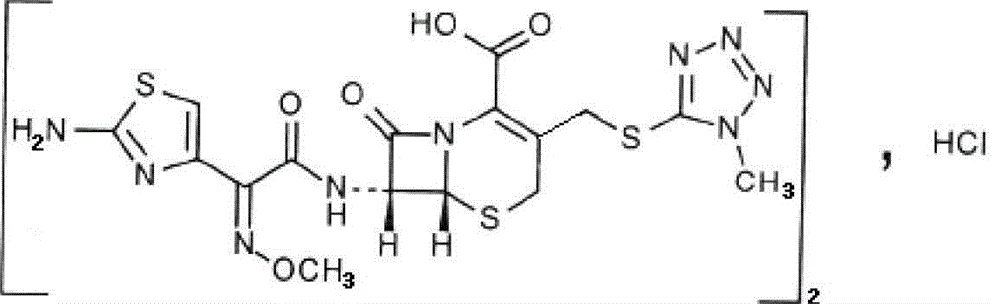
Cefmenoxime hydrochloride compound for injection and pharmaceutical composition thereof
A technology of cefmenoxime hydrochloride and its compound, which is applied in the field of cefmenoxime hydrochloride compound for injection and its pharmaceutical composition, can solve the problems of solubility and stability to be improved, and achieve high yield, good stability and high purity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
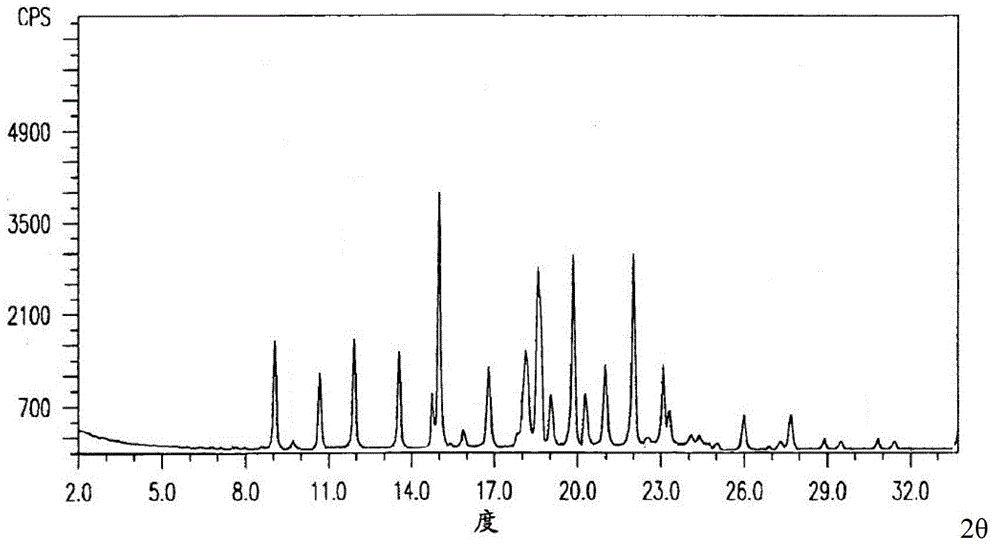
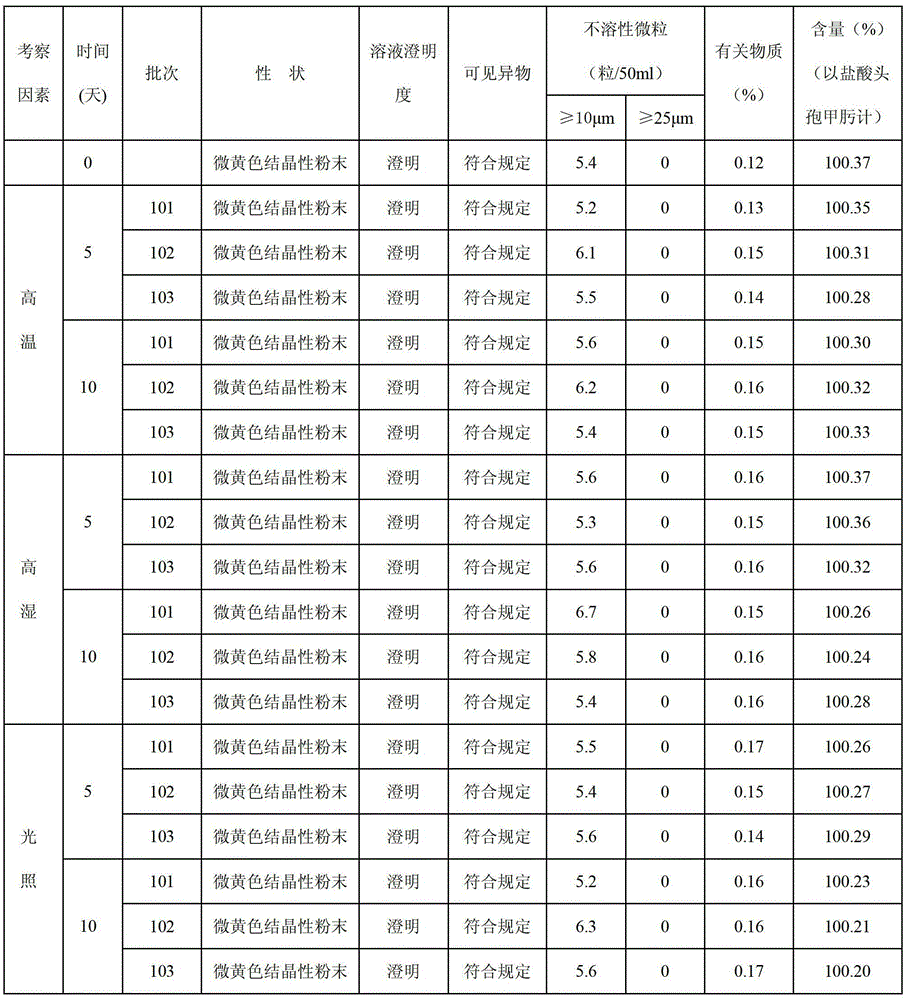
Image
Examples
Embodiment 1
[0041] The preparation of embodiment 1 cefmenoxime hydrochloride compound
[0042] (1) Take cefmenoxime hydrochloride solid, add water and stir to make a suspension, add 15% sodium hydroxide solution at 1 °C, stir and dissolve to obtain a clear liquid, then add a mixture of isopropanol and ethyl acetate solution; the volume ratio of isopropanol and ethyl acetate is 1:0.75; the volume ratio of the mixed solvent of isopropanol and ethyl acetate to the solid solution of cefmenoxime hydrochloride is 0.45:1;
[0043] (2) Add the clarified liquid to 0.01% activated carbon for decolorization, stir for 1 hour and then filter the filtrate;
[0044] (3) Heat the solution obtained in step (2) to 20°C, add hydrochloric acid at 1°C while stirring, adjust the pH value to 1.8, and stir at a speed of 90 rpm; stop stirring after adding the hydrochloric acid, and continue to cool the solution to 1° C., stand for crystal growth for 1 hour, obtain crystals, filter, wash, and vacuum-dry for 4 hou...
Embodiment 2
[0046] The preparation of embodiment 2 cefmenoxime hydrochloride compound
[0047] (1) Take cefmenoxime hydrochloride solid and add water to stir to make a suspension, add 15% sodium hydroxide solution at 0°C, stir and dissolve to obtain a clear liquid, then add a mixture of isopropanol and ethyl acetate solution; the volume ratio of isopropanol and ethyl acetate is 1:0.5; the volume ratio of the mixed solvent of isopropanol and ethyl acetate to the solid solution of cefmenoxime hydrochloride is 0.45:1;
[0048] (2) Add the clarified liquid to 0.01% activated carbon for decolorization, stir for 1.5 hours and then filter the filtrate;
[0049] (3) Heat the solution obtained in step (2) to 25°C, add hydrochloric acid at 1°C while stirring, adjust the pH value to 1.8, and stir at a speed of 120 rpm; stop stirring after adding the hydrochloric acid, and continue to cool the solution to 0° C., stand for crystal growth for 2 hours, obtain crystals, filter, wash, and vacuum-dry for ...
Embodiment 3
[0051] The preparation of embodiment 3 cefmenoxime hydrochloride compound
[0052] (1) Take cefmenoxime hydrochloride solid and add water and stir to make a suspension, add 15% sodium hydroxide solution at 5°C, stir and dissolve to obtain a clear liquid, then add isopropanol and ethyl acetate to mix solution; the volume ratio of isopropanol and ethyl acetate is 1:0.85; the volume ratio of the mixed solvent of isopropanol and ethyl acetate to the solid solution of cefmenoxime hydrochloride is 0.2:1;
[0053] (2) Add the clarified liquid to 0.01% activated carbon for decolorization, stir for 0.5 hours and then filter the filtrate;
[0054] (3) Heat the solution obtained in step (2) to 25°C, add hydrochloric acid at 5°C while stirring, adjust the pH value to 1.8, and stir at a speed of 120 rpm; stop stirring after adding the hydrochloric acid, and continue to cool the solution to 5° C., stand for crystal growth for 3 hours, obtain crystals, filter, wash, and vacuum-dry for 6 hou...
PUM
| Property | Measurement | Unit |
|---|---|---|
| width | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com