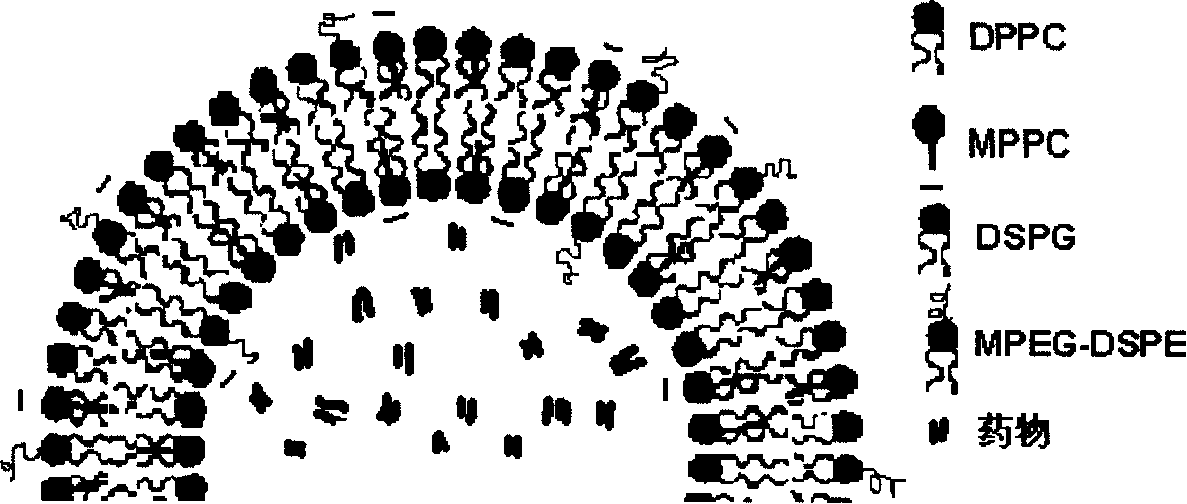
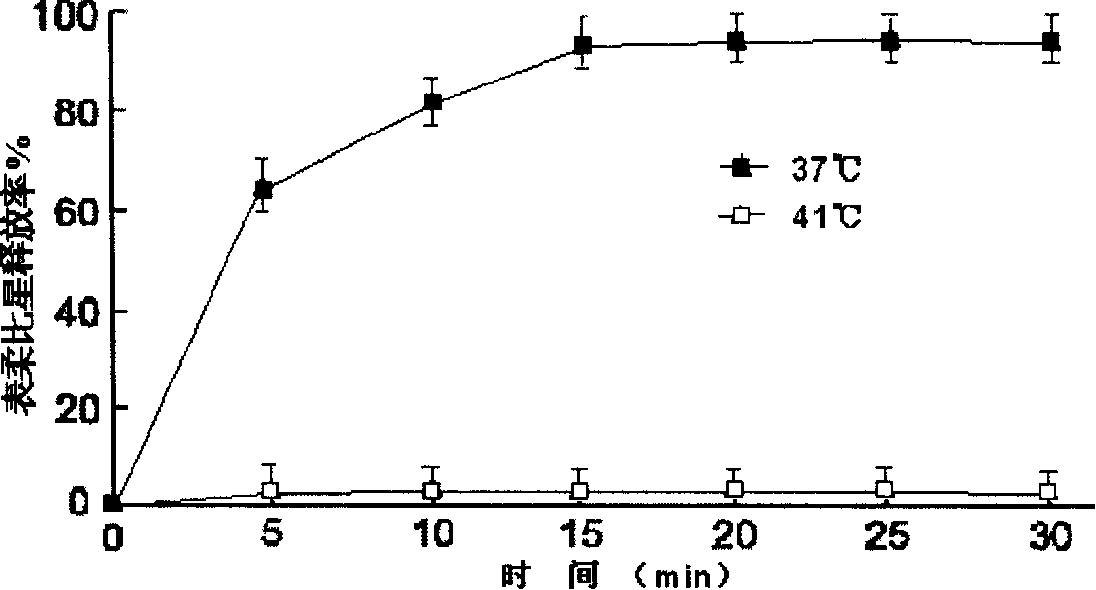
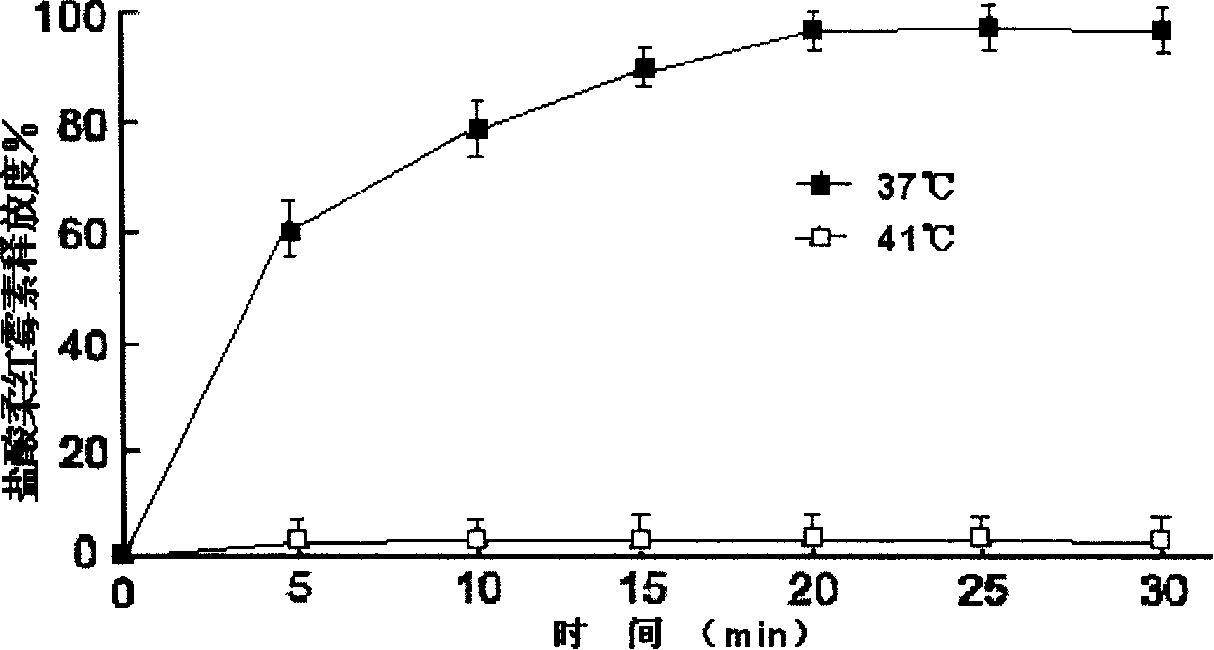
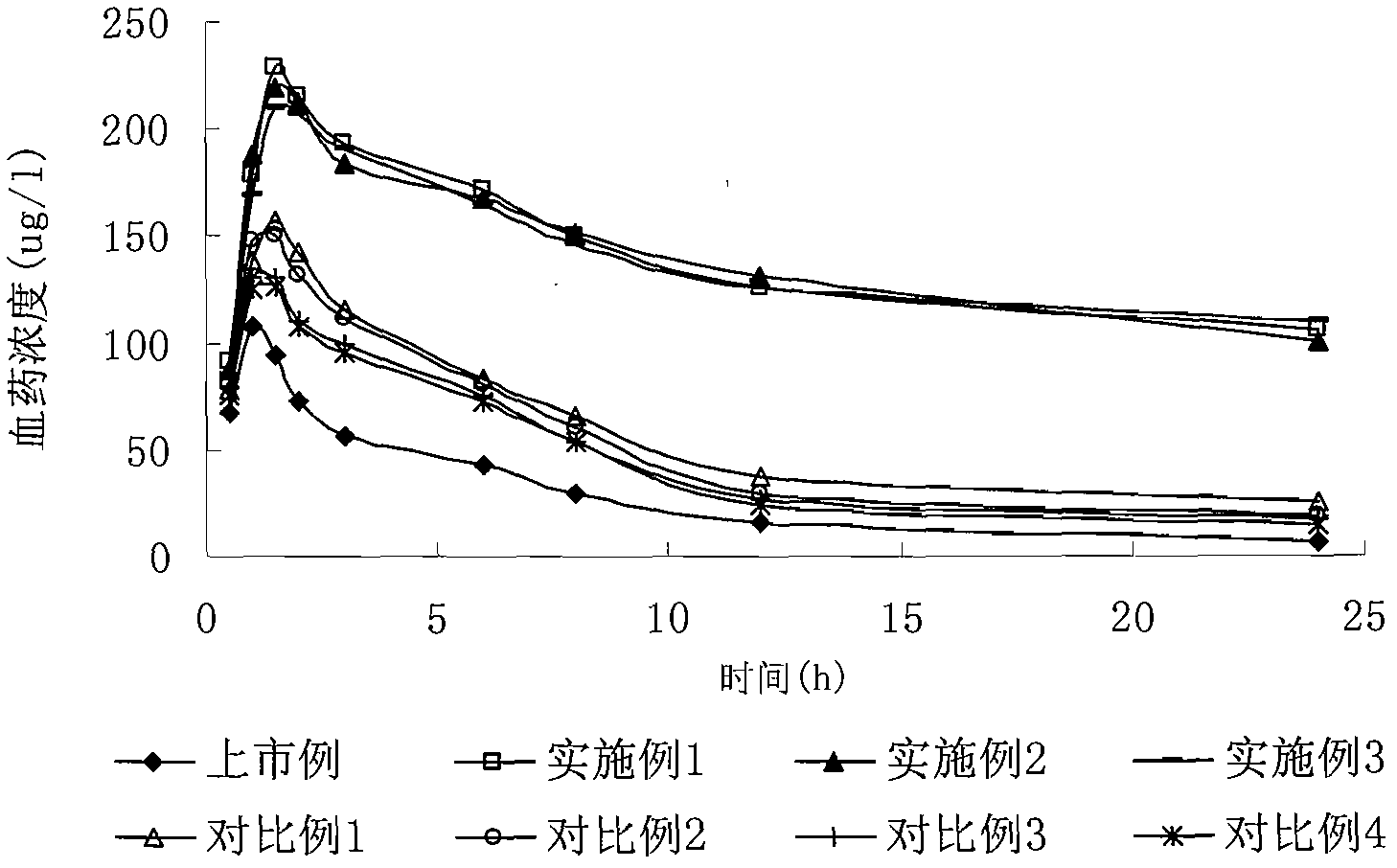
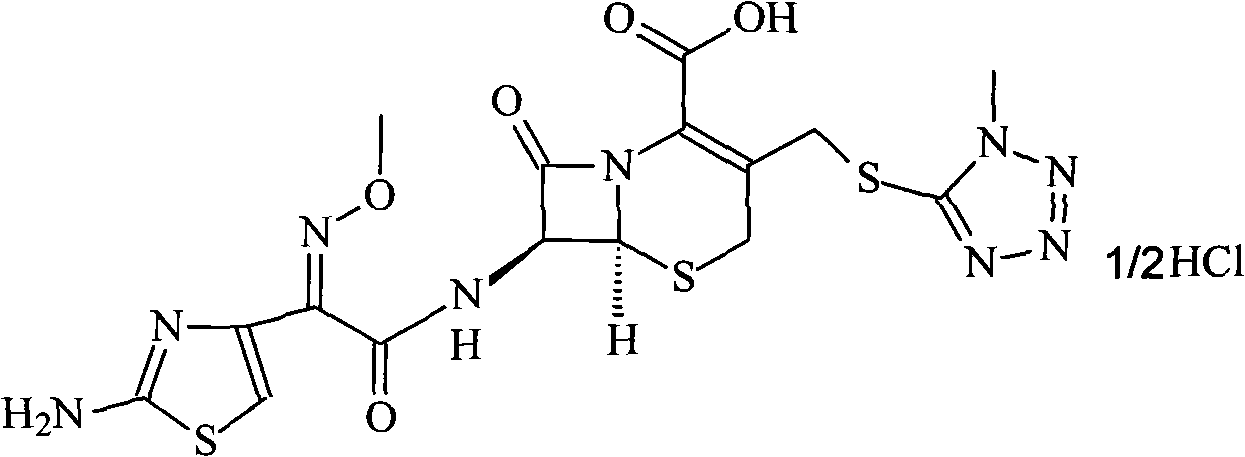
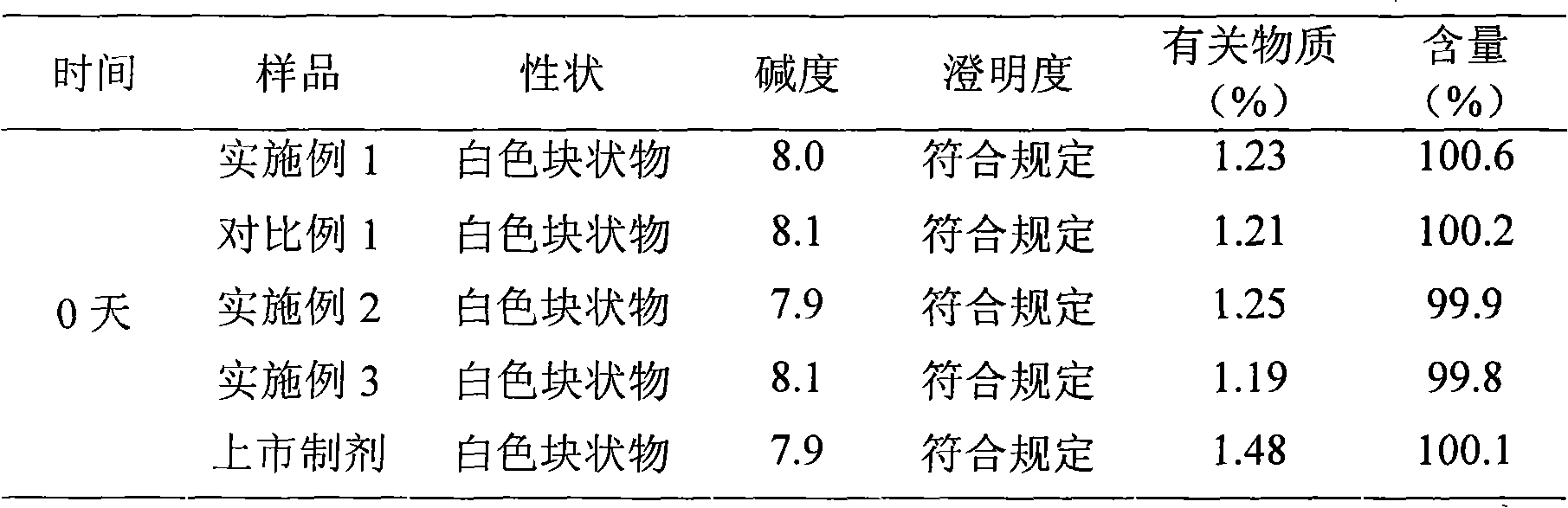
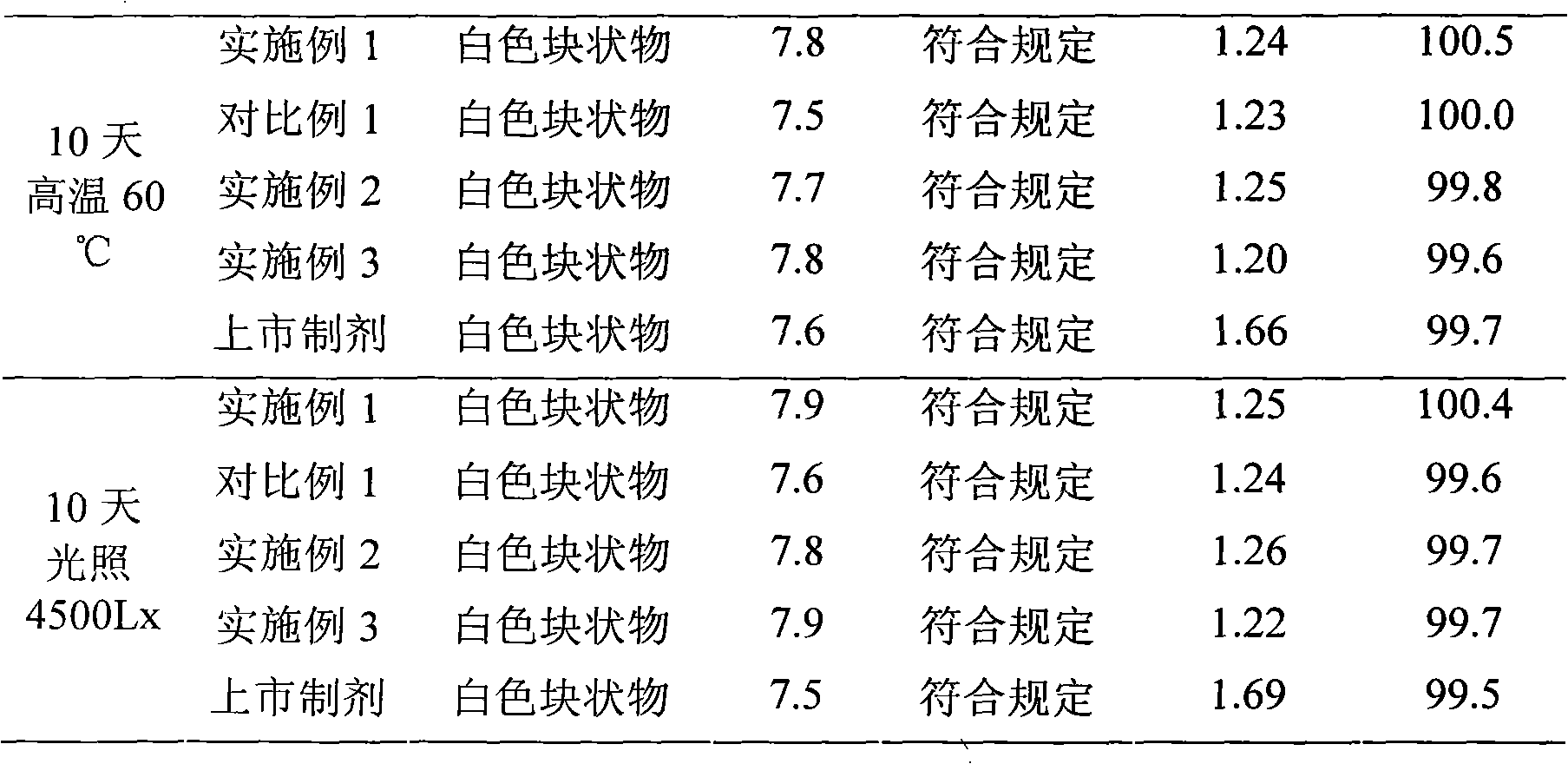
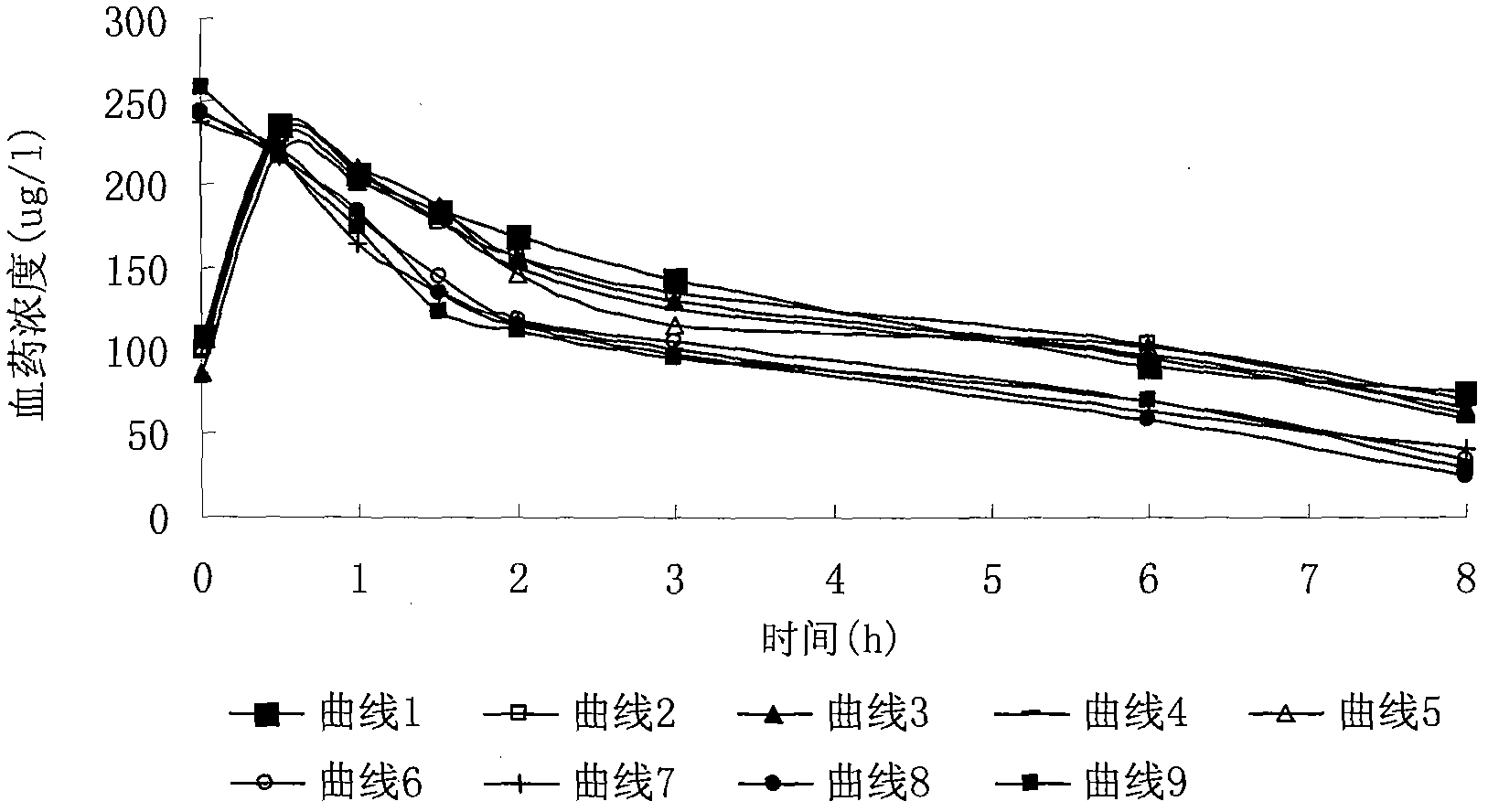
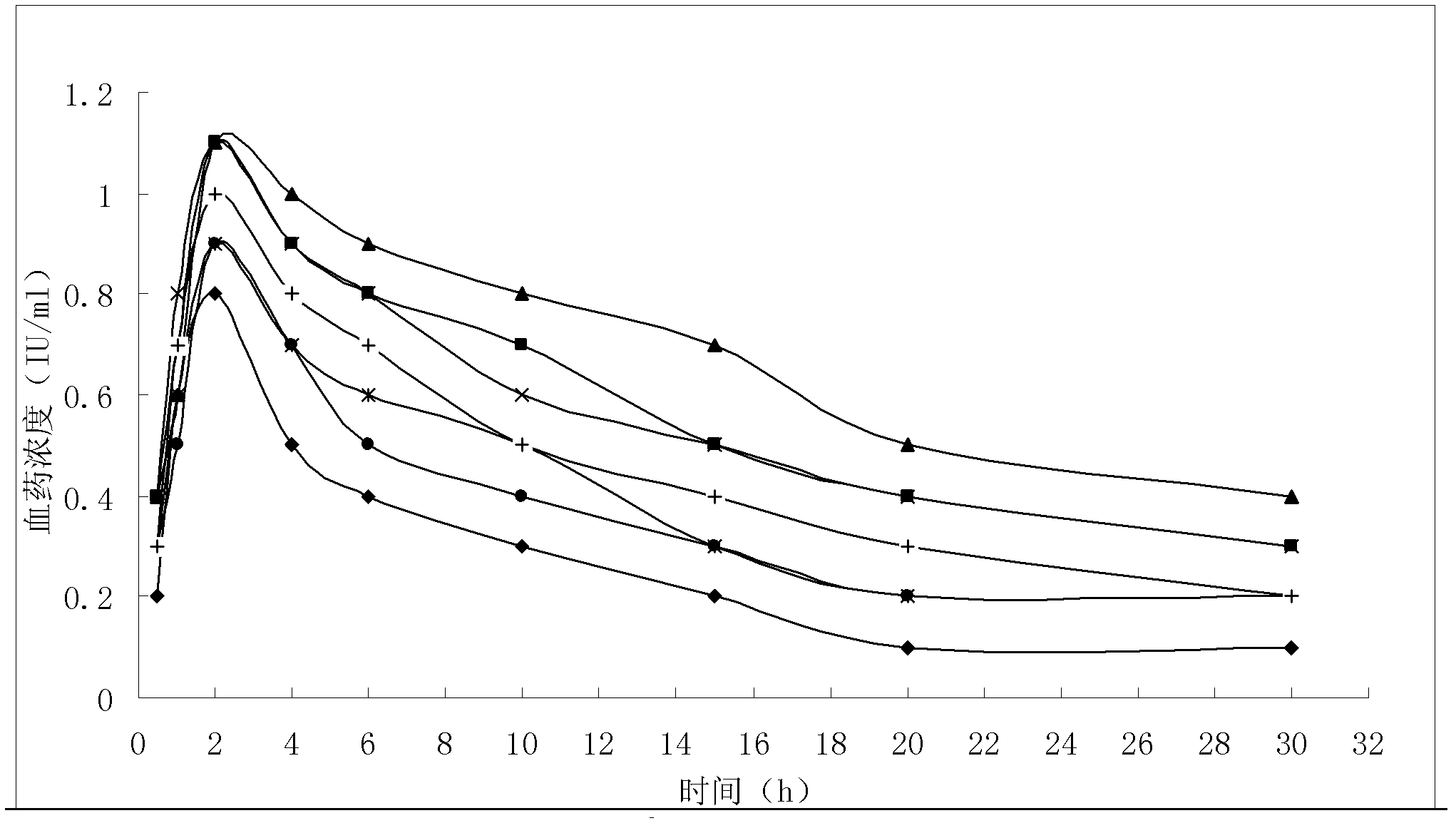
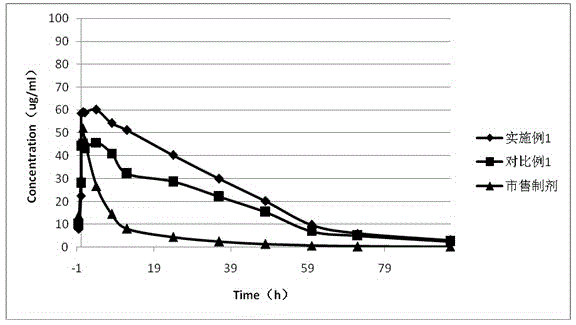
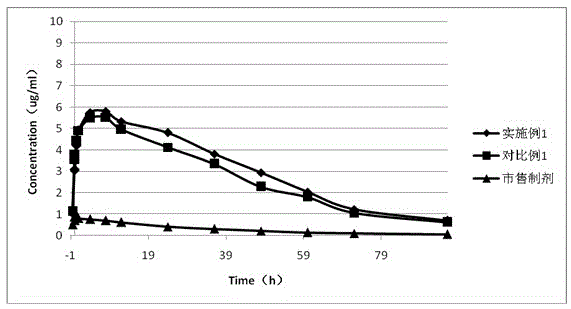
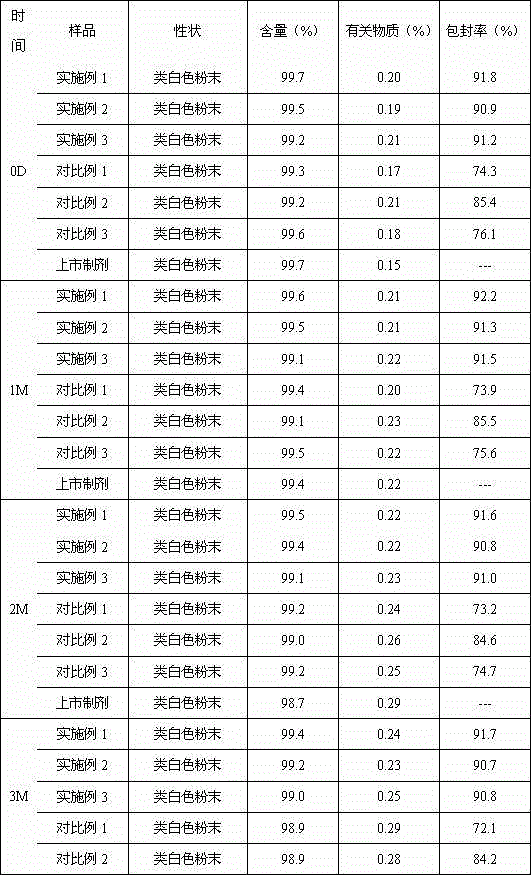
Patents
Literature
136 results about "Liposomal Injection" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
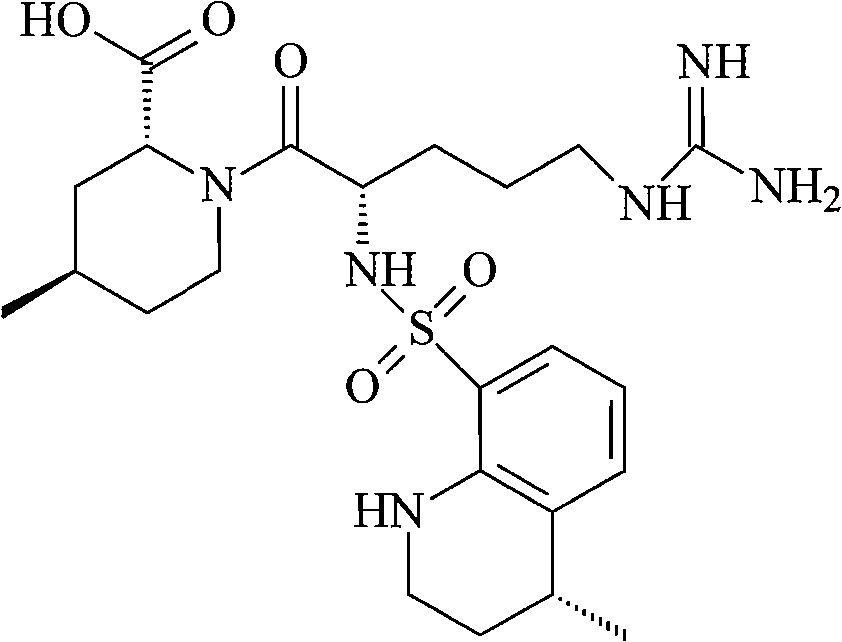
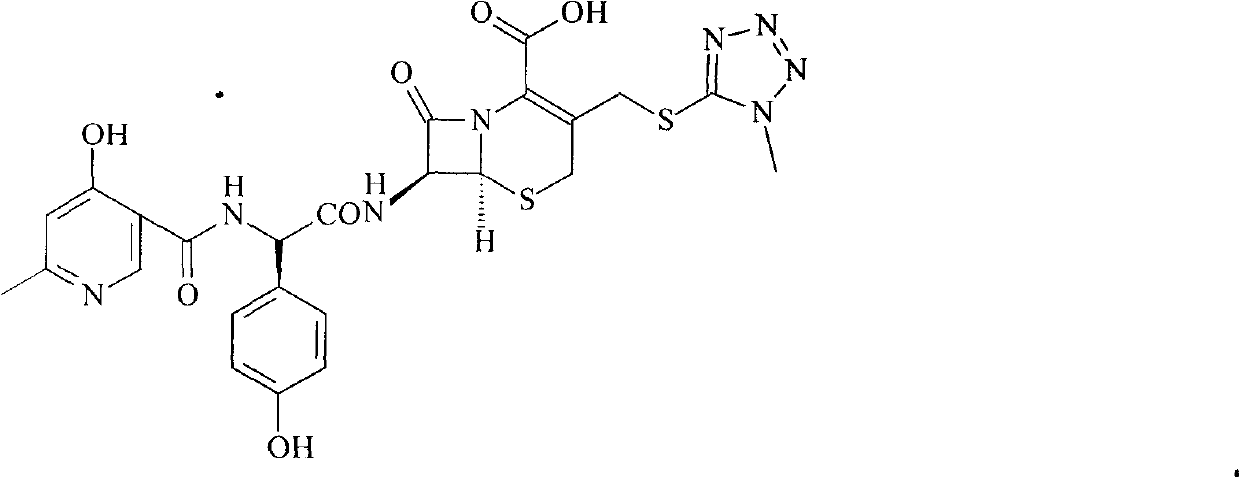
Doxorubicin hydrochloride liposome injection, in combination with bortezomib, is indicated for the treatment of patients with multiple myeloma who have not previously received bortezomib and have received at least one prior therapy.
Sirolimus lipidosome freeze-dried acanthopanax powder and technique of preparing the same
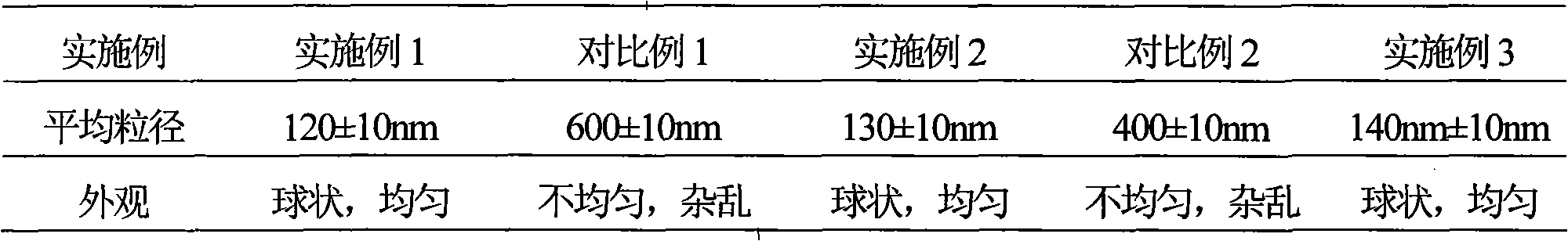
InactiveCN101129361AGood curative effectExtension of timeOrganic active ingredientsPowder deliveryDiffusion methodsFreeze-drying
The invention discloses a xiluomosi liposome freeze dried and making technique, which comprises the following steps: selecting liposome component, buffer, organic solvent, antioxidant and freeze-drying protective as raw material; adopting film diffusion method to make the liposome turbid liquor with xiluomosi through high-pressure or hypersonic dispersing method evenly; drying the liposome to improve the storage stability obviously; dispersing the freeze dried at random proportion in the water evenly without any sediment and impurity; making the packing rate of liposome at 96% with the grain size at 50-250nm; improving the drug effect greatly in comparison with oral agent; lengthening the circulating time in the blood; elevating the biological utility of drug.
Owner:山东华诺生物科技有限公司
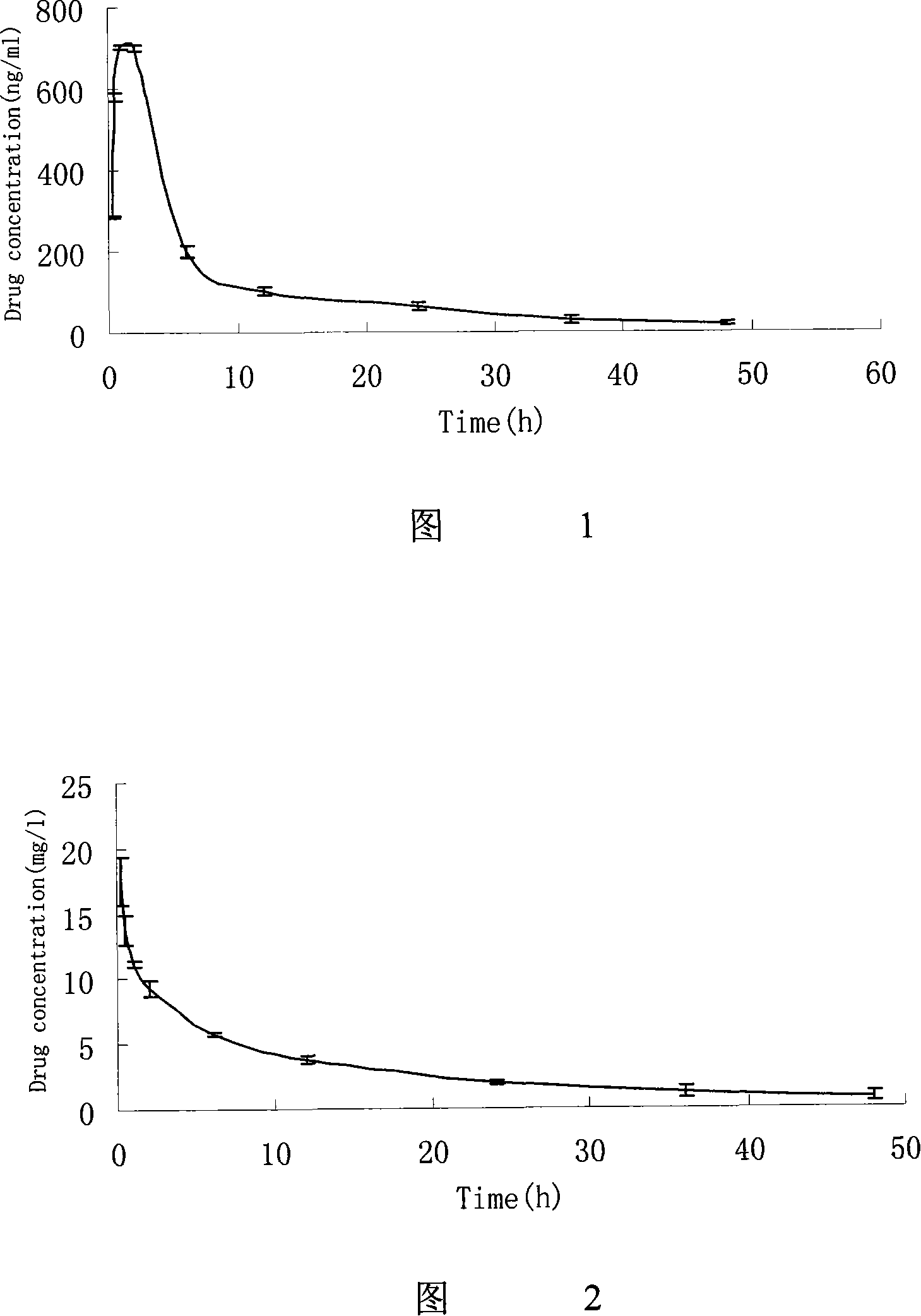
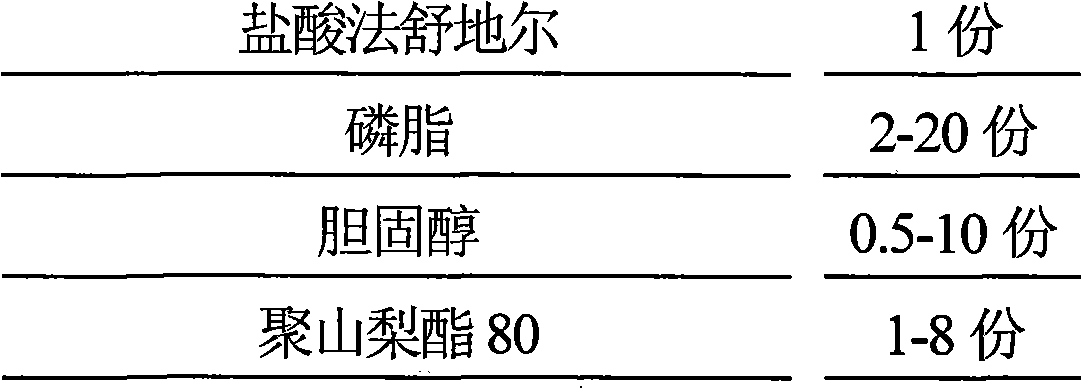
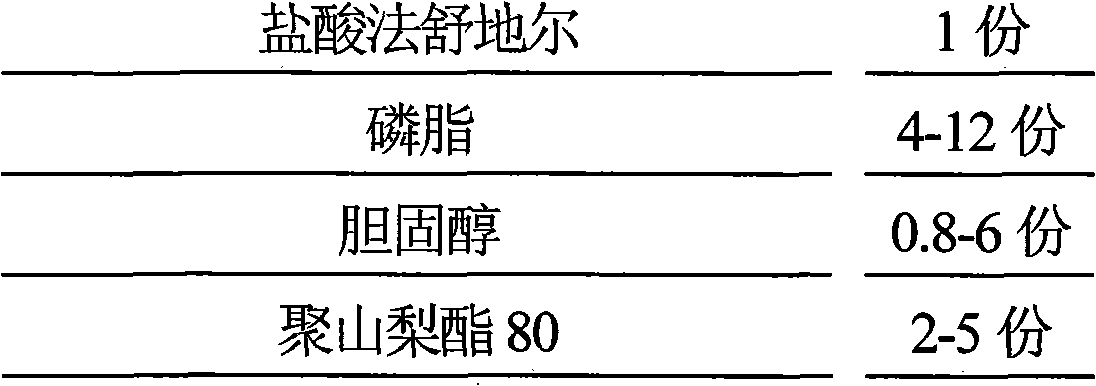
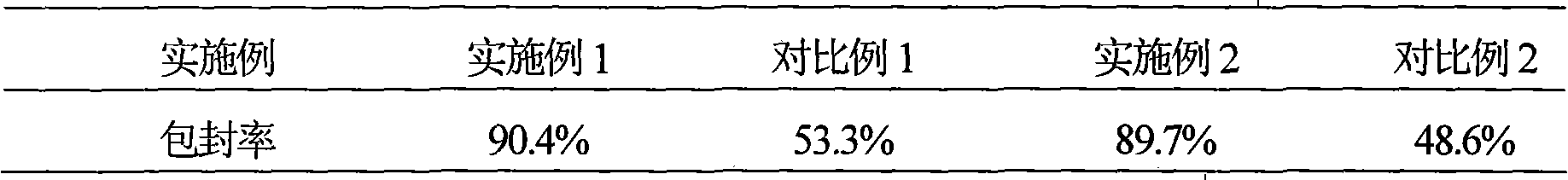
Hydrochloric acid Fasudil liposome injection and new application thereof
InactiveCN101601654AImprove stabilityHigh encapsulation efficiencyOrganic active ingredientsSkeletal disorderLeft vertebral arteryCervical spondylosis
The invention discloses hydrochloric acid Fasudil liposome injection and new application thereof. The injection mainly comprises the following components according to parts by weight: 1 part of hydrochloric acid Fasudil, 2 to 20 parts of phospholipid, 0.5 to 10 parts of cholesterol and 1 to 8 parts of polysorbate 80. Simultaneously, the hydrochloric acid Fasudil liposome injection also can be used for treating vertebral artery type cervical spondylosis.
Owner:HAINAN LINGKANG PHARMA CO LTD
Anthracene nucleus medicinal liposome injection and preparation method
InactiveCN101190188AGood storage stabilityIncreased drug release rateOrganic active ingredientsPowder deliverySide effectFreeze-drying
The invention discloses an anthracycline liposome injection, including the injection and a frozen powder injection and a preparation process of the injection. The frozen liposome consists of the anthracycline or an anthracycline hydrochloride, compound neutral phospholipids, surfactant, negative-charge phospholipids, buffers, PH regulators and freezing protection agent. The preparation process includes the following steps: preparation of a hollow liposome, a homogenized liposome, an anthracycline lopsome and an anthracycline lopsome suspension; adding the freezing protection agent; constant volume; sterilization; sub-package; freezing and drying; storage, etc. The liposome injection can be preserved for 12 months under the room temperature with better stability. And the encapsulation rate can be above 95 percent and a granule diameter is between 30 and 300mm. The side effect is low and the technique is simple, thus being convenient for industrial production.
Owner:北京天衡药物研究院有限公司
Argatroban liposome injection
InactiveCN102366410AHigh encapsulation efficiencyGood formulation stabilityDipeptide ingredientsPharmaceutical non-active ingredientsSide effectMedicine
The invention discloses an Argatroban liposome injection and a preparation method thereof. The Argatroban liposome injection with excellent quality is prepared by selecting Argatroban, sphingomyelin, octadecylamine, and tween 80 according to specific proportion. Compared with preparations in the prior art, the injection disclosed herein has improved stability and bioavailability, improved qualityof the preparation products, reduced toxic and side effect, stable drug release, and remarkable curative effect.
Owner:HAINAN LINGKANG PHARMA CO LTD
Liposome injection of amoxicillin sodium sulbactam sodium medicinal composition
InactiveCN101822669AImprove stabilityHigh encapsulation efficiencyAntibacterial agentsPowder deliveryDipalmitoylphosphatidylcholineFreeze-drying
The invention provides liposome injection of an amoxicillin sodium sulbactam sodium medicinal composition. The liposome injection consists of amoxicillin sodium, sulbactam sodium, liposome carriers, freeze-drying supporting agents and optional antioxidant, wherein the liposome carriers are dipalmitoyl phosphatidyl choline and deoxysodium cholate. The liposome injection has high stability; in the freeze-drying process, a phenomenon of breaking liposome caused by dehydration, fusion, ice crystal generation and the like does not occur; and the liposome can remain good encapsulation ratio after being hydrated and redissolved.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
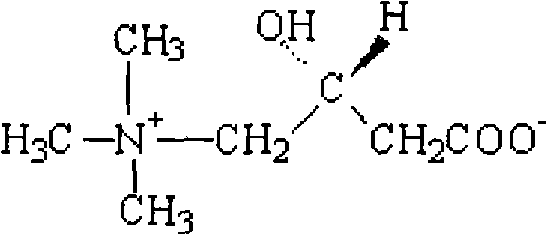
Levocarnitine liposomes injection
InactiveCN101637450AUnexpected effectImprove stabilityOrganic active ingredientsMetabolism disorderAcute toxicity testingCholesterol
The invention discloses levocarnitine liposomes injection, which is characterized by comprising the following active ingredients in parts by weight: 1 part of levocarnitine, 3-15 parts of soybean lecithin, 0.4-7.5 parts of cholesterol and 0.02-1 part of antioxidant, and a pharmaceutically acceptable carrier, wherein, the antioxidant is one or more of L-cysteine, thiourea, vitamin E and butylated hydroxyanisole and is most preferably the vitamin E. The invention also discloses a preferable preparation method of the levocarnitine liposomes injection, namely ammonium sulphate pH gradient method.The invention provides levocarnitine liposomes injection which has excellent stability, high entrapment rate and low leakage rate in the process of long-term storage. The acute toxicity tests, unusualtoxicity tests and heat source tests adopting the levocarnitine liposomes injection all conform to the specifications and are applicable to industrialized production.
Owner:HAINAN YONGTIAN PHARMA INST
Pemetrexed disodium liposome injection
InactiveCN103040748AInhibit aggregationLarge particle sizeOrganic active ingredientsPharmaceutical non-active ingredientsSolubilityMedicine
The invention discloses pemetrexed disodium liposome injection which is mainly made of pemetrexed disodium and a preparation method thereof, distearoyl phosphatidyl glycerol, dipalmitoyl phosphatidyl choline, PEG600, cholesterol and mannitol. The liposome injection has the advantages that the particle size of liposome is small, the liposome is uniformly distributed, the encapsulation efficiency is high, the leakage rate is low, the stability is good, the solubility of the pemetrexed disodium and the quality of injection products are improved, the toxic and side effect is reduced, and the curative effect is improved.
Owner:海南路易丹尼生物科技有限公司
Edaravone liposome injection and new application thereof
InactiveCN101601656AImprove solubilityImprove stabilityOrganic active ingredientsPharmaceutical non-active ingredientsCholesterolThrombus
The invention discloses an edaravone liposome preparation form. The edaravone liposome injection is prepared from excipients, such as edaravone, phospholipid, cholesterol, polysorbate 80, and the like. The invention further discloses new application of the edaravone liposome injection in treating thrombus type phlebitis.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
Liposome injection of pharmaceutical composition of amoxicillin sodium and clavulanate potassium
InactiveCN101804051AImprove stabilityHigh encapsulation efficiencyAntibacterial agentsPharmaceutical non-active ingredientsFreeze-dryingAntioxidant
The invention provides a liposome injection of a pharmaceutical composition of amoxicillin sodium and clavulanate potassium, which consists of amoxicillin sodium, clavulanate potassium, a liposome carrier, a lyoprotectant and a freely optional antioxidant, wherein the liposome carrier is egg yolk phosphatidylinositol and sodium taurocholate. The liposome injection has good preparation stability, liposome can avoid the fracture due to dehydration, fusion, ice crystal generation and the like during the freeze-drying process, and the liposome can still keep the good encapsulation rate after hydration and redissolution.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
Icaritin liposome and preparation method thereof
InactiveCN102133184AIncrease concentrationLow toxicityOrganic active ingredientsDigestive systemOrganic solventMass ratio
The invention provides icaritin liposome and a preparation method thereof. The lcaritin liposome mainly contains the following raw materials according to mass ratio: 0.01-5% of icaritin, 0.05-88% of phospholipid, 0-8% of plastification materials, 0-20% of propping agent and the balance of injection water. The preparation method comprises the following steps: completely dissolving the icaritin and the phospholipid in a organic solvent, then adopting a rotating film evaporation method for volatilizing the organic solvent completely; adding the injection water, continuously rotating a film under the normal pressure until the film is hydrated completely; emulsifying the mixture so as to obtain colostrum, conducting ultrasonic and homogenate on the colostrum, and then adopting the injection water for constant volume; and adjusting the PH value to be 4-8, then obtaining injecta preparation of the icaritin liposome, and further conducting vacuum cooling and drying on the icaritin liposome, then obtaining icaritin liposome powder preparation. Due to the icaritin liposome preparation, the icaritin treatment has better liver target property, and the stability and the bioavailability of a medicine are improved.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
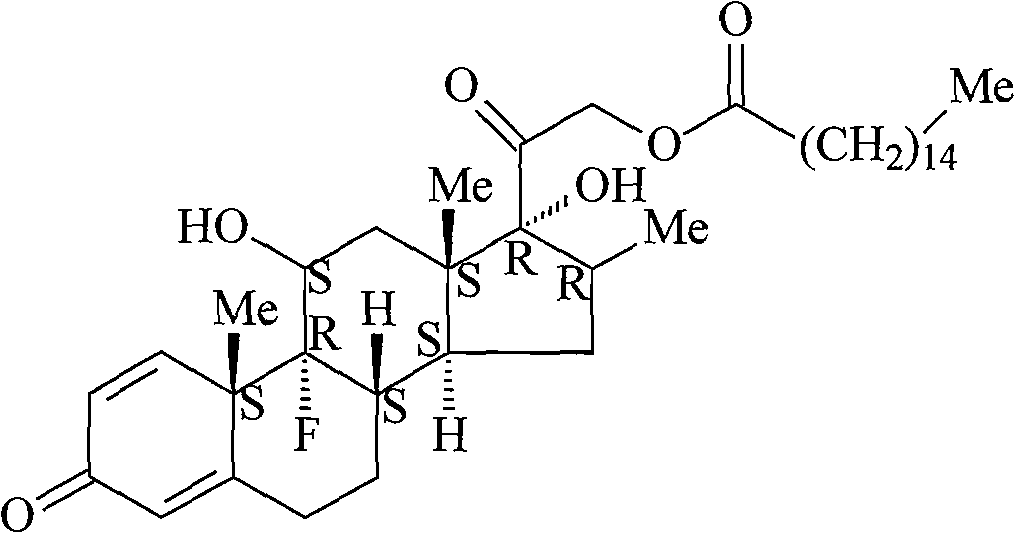
Dexamethasone palmitate acid liposome injection
InactiveCN102366411AWon't breakHigh encapsulation efficiencyOrganic active ingredientsPowder deliverySide effectCholesterol
The invention discloses a dexamethasone palmitate acid liposome injection and a preparation method thereof. The liposome injection is prepared from dexamethasone palmitate, dispermaceti phospholipids, cholesterol, tween80, glucose and dextran according to a certain weight ratio. The liposome injection provided by the invention has good preparation stability. During a freezing process, rupture of liposome due to fusion and ice crystals are prevented. After long-term storage, good entrapment rate is maintained in the liposome. With the injection and the method provided by the invention, dissolubility of dexamethasone palmitate is improved, quality of the preparation product is improved, toxic and side-effects are reduced, retention time of the medicine in systemic circulation is prolonged, bioavailability of the medicine is improved, and the curative effect is improved. The preparation method is simple, and the method is suitable for industrial productions.
Owner:HAINAN YONGTIAN PHARMA INST
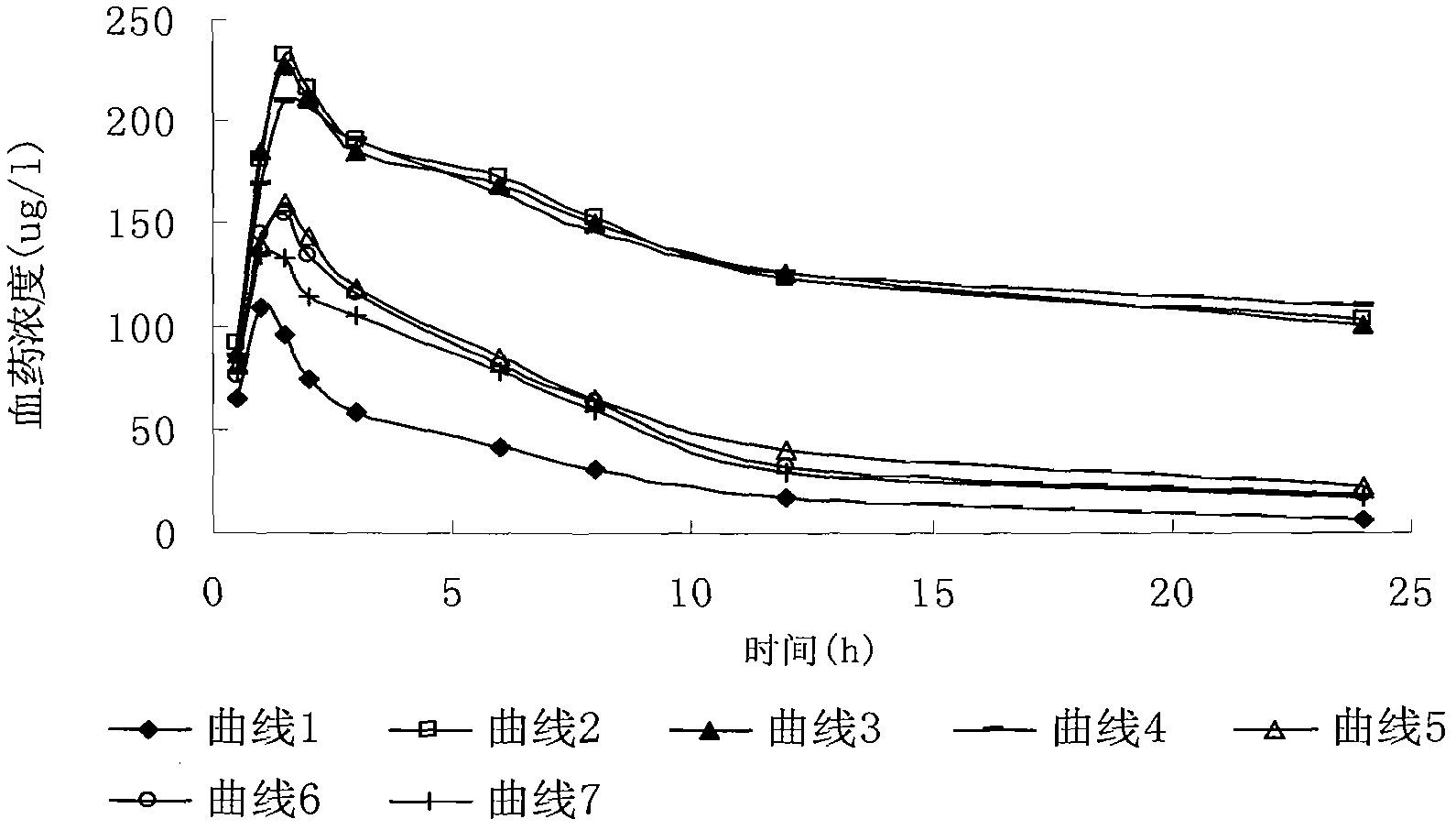
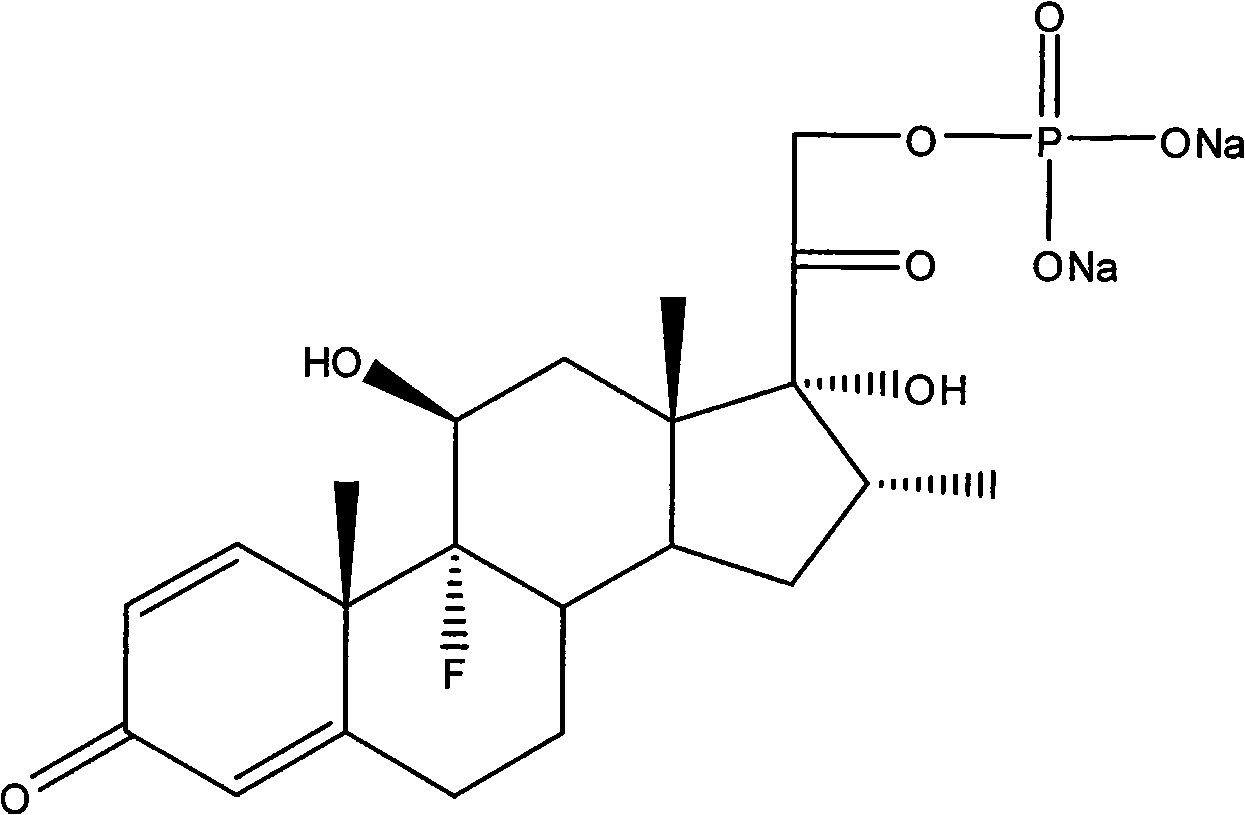
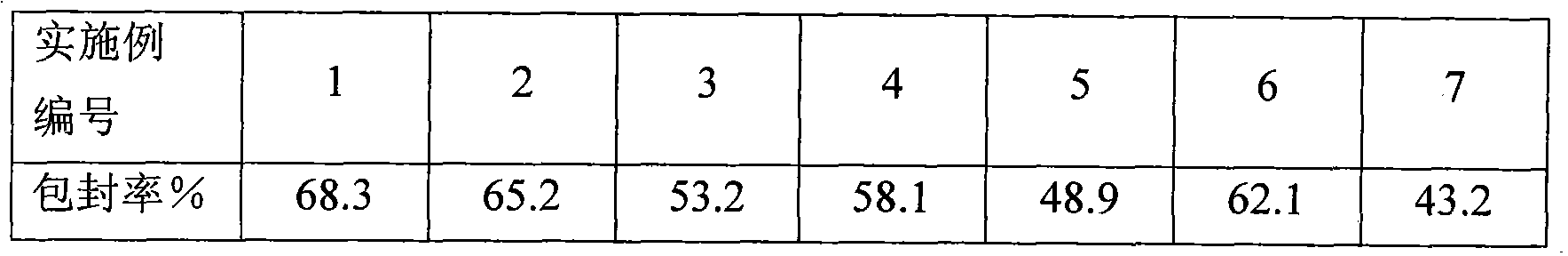
Dexamethasone sodium phosphate lipidosome injection
InactiveCN101926769AGood curative effectReduce dosageOrganic active ingredientsAntipyreticPh bufferingActive component
The invention relates to a lipidosome injection with dexamethasone sodium phosphate as an active component, which comprises dexamethasone sodium phosphate lipidosomes and one or more medical auxiliary materials for injection, wherein the liposome encapsulation rate of the active component is 40-70 percent, and the lipidosome injection comprises 0.025-0.5 percent of the dexamethasone sodium phosphate as the active component, 0.125-5 percent of phospholipid, 0.002-1 percent of lipophilic additive, a pH buffering agent with the pH value kept at 7-7.5 and the balance of water for injection.
Owner:TIANJIN JINYAO GRP
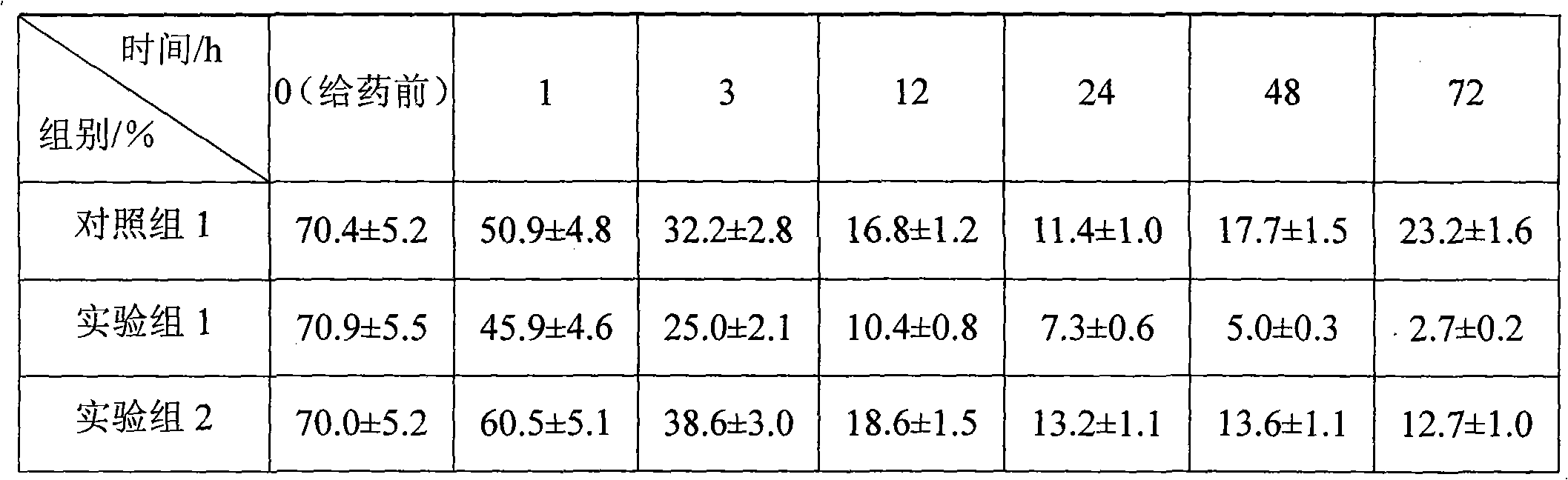
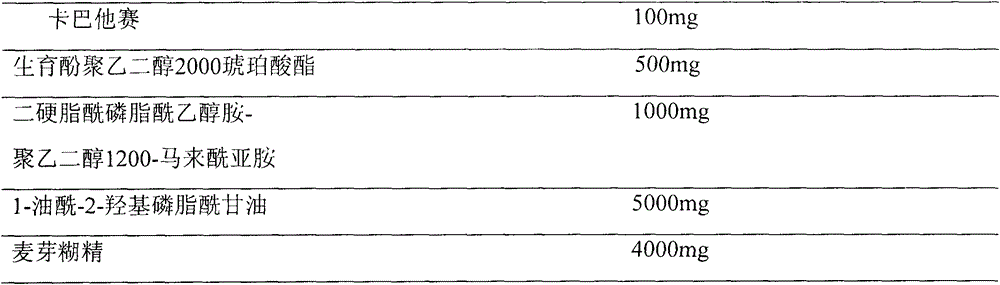
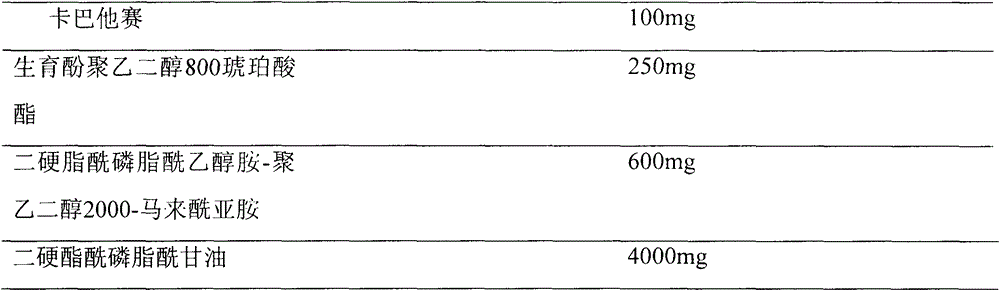
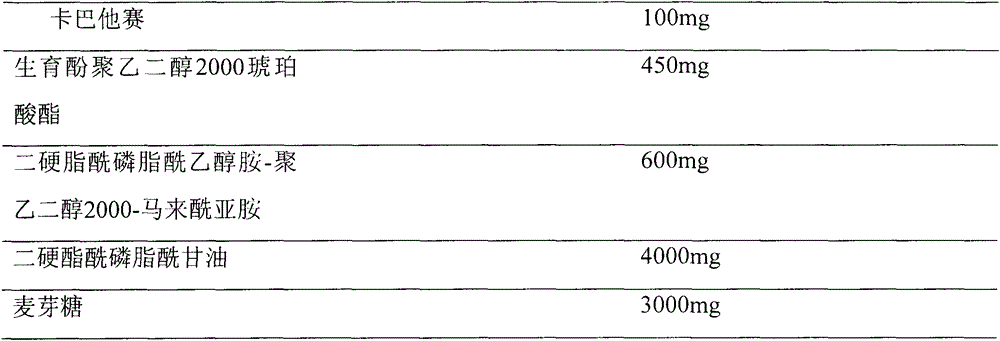
Cabazitaxel long-circulation liposome injection and preparation method thereof
ActiveCN104473873AHigh encapsulation efficiencyImprove bioavailabilityOrganic active ingredientsPharmaceutical non-active ingredientsMedicinePhospholipid
The invention provides cabazitaxel long-circulation liposome injection and a preparation method thereof. The cabazitaxel long-circulation liposome injection comprises cabazitaxel or a pharmaceutically acceptable salt thereof, tocopherol polyethylene glycol succinate, distearoyl-phosphatidylethanolamine-polyethylene glycol-maleimide and phospholipid. The cabazitaxel long-circulation liposome injection is prepared by a film method. The liposome not only has the advantages of reduced toxicity, simple and convenient clinical use and improved bioavailability, but also has good stability of storage and use.
Owner:南京西默思博检测技术有限公司
Carmustine precursor liposome powder injection formulation and process for preparing
InactiveCN1520808AAvoid slow breakdownAvoid side effectsAmide active ingredientsAntineoplastic agentsSide effectSterol
The precursor liposome powder for injection preparation has Carmustine in 3-12 wt% as main material and other components including phospholipid 19-25 wt%, sterol 3.5-6.5 wt% and support agent 54-73 wt%. Carmustine, phospholipid and sterol are compounded into solution; the solution is added into the support agent powder gradually and the mixture is vacuum dried to form Carmustine powder for injection preparation. Before use, the Carmustine powder for injection preparation is mixed with water for injection to form Carmustine liposome injection preparation. The injection preparation has Carmustine coated in double-layer liposome film of phospholipid and sterol and thus less toxic side effect of Carmustine to human body, so that it may be used continuously for raised cancer treating effect.
Owner:谷松兰 +4
Flumazenil liposome injection
InactiveCN102247324AWon't breakHigh encapsulation efficiencyOrganic active ingredientsNervous disorderSolubilitySide effect
The invention discloses a flumazenil liposome injection and a preparation method thereof. The liposome injection is prepared from flumazenil, cholesterol, phosphatidyl ethanolamine, soyasterol, tween 80, trehalose and polyvinylpyrrolidone in specific proportions by weight. The liposome injection disclosed by the invention has favorable preparation stability, the liposome can not be ruptured due to fusion, ice crystal and the like in the freezing process, and the liposome keeps the same favorable entrapment rate after long-term storage. The flumazenil liposome injection disclosed by the invention improves the solubility of flumazenil, improves the quality of the preparation product, reduces the toxic side effect, increases the retention time of the medicament in systemic circulation, improves the bioavailability of the medicament, obviously improves the curative effect, has a simple preparation method and is suitable for industrialized big production.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
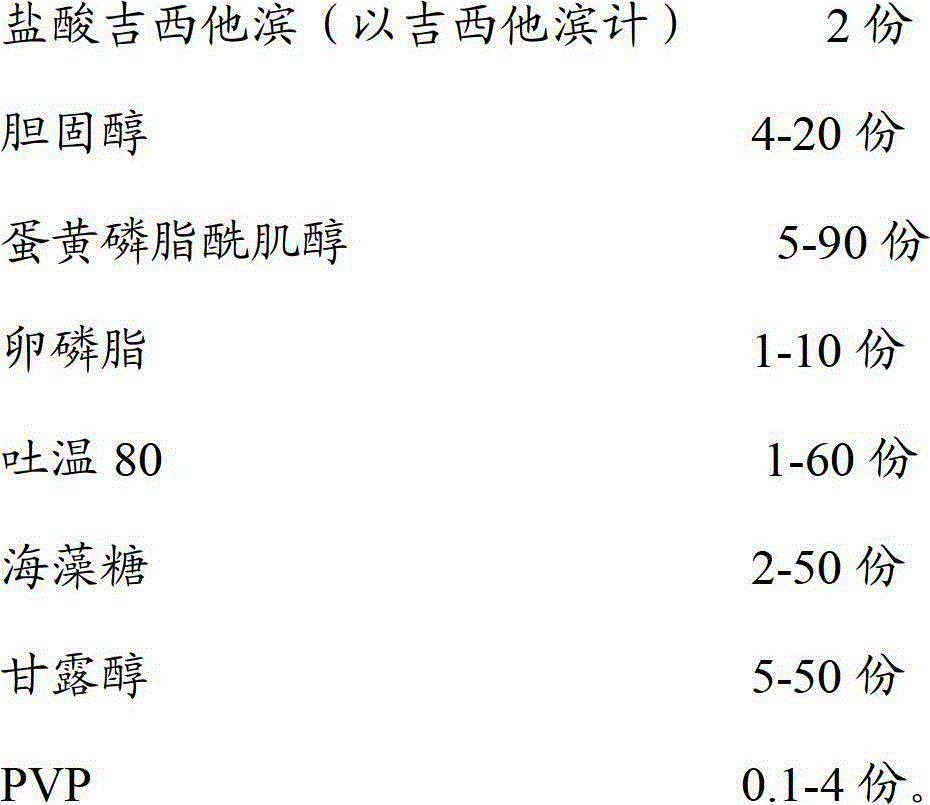
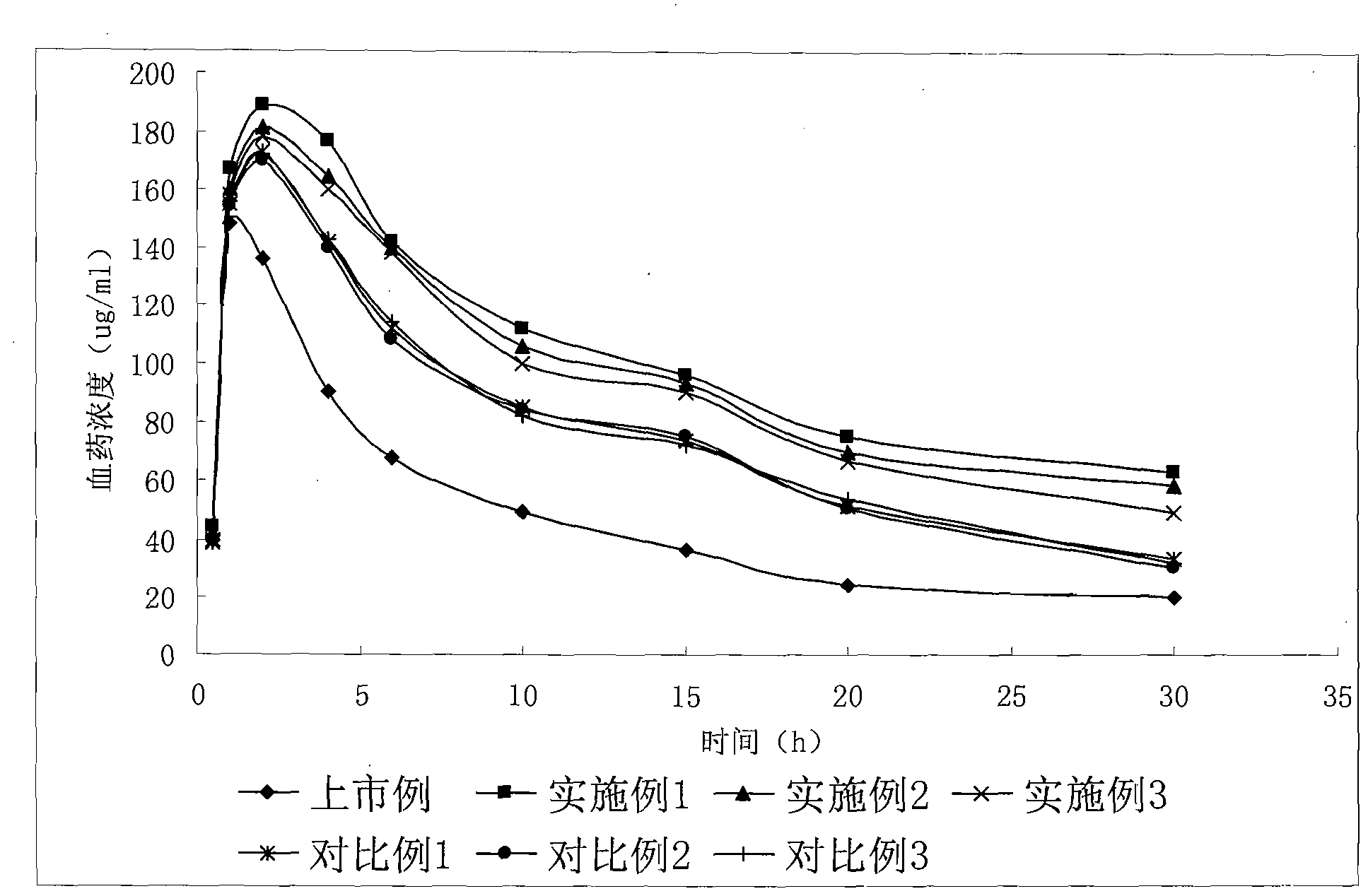
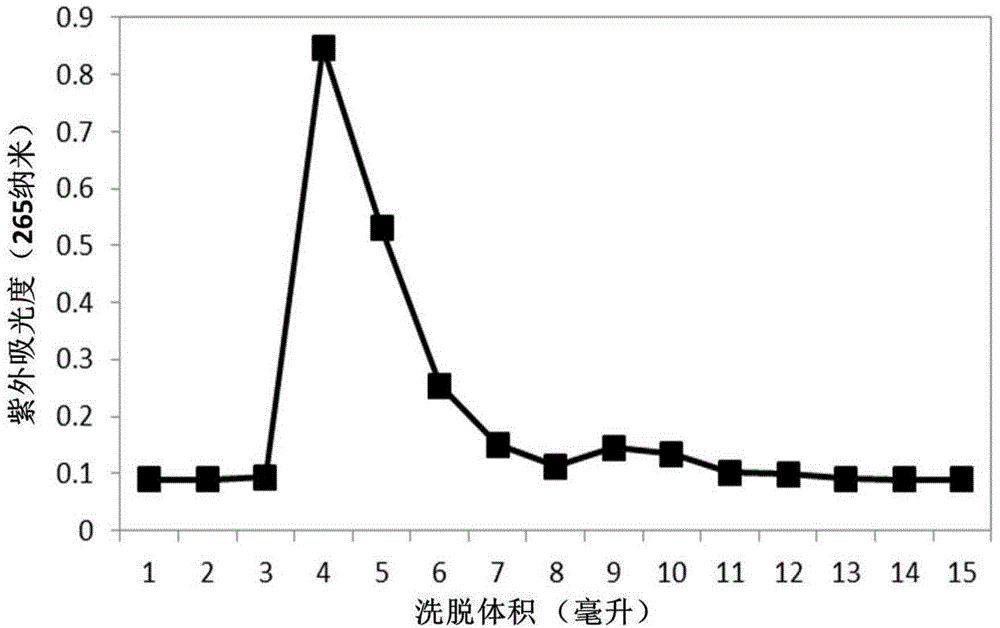
Gemcitabine hydrochloride liposome injection
InactiveCN102716089AWon't breakHigh encapsulation efficiencyOrganic active ingredientsPowder deliverySolubilitySide effect
The invention discloses a gemcitabine hydrochloride liposome injection and a preparation method thereof. The gemcitabine hydrochloride liposome injection is prepared from the following components in specific percentage by weight: gemcitabine hydrochloride, cholesterol, egg yolk phosphatidylinositol, phosphatidylcholine, tween 80, trehalose, mannitol and polyvinylpyrrolidone. The liposome injection is high in stability and cannot be cracked due to fusion, ice crystal and the like during freezing; and after long-term storage, liposome is still high in package rate. According to the gemcitabine hydrochloride liposome injection, the solubility of gemcitabine hydrochloride is improved; the quality of a product is improved; the toxic and side effects are reduced; the retention time of medicines during general circulation is prolonged; the biological utilization rate of the medicine is increased; the treating effect is obviously enhanced; the preparation method is simple; and the gemcitabine hydrochloride liposome injection is suitable for industrial large-scale production.
Owner:灵康药业集团股份有限公司
Liposome injection based on drug combination of mezlocillin sodium and sulbactam sodium
InactiveCN101804052AImprove stabilityHigh encapsulation efficiencyAntibacterial agentsPharmaceutical non-active ingredientsAntioxidantDissolution
The invention provides a liposome injection based on a drug combination of mezlocillin sodium and sulbactam sodium. The liposome injection comprises the following components: mezlocillin sodium, sulbactam sodium, liposome carriers, frozen and dried supporting agent and optional existing antioxidant, wherein the liposome carriers are particularly hydrogenated soybean phosphatidylcholine and octadecylamine. The liposome injection of the invention has good preparation stability, prevents the liposome from being cracked under the action of dehydration, fusion and ice crystal generation in the freezing-drying process, and keeps good entrapment rate of the liposome after re-dissolution through hydration.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
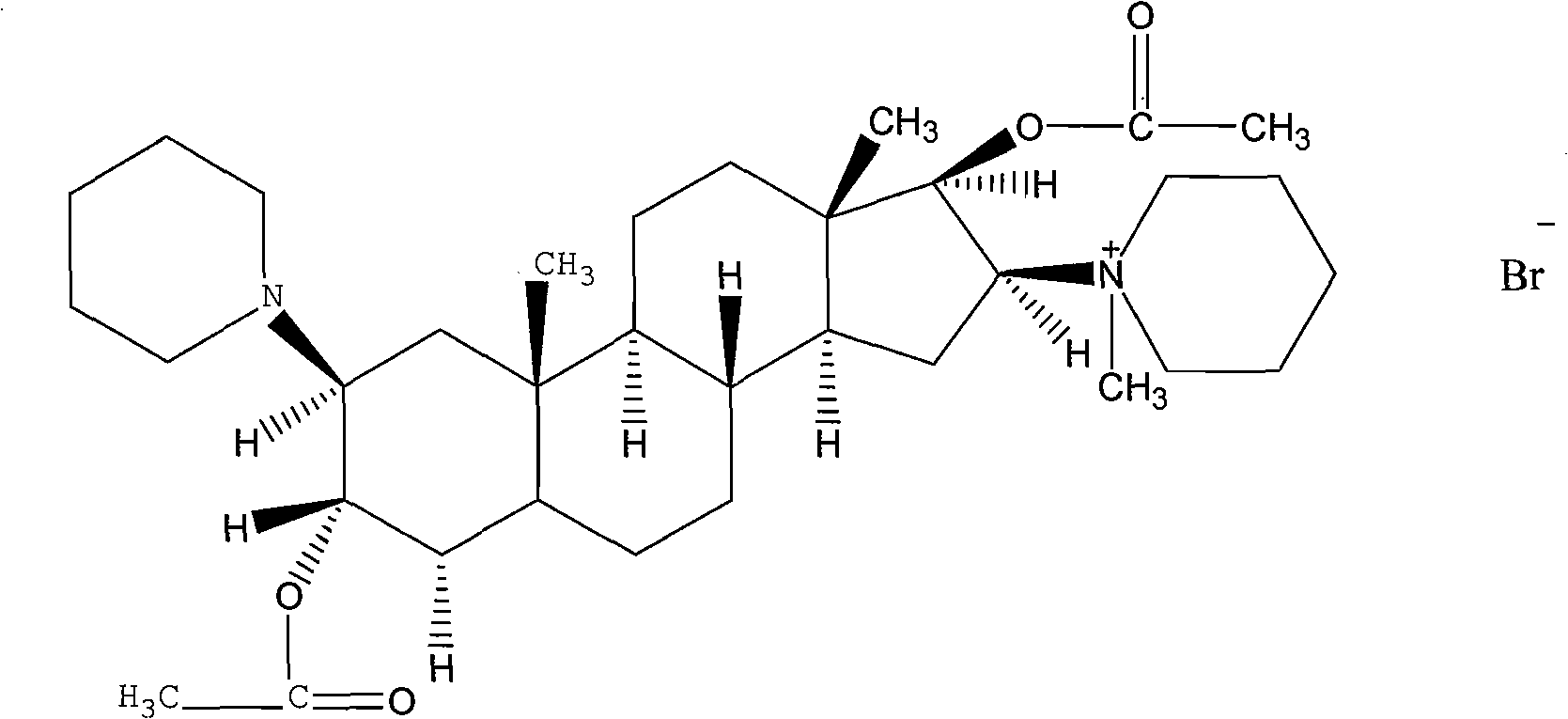
Vecuronium bromide liposome injection
InactiveCN102319215AImprove stabilityProtection formOrganic active ingredientsMuscular disorderSolubilitySide effect
The invention discloses a vecuronium bromide liposome injection and a preparation method thereof. The liposome injection is prepared from vecuronium bromide, cholesterol, phosphatidyl ethanolamine, soyabean lecithin, twain 80, trehalose, mannitol and polyvinylpyrrolidone in a specific weight ratio. The liposome injection has high preparation stability, and the liposome is not cracked due to fusion, ice crystals and the like in the refrigerating process and keeps high entrapment efficiency even after being stored for a long-time. Due to the adoption of the injection, the solubility of the vecuronium bromide is improved, the quality of a preparation product is improved, toxic and side effects are reduced, the retention time of a medicament is increased during systemic circulation, the bioavailability of the medicament is enhanced, the curative effect is improved remarkably; and moreover, the preparation method is simple and is suitable for industrial production.
Owner:HAINAN LINGKANG PHARMA CO LTD
Sorafenib lipidosome freeze-dried injection for injection and preparation method thereof
InactiveCN104523607AImprove bioavailabilitySmall dosePowder deliveryLyophilised deliveryCholesterolFreeze-drying
The invention discloses a sorafenib lipidosome freeze-dried injection for injection and a preparation method thereof. The freeze-dried injection is prepared from the following components in molar ratio: 1 mol of sorafenib, 5-60 mol of phospholipid, 1-30 mol of cholesterol, 1-60 mol of phosphatidyl glycerol, 1-10 mol of a polyethylene glycol surfactant and 50-400 mol of a freeze-drying protecting agent. The preparation method comprises the following steps: sequentially dissolving phospholipid, cholesterol, phosphatidyl glycerol, polyethylene glycol surfactant and sorafenib in an organic solvent in proportion; then, carrying out rotary and vacuum evaporation on the obtained organic solution in a round bottom glass bottle, so that a uniform thin film is formed on the inner wall of the glass bottle; then, adding a buffer liquid with the pH of 7-10 into the glass bottle to be continuously stirred to form a suspension; reducing the grain size of the suspension by an ultrasonic or high-pressure homogenizing method; and adding the freeze-drying protecting agent and freeze-drying. The sorafenib lipidosome disclosed by the invention remarkably improves the bioavailability and is stable in property, and the encapsulation efficiency can reach over 96%.
Owner:TIANJIN ZHONGXI MEIHUA BIOMEDICAL TECH
Thymalfasin liposome preparation for injecting
InactiveCN102579347ARound shape and not aggregatedImprove stabilityPeptide/protein ingredientsDigestive systemSolubilitySucrose
The invention discloses a thymalfasin liposome preparation for injecting and a preparation method thereof. The thymalfasin liposome preparation for injecting is prepared from thymalfasin, cholesterol, egg yolk lecithin, phosphatidyl inositol, sucrose ester and a pharmaceutically-acceptable carrier in a specific weight ratio, wherein the carrier is preferably mannitol and fucose. A liposome injection disclosed by the invention has high preparation stability, a liposome is prevented from cracking due to fusion, ice crystals and the like in a cooling process, and the liposome still keeps high packing rate and high stability after long-time storage. Due to the adoption of the thymalfasin liposome preparation, the solubility of thymalfasin is increased, the quality of a preparation product is improved, toxic and side effects are reduced, the retaining time of a medicament in body circulation is prolonged, the bioavailability of the medicament is increased, and a curative effect is increased remarkably; and moreover, the preparation method is simple, and is suitable for industrial production.
Owner:HAINAN LINGKANG PHARMA CO LTD
Gastrodin multiphase liposome injection
InactiveCN102626390AHigh encapsulation efficiencySmall toxicityOrganic active ingredientsSenses disorderCholesterolSoybean Phospholipids
The invention discloses a gastrodin multiphase liposome injection and a preparation method thereof. Gastrodin, soybean phosphatidylinositol, cholesterol, Twain 80, polyoxyethylene 40, hydrogenated castor oil, trehalose and EDTA-2Na which have specific weight ratios are prepared into the gastrodin multiphase liposome injection. The gastrodin multiphase liposome injection provided by the invention has excellent preparation stability and meets the demand of intravenous injection; after the gastrodin multiphase liposome injection is stored for a long time, the excellent encapsulation of the liposome is kept; the solubility of gastrodin is increased; the quality of preparation product is increased; the toxic side effect is reduced; the retention time of medicine in systemic circulation is increased; the bioavailability of the medicine is increased; the curative effect is obviously increased; and the preparation method is simple and is suitable for industrial production in bulk.
Owner:灵康药业集团股份有限公司
Tropisetron hydrochloride liposome injection
InactiveCN102327222AQuality improvementImprove bioavailabilityDigestive systemLiposomal deliverySide effectCholesterol
The invention provides a tropisetron hydrochloride liposome injection. The tropisetron hydrochloride liposome injection is mainly prepared from tropisetron hydrochloride, glycocholatesodium, cholesterol, gelatin, antioxygen and additive. The liposome injection improves the quality of preparation products, reduces toxic and side effects, improves bioavailability, and is high in preparation stability; and liposome is not broken due to dehydration, fusion, crystallization and the like during freeze-drying, and the liposome still keeps a good entrapment rate after the liposome is subjected to hydration and dissolution.
Owner:HAINAN LINGKANG PHARMA CO LTD
Amoxicillin sodium flucloxacillin sodium medicine compound liposome injection
InactiveCN101810610AImprove stabilityHigh encapsulation efficiencyAntibacterial agentsPowder deliveryCholesterolFreeze-drying
The invention provides an amoxicillin sodium flucloxacillin sodium medicine compound liposome injection which comprises amoxicillin sodium, flucloxacillin sodium, liposome carrier, lyoprotectant and optionally existing preservative, wherein the liposome carrier is soy lecithin and cholesterol. The liposome injection has good preparation stability, the liposome during freeze drying process does not crack caused by dehydration, fusion, ice crystal formation and the like; and likewise, the liposome keeps good encapsulation efficiency after hydration redissolution.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
Cefmenoxime hydrochloride/anhydrous sodium carbonate pharmaceutical composition liposome injection
InactiveCN101785758ATargetedGood clinical effectAntibacterial agentsOrganic active ingredientsCefmenoxime HydrochlorideSodium carbonate anhydrous
The invention provides a cefmenoxime hydrochloride / anhydrous sodium carbonate pharmaceutical composition liposome injection and a preparation method thereof. The cefmenoxime hydrochloride / anhydrous sodium carbonate pharmaceutical composition comprises the following components according to parts by weight: 1 part of cefmenoxime hydrochloride, 0.36-0.44 part of anhydrous sodium carbonate, 1-3 parts of liposome matrix and 0-1 part of additive. The prepared preparation has better stability and ensures medication safety.
Owner:HAINAN LINGKANG PHARMA CO LTD
Monosialotetrahexosyl ganglioside sodium liposome injection
InactiveCN102366408AHigh encapsulation efficiencyGood formulation stabilityOrganic active ingredientsNervous disorderSide effectSterol
The invention discloses a monosialotetrahexosyl ganglioside sodium liposome injection and its preparation method. The liposome injection is made from monosialotetrahexosyl ganglioside sodium, hydrogenated egg yolk lecithin, soybean derived sterol, sodium deoxycholate, poloxamer 188, sodium bisulfite and other auxiliary materials. The liposome injection provided by the invention has good stability; the liposome will not break due to fusion and ice crystals during the freezing process and will also maintain good entrapment rate after stored for a long time. According to the invention, the quality of the preparation product is improved, toxic and side effects are minimized, retention time of the medicament in systematic circulation is increased, bioavailability and blood-brain barrier permeability of the medicament are raised and the curative effect is obviously enhanced. In addition, the preparation method is simple and is suitable for industrial production.
Owner:HAINAN LINGKANG PHARMA CO LTD
Cefpiramide, sodium benzoate, sodium bicarbonate pharmaceutical composite lipidosome injection
InactiveCN101766571AMature production processEasy to implementAntibacterial agentsOrganic active ingredientsSodium bicarbonateAntioxidant
The invention discloses a cefpiramide, sodium benzoate, sodium bicarbonate pharmaceutical composite lipidosome injection and a preparation method thereof. The cefpiramide, sodium benzoate, sodium bicarbonate pharmaceutical composite lipidosome injection includes cefpiramide, sodium benzoate, sodium bicarbonate, lipidosome matrix, osmotic pressure regulator and antioxidant, wherein, the lipidosome matrix is the mixture of phospholipids, cholesterin and Tween-80. Preferentially, the mass ratio of the phospholipids, the cholesterin and the Tween-80 in the lipidosome matrix is 1-3:2:1; the mass ratio of the cefpiramide and the lipidosome matrix is 1:2-11, preferentially as 1:2-11; the mass ratio of the cefpiramide, the sodium benzoate and the sodium bicarbonate is 1:0.05-0.06:0.6-0.8.
Owner:HAINAN LINGKANG PHARMA CO LTD
Ambroxol hydrochloride liposome injection
InactiveCN102429879AImprove stabilityProtection formPowder deliveryOrganic active ingredientsSolubilitySide effect
The invention discloses an ambroxol hydrochloride liposome injection and a preparation method thereof. The liposome injection is prepared from ambroxol hydrochloride, cholesterol, soybean phosphatidylserine, sojasterol, Tween 80, trehalose and PVP (Polyvinylpyrolidone) in a specific weight ratio. The liposome injection has high preparation stability, and a liposome does not crack due to fusion, ice crystals and the like and keeps high entrapment rate simultaneously after being stored for a long time; the solubility of the ambroxol hydrochloride is increased, the quality of a preparation product is enhanced, the toxic and side effects are reduced, the retention time of a medicament in systemic circulation is increased, the bioavailability of the medicament is enhanced, and the curative effect is enhanced remarkably; and moreover, a preparation method is simple, and is suitable for industrial mass production.
Owner:HAINAN LINGKANG PHARMA CO LTD
Calcium heparin liposome preparation for injection
InactiveCN102552149ARound shape and not aggregatedImprove stabilityOrganic active ingredientsPharmaceutical non-active ingredientsSolubilityCholesterol
The invention discloses a calcium heparin liposome preparation for injection and a preparation method thereof. The liposome injection is made from the following components in a specific weight ratio: calcium heparin, cholesterol, phosphatidyl glycerol, lecithin, polyoxyethylene (40) hydrogenated castor oil, trehalose, sorbitol and polyvinylpyrrolidone. The liposome injection has good stability; the liposomes are not liable to be dehydrated, fused, crystallized or broken during the freezing process; and the liposomes maintain good encapsulation efficiency and stability after long-term storage.The solubility of calcium heparin is increased, the quality of the product is improved, the toxic and adverse effects are reduced, the retention time of drugs in the systemic circulation is increased, the bioavailability of drugs is improved, and the efficacy is greatly improved. Besides, the preparation method is simple and suitable for industrial production.
Owner:灵康药业集团股份有限公司
Long-circulating rabeprazole liposome composition and preparation method and application thereof
InactiveCN104546718AGood resolubilityHigh encapsulation efficiencyOrganic active ingredientsPowder deliveryCholesterolMetal chelate
The invention relates to the field of medicinal preparations, specifically to a long-circulating rabeprazole liposome composition and its preparation method and application. The composition comprises rabeprazole, lecithin, cholesterol, Phosphatidylethanolamine Pegol, an antioxidant, metal chelate and a freeze-drying protective additive. The liposome injection provided by the invention has good re-dissolubility. After redissolution, particle size is uniform and stability is excellent. Quality of a preparation product is enhanced, half-life period of rabeprazole in the blood circulation system is prolonged, and bioavailability is higher.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Traditional Chinese medicine pro-liposome injection for treating liver cancer and preparation process thereof
InactiveCN101607068AEasy to storeEasy to transportDigestive systemPharmaceutical non-active ingredientsWater useAdemetionine
The invention provides a traditional Chinese medicine pro-liposome injection for treating liver cancer and a preparation process thereof, belonging to the technical field of traditional Chinese medicine preparation. A prescription of the injection comprises 16 to 25 portions of the bunge pricklyash seed olein and 4 to 6 portions of the zedoary turmeric oil by weight. The preparation method comprises the following steps: the bunge pricklyash seed olein medicine materials and the zedoary turmeric oil are prepared to a pro-liposome taking sorbitol as a carrier after being mixed according to a certain ratio and a pro-liposome method. The pro-liposome is shaken by adding water used for injection before being used, and a liposome suspension is formed by hydration to be fed through intravenus drip. A product is a novel pure Chinese drug compound preparation, strengthens the targeting of the drug, enhances the bioavailability, increases the stability of the drug, conceals unpleasant odors, is beneficial to preservation and transportation, has rich raw material sources, low cost and simple preparation technology, and is easy to expand production.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com