOrganic active ingredientsCefmenoxime HydrochlorideSodium carbonate anhydrous
The invention provides a cefmenoxime hydrochloride / anhydrous sodium carbonate pharmaceutical composition liposome injection and a preparation method thereof. The cefmenoxime hydrochloride / anhydrous sodium carbonate pharmaceutical composition comprises the following components according to parts by weight: 1 part of cefmenoxime hydrochloride, 0.36-0.44 part of anhydrous sodium carbonate, 1-3 parts of liposome matrix and 0-1 part of additive. The prepared preparation has better stability and ensures medication safety.
Owner:HAINAN LINGKANG PHARMA CO LTD
Medicinal composition containing injection cefmenoxime hydrochloride and compound amino acid injection
InactiveCN103083322AImprove bioavailabilityIncrease profitAntibacterial agentsOrganic active ingredientsAmino Acid InjectionBioavailability
The invention relates to a medicinal composition containing injection cefmenoxime hydrochloride and a compound amino acid injection, and especially relates to a medicinal composition packaged in a combination manner and comprising injection cefmenoxime hydrochloride and the compound amino acid injection. When the medicinal composition is used, the injection cefmenoxime hydrochloride is added to the compound amino acid injection, and the medicinal composition is intravenously dripped for 30min-2h. Compared with a compatible mixture of the injection cefmenoxime hydrochloride and the compound amino acid injection, the combination packaged medicinal composition allows the steps to be simplifies, the safety to be improved, the stability of cefmenoxime hydrochloride to be large and the clinical application quality and the bioavailability of the medicinal composition to be improved.
Owner:海南路易丹尼生物科技有限公司
Preparation method of cefmenoxine hydrochloride dry powder
InactiveCN103554136AImprove liquidityCrystal sinkOrganic chemistryActivated carbonPhysical chemistry
The invention relates to a preparation method of cefmenoxine hydrochloride dry powder. The method comprises the following steps: 1) adding cefmenoxine hydrochloride into water, adjusting by using water containing soda until the cefmenoxine hydrochloride is dissolved, adding active carbon for decoloring, and filtering; 2) adding methanol into a filtrate, adding acid to adjust the PH value to 0.5-4.0, and separating out crystal; 3) heating to 30-45 DEG C, growing the grain for 1-30 minutes, cooling to 15-29 DEG C, growing the grain for 1-30 minutes, cooling to 0-14 DEG C, growing the grain for 1-3 hours, filtering and drying, so as to obtain the cefmenoxine hydrochloride dry powder.
Owner:HARBIN PHARMA GRP CO LTD GENERAL PHARMA FACTORY
Method for synthesizing cefmenoxime hydrochloride
InactiveCN104447798AEasy to recycleImprove conversion rateOrganic chemistryCefmenoxime HydrochlorideHydrochloride
The invention discloses a method for synthesizing cefmenoxime hydrochloride. The method comprises the following steps: dissolving 7-ATCA or 7-ATCA hydrochloride and AE-active ester in dichloromethane, and dropwise adding organic alkali at -5-15 DEG C; adding water to extract for multiple times after the reaction is completed, decolorizing the water phase, adding a hydrophilic solvent, and dropwise adding hydrochloric acid at 10-35 DEG C to crystallize and separate cefmenoxime hydrochloride. According to the method for synthesizing cefmenoxime hydrochloride, the condensation system reacts by adopting a single solvent with high conversion rate, and the solvent is convenient to reclaim; the method for synthesizing cefmenoxime hydrochloride is direct crystallization and separation, and adsorption and other separation methods are not needed, so that cefmenoxime hydrochloride has good crystalline form, is easy to separate and dry, simple in synthesizing route and low in cost, and is suitable for industrial production.
Owner:苏州盛达药业有限公司
Cefmenoxime hydrochloride compound entity used for children and preparation thereof
InactiveCN105001239AIncrease internal pressureImprove solubilityPowder deliveryOrganic chemistryTemperature control modeStructural formula
The invention provides a cefmenoxime hydrochloride compound entity used for children. The structural formula is as shown in specifications. The cefmenoxime hydrochloride compound entity is prepared through the steps that step1, water is added into a cefmenoxime hydrochloride crude product, sodium carbonate is added, stirring is performed for dissolving, an extracting agent is added, and then the cefmenoxime hydrochloride crude product is transferred into a pressure-resistant container in a filling mode, vibrated in a sealed mode after being defoamed and taken out after being frozen in a temperature control mode; step2, an organic phase is removed, after a solid is melted, active carbon is added, stirring is performed for discoloring, and filtering is performed; step3, pH of filtrate is adjusted to 1.5-2.3 through hydrochloride acid, and then crystal growing, filtering, washing with water and vacuum drying at 40 DEG C are performed to obtain the cefmenoxime hydrochloride compound entity. The cefmenoxime hydrochloride prepared through the method has the advantages of having few impurities, being high in purity and the like compared with a traditional technology.
Owner:ZHEJIANG CHANGDIAN PHARMA
New crystal form cefmenoxine hydrochloride compound prepared by adopting particle process crystal product molecular assembling and morphology optimizing technology and preparation
InactiveCN105566352AHigh purityImprove color gradeAntibacterial agentsOrganic active ingredientsCefmenoxime HydrochlorideHydrochloride
The invention discloses a new crystal form cefmenoxine hydrochloride compound and a crystallization preparation method thereof. The new crystal form cefmenoxine hydrochloride compound is prepared by adopting the particle process crystal product molecular assembling and morphology optimizing technology. The compound has the advantages of being high in purity and good in fluidity and stability. The invention also discloses a preparation which is prepared from the cefmenoxine hydrochloride compound, namely cefmenoxine hydrochloride for injection.
Owner:HAINAN LINGKANG PHARMA CO LTD +1
Cefmenoxime hydrochloride compound and synthesizing method thereof
ActiveCN102731531AQuality assuranceHigh purityOrganic chemistryCefmenoxime HydrochlorideMedicinal chemistry
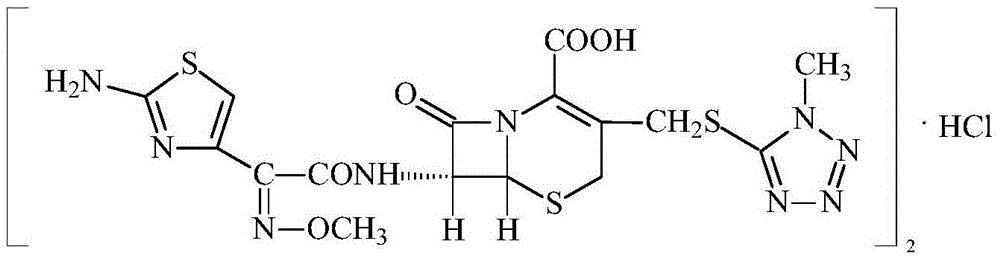
The invention relates to the field of pharmacy, and especially related to a cefmenoxime hydrochloride compound and a synthesizing method thereof. The cefmenoxime hydrochloride compound has a formula shown below, and is prepared with a method comprising the steps that: (1) a cefoperazone precursor 7-ATCA.HCl and AE active ester are subjected to a condensation reaction under the existence of CH2Cl2and an alkalizing agent; and the obtained product is subjected to extraction, decolorization, press-filtration, neutralization, rejection filtration, and drying, such that cefmenoxime acid CMX-H is prepared; (2) the cefmenoxime acid is completely dissolved by using sodium carbonate; the obtained product is subjected to decolorization, press-filtration, neutralization, decolorization, filtration, crystallization, washing, and drying, such that a cefmenoxime hydrochloride half-finished product is prepared; a jet mill is started; and the material is crushed and is filled in aluminum bottles. According to the invention, cefmenoxime acid is prepared into crystals, such that the product purity is greatly improved, and the product quality is ensured. Also, the method provided by the invention is advantaged in small three-waste volume, simple preparation process, and low cost. Therefore, the method is suitable for the industrialized productions of our nation.
Owner:ZHEJIANG JIANFENG PHARM CO LTD
Micro-balloon injection for pharmaceutical composition of cefmenoxime hydrochloride / anhydrous sodium carbonate
InactiveCN101822644AImprove stabilityHigh encapsulation efficiencyAntibacterial agentsOrganic active ingredientsMicrospherePolyethylene glycol
The invention discloses a micro-balloon injection for a pharmaceutical composition of cefmenoxime hydrochloride / anhydrous sodium carbonate, which is characterized by comprising cefmenoxime hydrochloride, anhydrous sodium carbonate, polylactic acid- polyethylene glycol block copolymer, poloxamer 188, glycerol and mannitol. The optimal scheme of the invention is the micro-balloon injection for the pharmaceutical composition of cefmenoxime hydrochloride / anhydrous sodium carbonate, which is characterized by comprising 1 part of cefmenoxime hydrochloride, 0.12-0.18 part of anhydrous sodium carbonate, 1.2-4.5 parts of polylactic acid- polyethylene glycol block copolymer, 0.8-2 part(s) of poloxamer 188, 0.5-1 part of glycerol and 3-6 parts of mannitol. Compared with the prior art, the micro-balloon injection for the pharmaceutical composition of cefmenoxime hydrochloride / anhydrous sodium carbonate prepared by the invention has high stability, the preparation process is simple and is suitable for industrial production, and has high encapsulation efficiency. As anhydrous sodium carbonate is used as a latent solvent, the re-dissolution is good, and has an appropriate slow-release effect.
Owner:HAINAN LINGKANG PHARMA CO LTD
Method for preparing cefmenoxime hydrochloride freeze-dried powder injection
InactiveCN101721378AImprove sterilityImprove stabilityAntibacterial agentsOrganic active ingredientsActivated carbonArginine
The invention discloses a method for preparing a cefmenoxime hydrochloride freeze-dried powder injection, which comprises the following steps of: dissolving cefmenoxime hydrochloride and arginine together; quickly cooling and freezing solution decolorized by activated carbon in a freeze dryer; vacuumizing; and gradually raising the temperature to room temperature. The obtained cefmenoxime hydrochloride freeze-dried powder injection has the advantages of over 98 percent of average yield, less than 0.5 percent of moisture content, stable medicament quality and full appearance.
Owner:海南澳合医药有限公司
Anti-infective medicinal composition for injection and preparation method thereof
InactiveCN101816660AImprove securityImprove solubilityAntibacterial agentsPowder deliveryOptimal weightDissolution
The invention belongs to the technical field of medicaments, and discloses an anti-infective medicinal composition of cefmenoxime hydrochloride, Na2HPO4 / NaH2PO4 buffer salt and PVPK-30 and a preparation method thereof. The weight ratio of the cefmenoxime hydrochloride to the Na2HPO4 / NaH2PO4 buffer salt to the PVPK-30 in the medicinal composition are 100 to 0.1-10 to 0.1-10; and the optimal weight ratio of the cefmenoxime hydrochloride to the Na2HPO4 / NaH2PO4 buffer salt to the PVPK-30 is 100 to 1 to 1. The medicinal composition solves the problems of poor dissolubility and stability of the cefmenoxime hydrochloride in the prior art, has stable characteristic, convenient storage and high dissolution velocity, and enhances the clinical medication safety.
Owner:深圳四环医药有限公司 +1
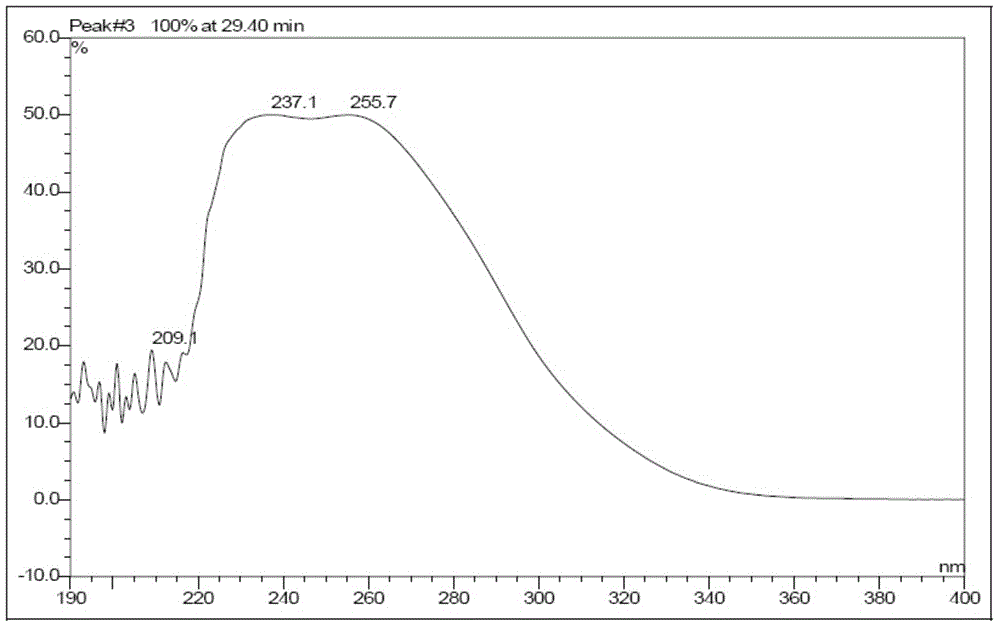
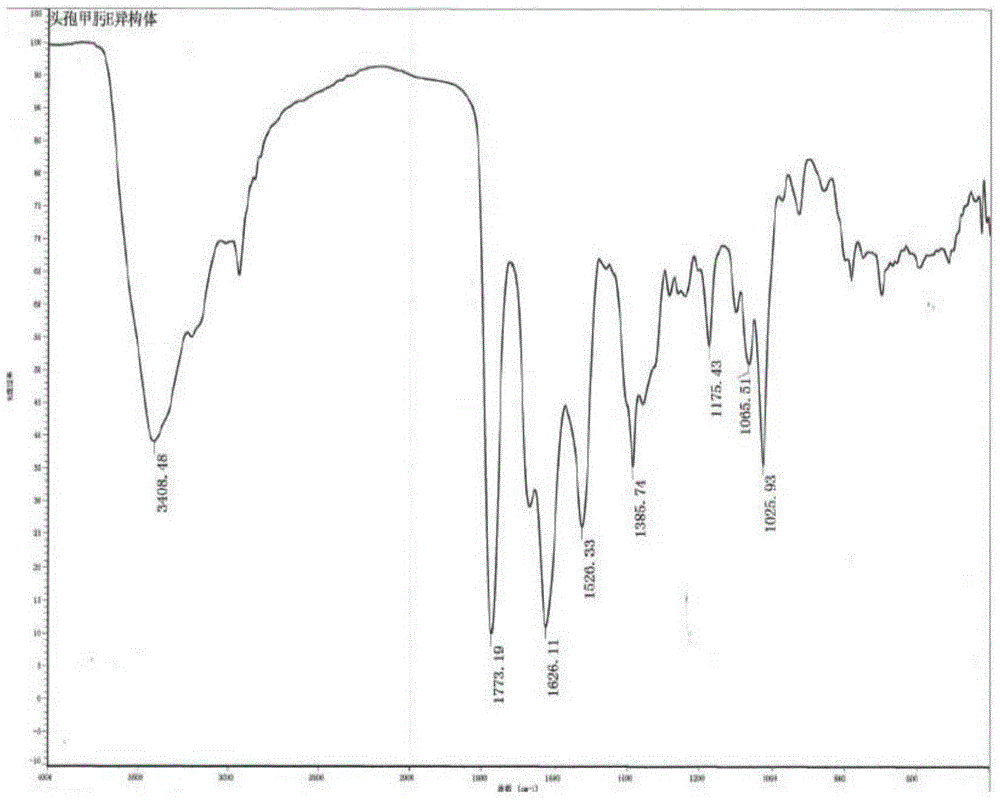
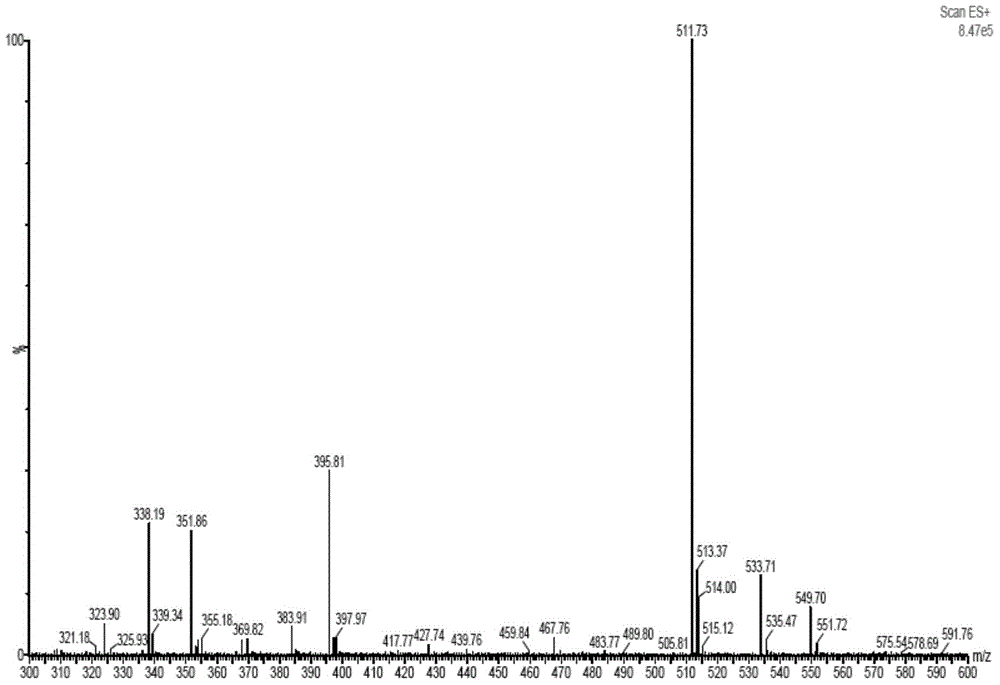
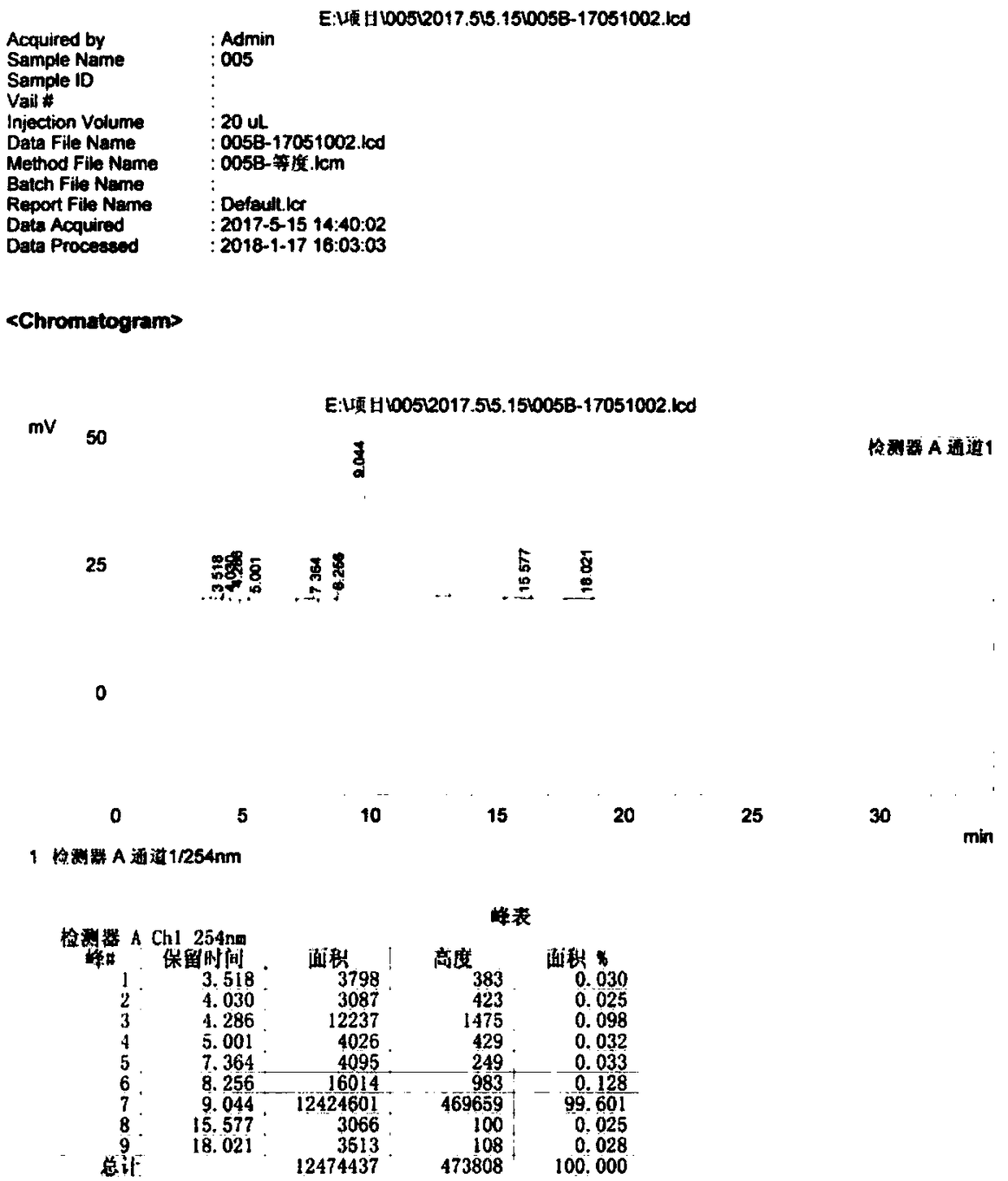
Method of preparing cefmenoxime E isomers
The invention relates to a method of preparing cefmenoxime E isomers and is applicable to the need of pharmaceutical enterprises. The method comprises the steps: step (1), suspending 3-[[(1-methyl-1H-tetrazole-5-yl) sulfur] methyl]-7-amino-carboxylate hydrochloride dehydrate (7-ATCA.HCl) and trans AE active ester in a mixed system of water and organic solvent, adding alkaline, stirring and reacting to generate the cefmenoxime E isomers, in which the molar rate of the 3-[[(1-methyl-1H-tetrazole-5-yl) sulfur] methyl]-7-amino-carboxylate hydrochloride dehydrate, the trans AE active ester and the alkaline is 1: 1.1-1.3: 2.5-2.8, and the reaction time is 1-3 hours; step (2) after the reaction is finished, passivating, regulating the pH value to be 2.0-3.0, crystallizing, filtering and drying in vacuum. The prepared cefmenoxime E isomers have high purity which is not less than 97%, and can be used as reference substances for quality control of cefmenoxime hydrochloride bulk drug and preparations thereof.
Owner:GUANGZHOU BAIYUSN TIANXIN PHARMA
Preparation method of cefmenoxime hydrochloride
ActiveCN108383857AReasonable temperature controlReduce degradationOrganic chemistryChemistryCefmenoxime Hydrochloride
The invention discloses a preparation method of cefmenoxime hydrochloride. The method includes the steps: performing trimethylsilyl protection on methoxyiminoacetic acid; performing carboxyl activation by the aid of activating reagents; performing reaction with 7-ATCA hydrochloride; performing acidification by the aid of hydrochloric acid, growing the grain in a cooling manner to obtain the cefmenoxime hydrochloride. The purity of the cefmenoxime hydrochloride prepared by the method is higher than 99.5%, and the mole yield of products is 88% or more. According to the method, raw materials arecheap and easy to obtain, the purity of the products is high, yield is high, and industrial production can be achieved.
Owner:山东四环药业股份有限公司
Medicinal composition of cefmenoxime hydrochloride
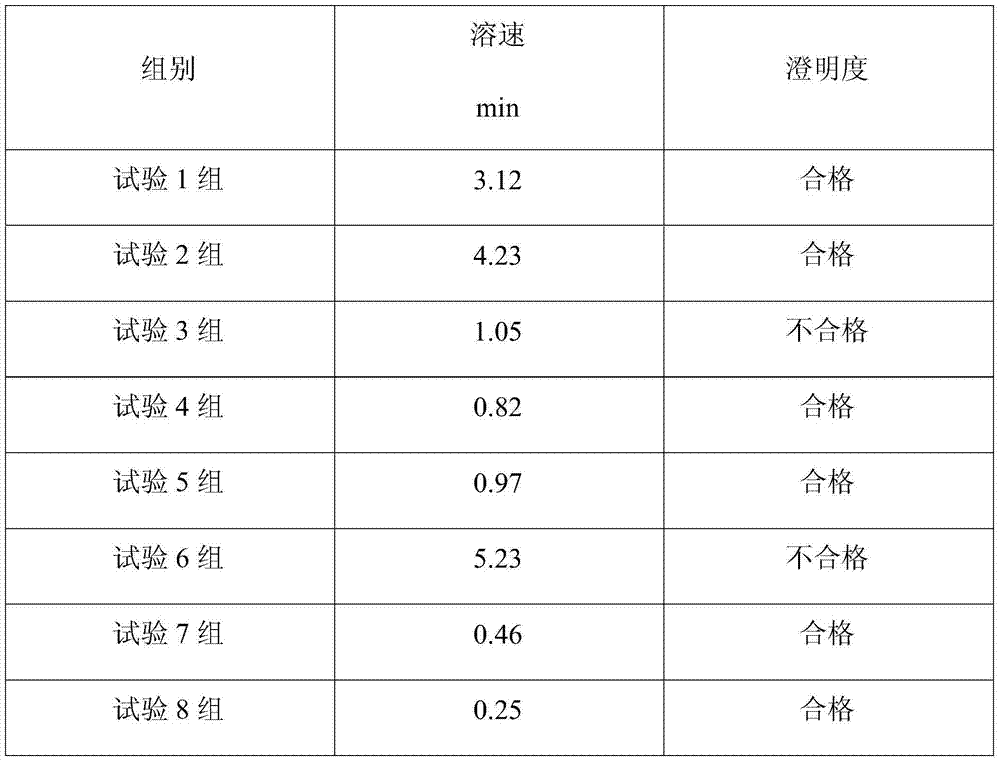
The invention belongs to the technical field of medicines, discloses a medicinal composition of cefmenoxime hydrochloride, and in particular relates to a medicinal composition containing cefmenoxime hydrochloride, sodium carbonate and ethylenediamine. The medicinal composition is obtained by a screening test, and dissolution speed tests, clarity and medicine stability tests indicate that a powder injection prepared from the medicinal composition is high in re-dissolution speed and has qualified clarity and small content of impurities and cefmenoxime polymer and high quality.
Owner:ZHEJIANG WHITESON PHARMA
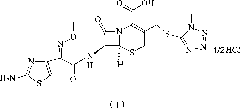
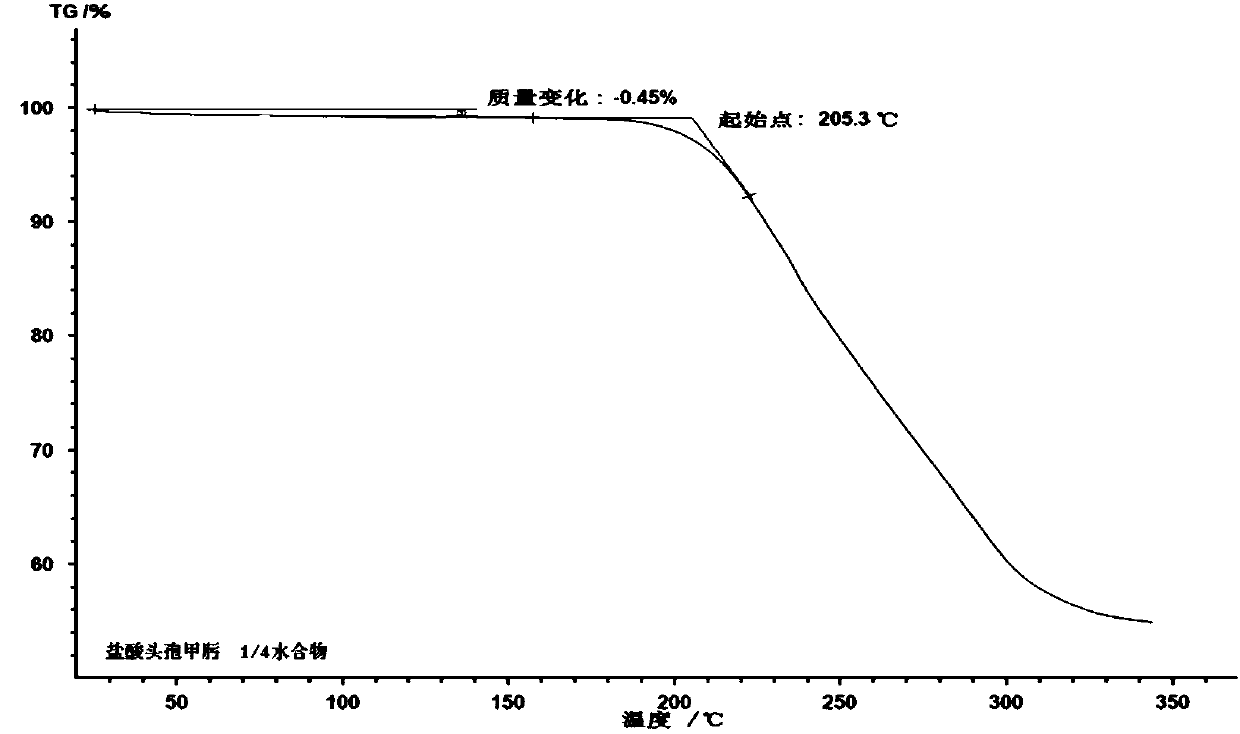
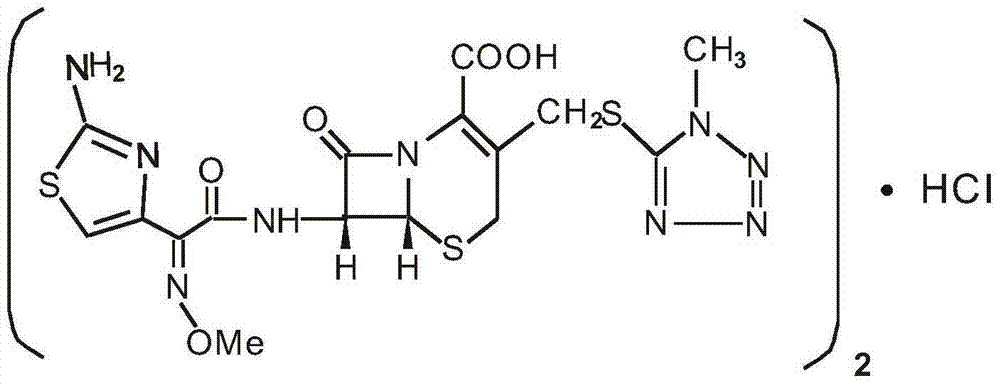
1/4 water cefmenoxime hydrochloride compound and pharmaceutical composition thereof
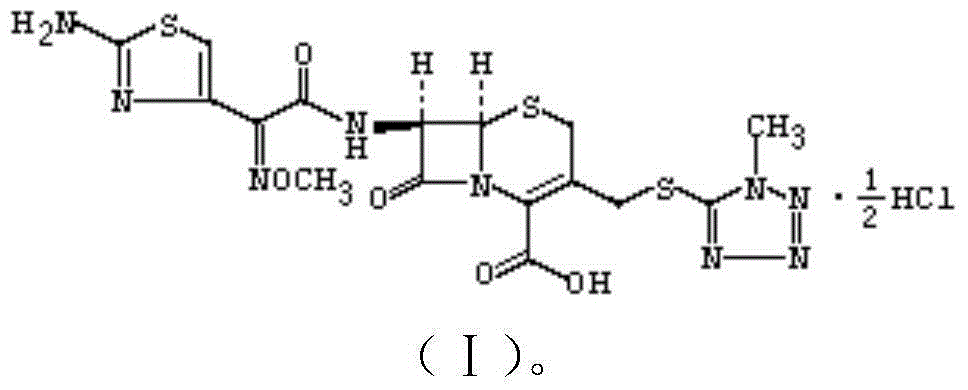
The invention discloses a 1 / 4 water cefmenoxime hydrochloride compound and a pharmaceutical composition thereof. Cefmenoxime hydrochloride per mole contains 1 / 4 mole of water. 7 amino cephalosporanicacid (7ACA) is taken as a starting raw material, is firstly reacted with 5-fluorenyl 1 methylol 1H-tetrazolium (MMT) at a C-3 position to form 3-(1-methyl-1-tetrazolium-5H-group) thiomethyl-7-aminocephalosporanic acid (7ACT), is reacted with MAEM in a C-7 position to synthesize cefmenoxime, and then is formed into salt with hydrochloric acid to obtain the 1 / 4 water cefmenoxime hydrochloride compound. The operation is simple, reactants are easily available, reaction conditions are mild, and the yield is high. The 1 / 4 water cefmenoxime hydrochloride compound has low moisture absorbing property,low impurity content, good fluidity, good thermodynamic stability and wide application prospect.
Owner:HAINAN LINGKANG PHARMA CO LTD
Method for preparing cefmenoxine hydrochloride raw material
The invention belongs to the technical field of medicines, and discloses a method for preparing a cefmenoxine hydrochloride raw material. The method comprises the following steps: enabling 7-ACT as a raw material to react with AE active ester, and adjusting with hydrochloric acid so as to generate a crude product of cefmenoxine hydrochloride; adding L-glutamic acid at normal temperature, heating, adding diethanol amine, cooling, and adding ethyl acetate, thereby obtaining a novel cefmenoxine hydrochloride raw material. By adopting the method disclosed by the invention, the content of macromolecule impurities in cefmenoxine hydrochloride is smaller than 0.1%, and the cefmenoxine hydrochloride raw material can be completely dissolved within 5 seconds.
Owner:ZHEJIANG WHITESON PHARMA
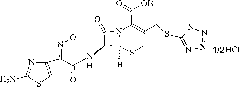
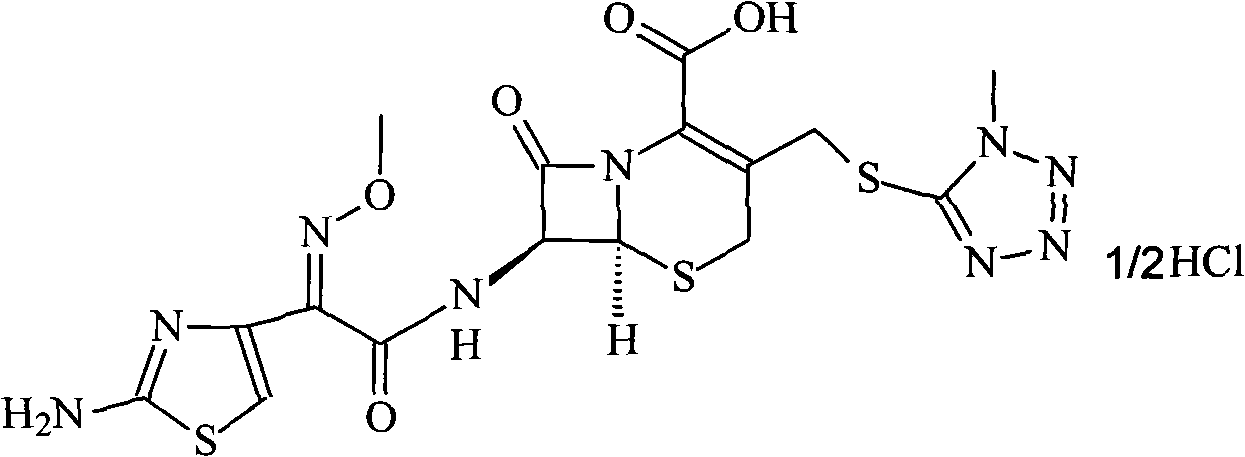
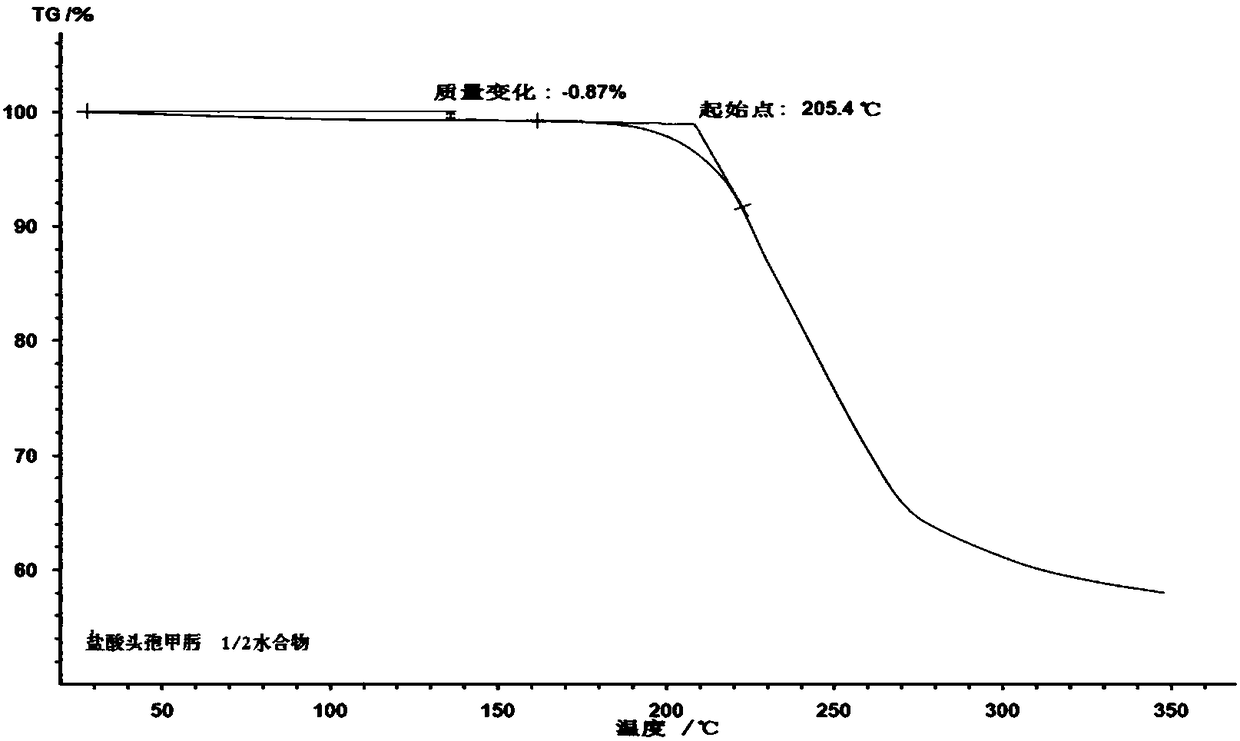
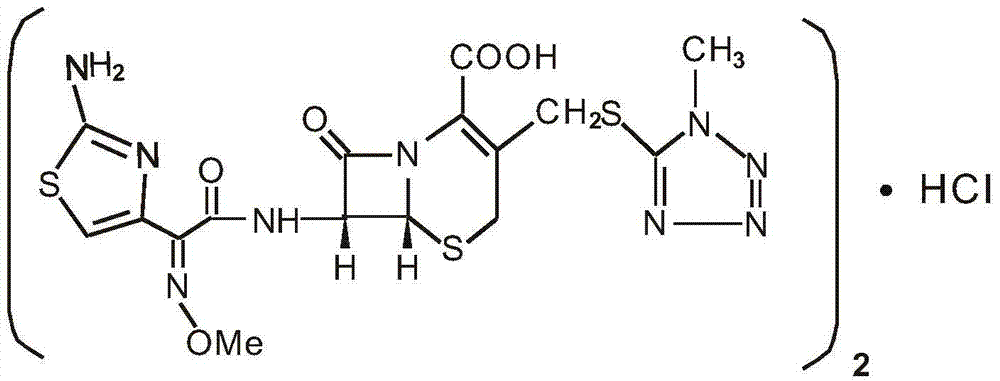
Cefmenoxime hydrochloride compound containing 1/2 of water and pharmaceutical composition preparation thereof
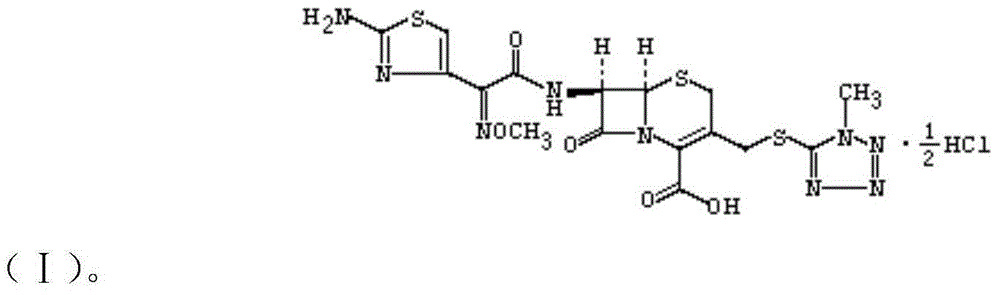
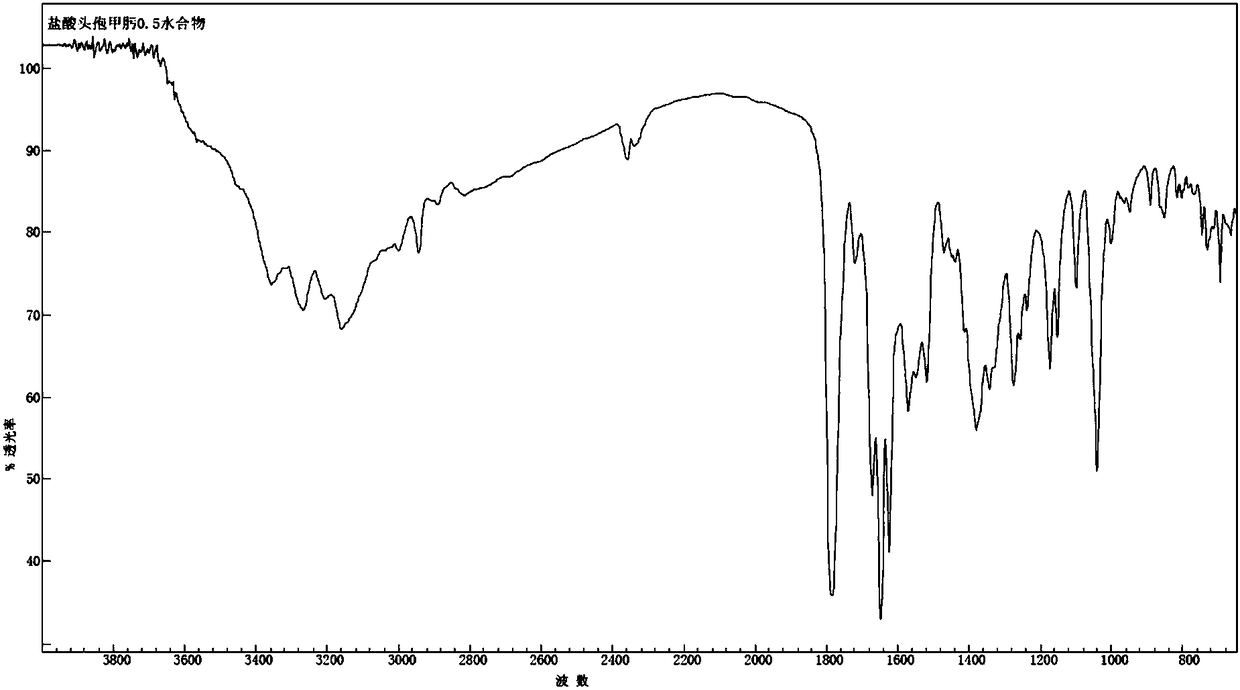
The invention discloses a cefmenoxime hydrochloride compound containing 1 / 2 of water and a pharmaceutical composition preparation thereof. Each mole of cefmenoxime hydrochloride contains 1 / 2 mole of water. 7-amino-cephalosporanic acid (7ACA) is taken as a starting material, firstly reacts with 5-sulfydryl-1-methyl-1H-tetrazole (MMT) to generate 3-(1-methyl-1-tetrazole-5H) methyl sulfide-7-amino-cephalosporanic acid (7ACT) at C-3 position, then reacts with MAEM to synthesize cefmenoxime at C-7 position, and then reacts with hydrochloric acid to form salt, so that the cefmenoxime hydrochloride compound containing 1 / 2 of water is obtained. The operation is simple, the reactants are obtained easily, the reaction conditions are mild, and the yield is high. The cefmenoxime hydrochloride compoundcontaining 1 / 2 of water has low hygroscopicity, low impurity content, good mobility, good thermodynamic stability and wider application prospects.
Owner:陈立平
Medicinal composition of cefmenoxime hydrochloride
Owner:ZHEJIANG WHITESON PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com