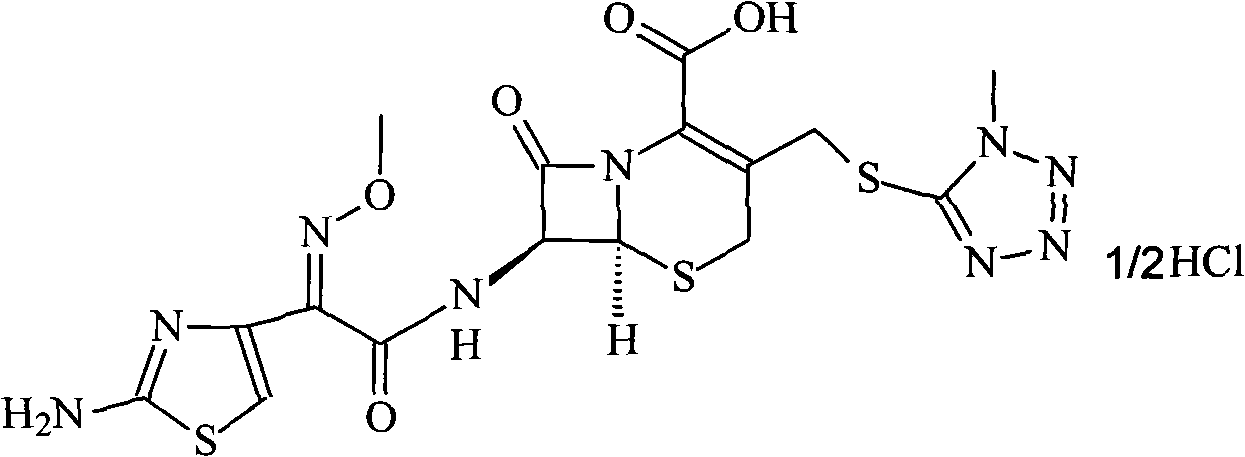
Cefmenoxime hydrochloride/anhydrous sodium carbonate pharmaceutical composition liposome injection
A technology of cefmenoxime hydrochloride and anhydrous sodium carbonate, applied in the field of medicine, can solve the problems of light, heat sensitivity, poor stability, influence drug safety, etc., and achieves short production cycle, improved drug safety, and increased in vitro stability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
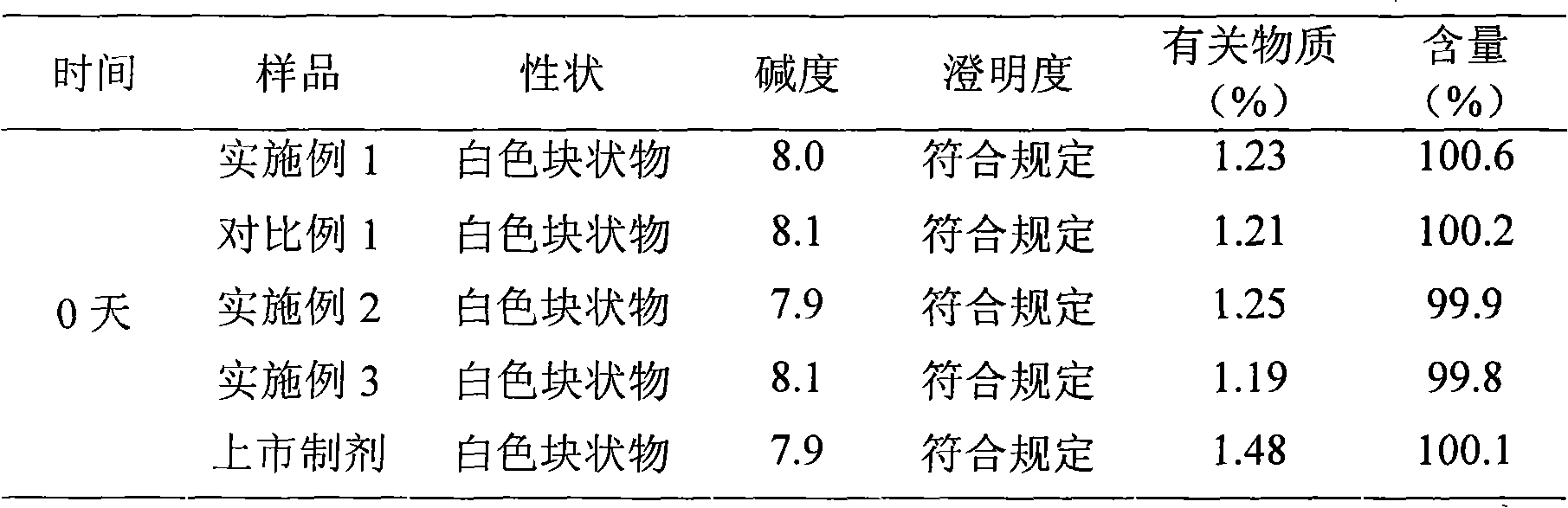
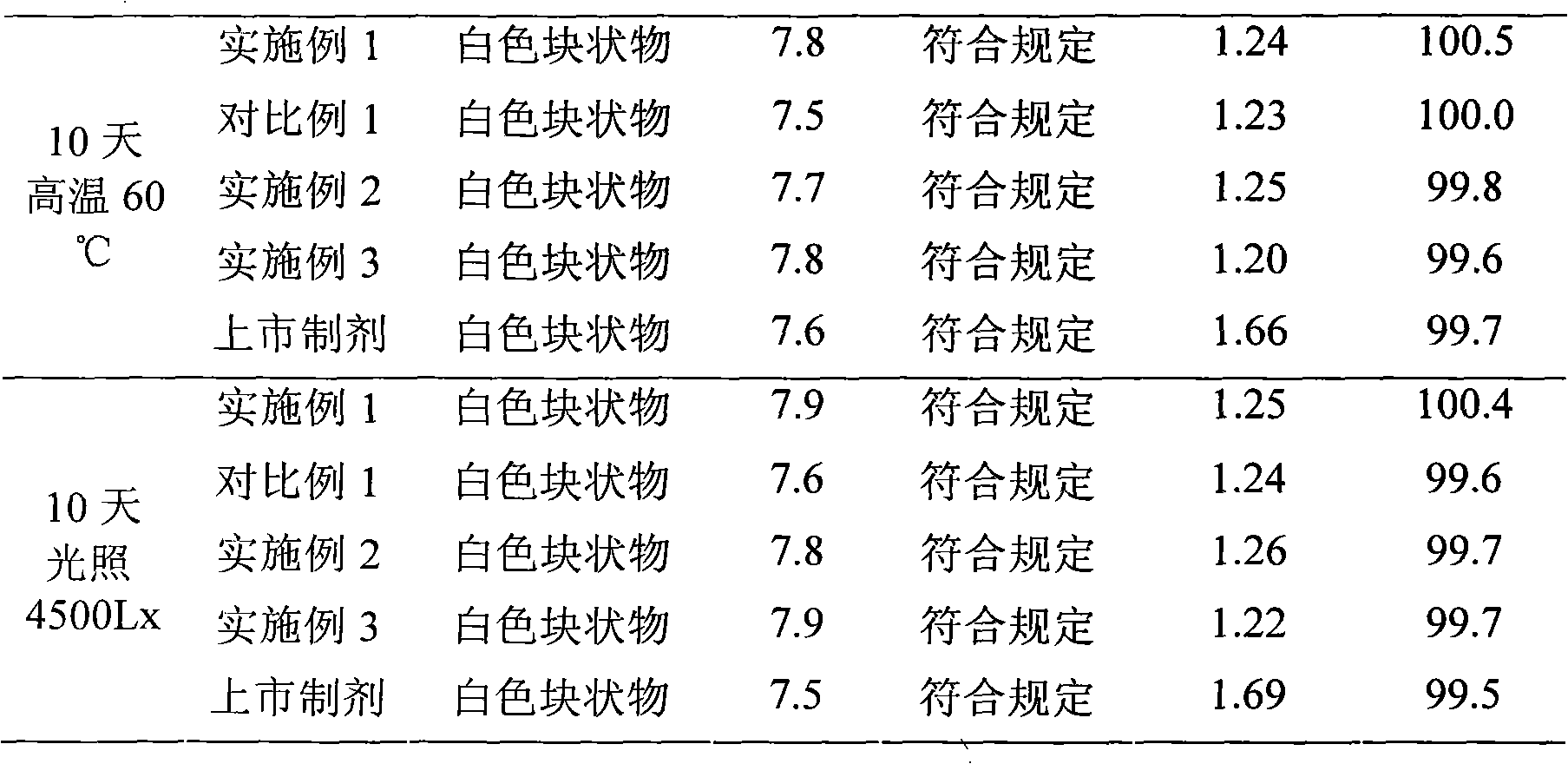
Embodiment 1
[0030] The preparation of embodiment one cefmenoxime hydrochloride liposome injection
[0031] Prescription (specification 0.05g)
[0032] Cefmenoxime Hydrochloride 5g
[0033] Anhydrous sodium carbonate 2g
[0034] Soy Lecithin 20g
[0035] Cholesterol 20g
[0036] Tween 80 10g
[0037] Isopropanol 300ml
[0038] Lactose 7g
[0040] Phosphate buffer 200ml
[0041] Preparation Process:
[0042] (1) 20g phospholipids, 20g cholesterol, 10g Tween 80 and 3g sodium sulfite are dissolved in 300ml isopropanol, mix uniformly, remove the organic solvent under reduced pressure on a rotary thin film evaporator, and obtain a phospholipid film;
[0043] (2) Add 200ml of sodium dihydrogen phosphate-disodium hydrogen phosphate buffer solution with a pH value of 6.3 to the prepared phospholipid film, shake and stir to completely hydrate the phospholipid film, high-speed homogeneous emulsification, and 0.45 μm microporous membrane Filter to obtain a blank l...
Embodiment 2
[0062] The preparation of embodiment two cefmenoxime hydrochloride liposome injection
[0063] Prescription (specification 0.1g)
[0064] Cefmenoxime Hydrochloride 10g
[0065] Anhydrous sodium carbonate 3.6g
[0067] Cholesterol 7g
[0068] Tween 80 28g
[0069] Ethanol 300ml
[0070] Mannitol 10g
[0071] Vitamin E 8g
[0072] Citrate buffer 200ml
[0073] Preparation Process:
[0074] (1) 35g egg yolk lecithin, 7g cholesterol, 28g Tween 80 and 8g vitamin E are dissolved in 300ml ethanol, mix uniformly, remove the organic solvent under reduced pressure on a rotary thin film evaporator, and obtain a phospholipid film;
[0075] (2) Add 200ml of citric acid-sodium citrate buffer solution with a pH value of 7 to the prepared phospholipid film, shake and stir to completely hydrate the phospholipid film, high-speed homogeneous emulsification, and filter with a 0.45um microporous membrane , to prepare a blank liposome suspension;
[0076] (...
Embodiment 3
[0078] The preparation of embodiment three cefmenoxime hydrochloride liposome injection
[0079] Prescription (specification 0.2g)
[0080] Cefmenoxime Hydrochloride 20g
[0081] Anhydrous sodium carbonate 8.8g
[0082] Soy Lecithin 18g
[0083] Cholesterol 27g
[0084] Tween 80 3.6g
[0085] Ethanol 200ml
[0086] Mannitol 10g
[0088] Phosphate buffer 200ml
[0089] Preparation Process:
[0090] (1) 18g soybean lecithin, 27g cholesterol, 3.6g Tween 80 and 6g sodium bisulfite are dissolved in 200ml ethanol, mix uniformly, remove the organic solvent under reduced pressure on a rotary thin film evaporator, and obtain a phospholipid film;
[0091] (2) Add sodium dihydrogen phosphate-disodium hydrogen phosphate buffer solution with a pH value of 5.0 to the prepared phospholipid film, shake and stir to completely hydrate the phospholipid film, high-speed homogeneous emulsification, and filter with a 0.45um microporous membrane , to prepare a ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com